Abstract
Stoichiometric amounts of pure reverse gyrase, a type I topoisomerase from the archaebacterium Sulfolobus acidocaldarius were incubated at 75 degrees C with circular DNA containing a single-chain scission. After covalent closure by a thermophilic ligase and removal of bound protein molecules, negatively supercoiled DNA was produced. This finding, obtained in the absence of ATP, contrasts with the ATP-dependent positive supercoiling catalyzed by reverse gyrase and is interpreted as the result of enzyme binding to DNA at high temperature. Another consequence of reverse gyrase stoichiometric binding to DNA is the formation of a cleavable complex which results in the production of single-strand breaks in the presence of detergent. Like eubacterial type I topoisomerase (protein omega), reverse gyrase is tightly attached to the 5' termini of the cleaved DNA. In the light of these results, a comparison is tentatively made between reverse gyrase and the eubacterial type I (omega) and type II (gyrase) topoisomerases.
Full text
PDF
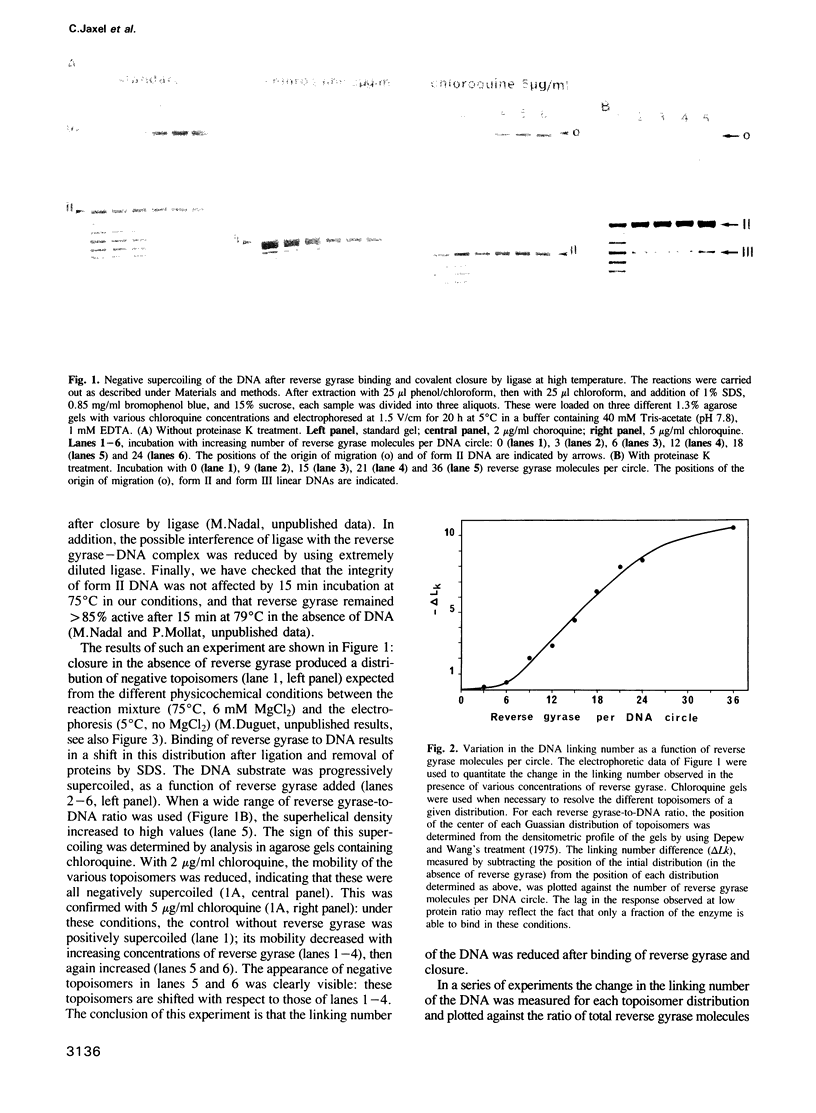
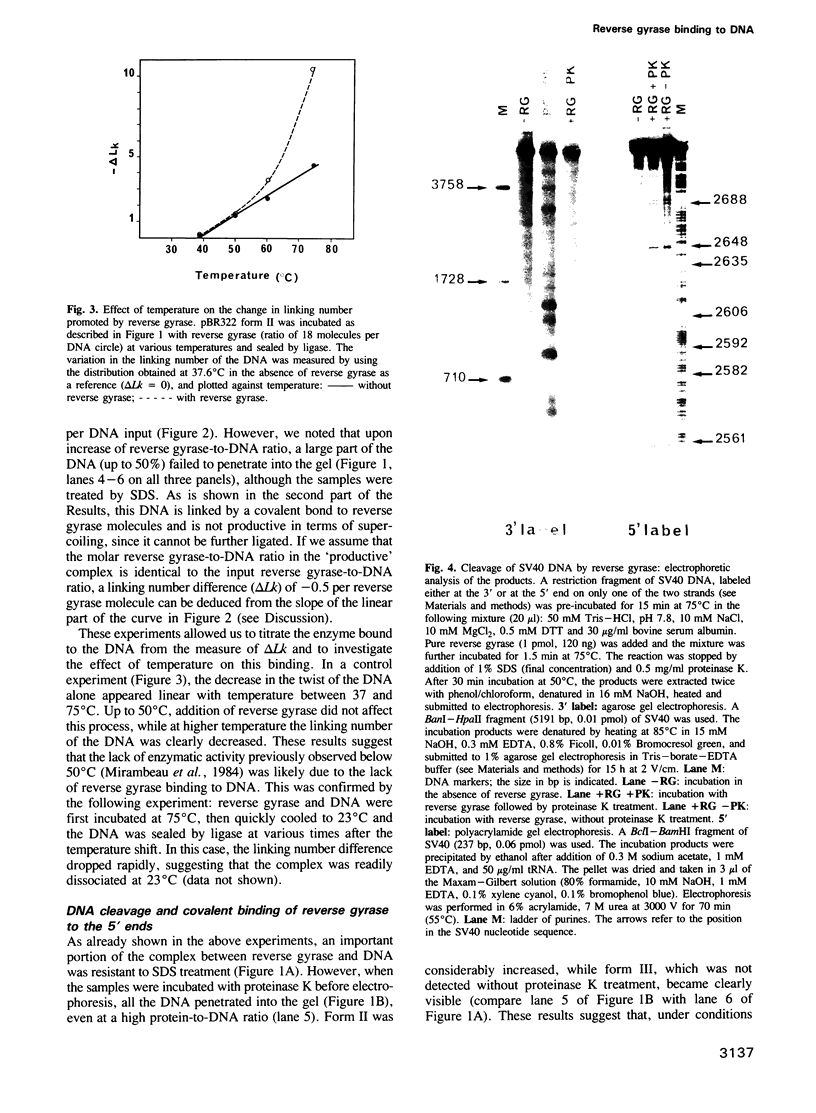


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai R. SV40 DNA: quantitative conversion of closed circular to open circular form by an ethidium bromide-restricted endonuclease. J Mol Biol. 1973 Mar 15;74(4):739–742. doi: 10.1016/0022-2836(73)90062-4. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Cozzarelli N. R. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1979 Nov 30;206(4422):1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Evidence for an intermediate with a single-strand break in the reaction catalyzed by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3488–3491. doi: 10.1073/pnas.73.10.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Mechanism of the reaction catalyzed by the DNA untwisting enzyme: attachment of the enzyme to 3'-terminus of the nicked DNA. J Mol Biol. 1978 Jan 25;118(3):441–446. doi: 10.1016/0022-2836(78)90238-3. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguet M., Lavenot C., Harper F., Mirambeau G., De Recondo A. M. DNA topoisomerases from rat liver: physiological variations. Nucleic Acids Res. 1983 Feb 25;11(4):1059–1075. doi: 10.1093/nar/11.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre P., Mirambeau G., Jaxel C., Nadal M., Duguet M. High positive supercoiling in vitro catalyzed by an ATP and polyethylene glycol-stimulated topoisomerase from Sulfolobus acidocaldarius. EMBO J. 1985 Aug;4(8):2123–2128. doi: 10.1002/j.1460-2075.1985.tb03902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Fisher L. M., Ohmori H., O'Dea M. H., Mizuuchi K. DNA gyrase: site-specific interactions and transient double-strand breakage of DNA. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):391–398. doi: 10.1101/sqb.1981.045.01.053. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Micrococcus luteus DNA gyrase: active components and a model for its supercoiling of DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A., Gellert M. The DNA dependence of the ATPase activity of DNA gyrase. J Biol Chem. 1984 Dec 10;259(23):14472–14480. [PubMed] [Google Scholar]
- Mirambeau G., Duguet M., Forterre P. ATP-dependent DNA topoisomerase from the archaebacterium Sulfolobus acidocaldarius. Relaxation of supercoiled DNA at high temperature. J Mol Biol. 1984 Nov 5;179(3):559–563. doi: 10.1016/0022-2836(84)90080-9. [DOI] [PubMed] [Google Scholar]
- Nadal M., Jaxel C., Portemer C., Forterre P., Mirambeau G., Duguet M. Reverse gyrase of Sulfolobus: purification to homogeneity and characterization. Biochemistry. 1988 Dec 27;27(26):9102–9108. doi: 10.1021/bi00426a006. [DOI] [PubMed] [Google Scholar]
- Nakasu S., Kikuchi A. Reverse gyrase; ATP-dependent type I topoisomerase from Sulfolobus. EMBO J. 1985 Oct;4(10):2705–2710. doi: 10.1002/j.1460-2075.1985.tb03990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesarev A. I. Positive supercoiling catalysed in vitro by ATP-dependent topoisomerase from Desulfurococcus amylolyticus. Eur J Biochem. 1988 Apr 15;173(2):395–399. doi: 10.1111/j.1432-1033.1988.tb14012.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Cozzarelli N. R. The intrinsic ATPase of DNA gyrase. J Biol Chem. 1980 Jul 10;255(13):6299–6306. [PubMed] [Google Scholar]
- Takahashi M., Yamaguchi E., Uchida T. Thermophilic DNA ligase. Purification and properties of the enzyme from Thermus thermophilus HB8. J Biol Chem. 1984 Aug 25;259(16):10041–10047. [PubMed] [Google Scholar]
- Tse Y., Wang J. C. E. coli and M. luteus DNA topoisomerase I can catalyze catenation of decatenation of double-stranded DNA rings. Cell. 1980 Nov;22(1 Pt 1):269–276. doi: 10.1016/0092-8674(80)90174-9. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]