Abstract
In Alzheimer’s disease (AD) basic research and drug discovery, mouse models are essential resources for uncovering biological mechanisms, validating molecular targets and screening potential compounds. Both transgenic and non-genetically modified mouse models enable access to different types of AD-like pathology in vivo. Although there is a wealth of genetic and biochemical studies on proposed AD pathogenic pathways, as a disease that centrally features cognitive failure, the ultimate readout for any interventions should be measures of learning and memory. This is particularly important given the lack of knowledge on disease etiology – assessment by cognitive assays offers the advantage of targeting relevant memory systems without requiring assumptions about pathogenesis. A multitude of behavioral assays are available for assessing cognitive functioning in mouse models, including ones specific for hippocampal-dependent learning and memory. Here we review the basics of available transgenic and non-transgenic AD mouse models and detail three well-established behavioral tasks commonly used for testing hippocampal-dependent cognition in mice – contextual fear conditioning, radial arm water maze and Morris water maze. In particular, we discuss the practical considerations, requirements and caveats of these behavioral testing paradigms.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized clinically by progressive cognitive decline (reviewed in [1]). Currently, AD is the most common type of dementia worldwide; and since age is the biggest risk factor, the prevalence is expected to greatly increase over the next few decades with aging population structures. Unfortunately, despite decades of research, the etiology of AD is unknown, and many fundamental questions remain unanswered. Continuing research into the basic underlying biology of AD as well as renewed efforts in developing disease-modifying drugs are necessary to address this problem. In both the basic research and translational arenas, animal models of the disease are critical. In particular, genetic and non-genetic mouse models of AD pathology have become key research tools for discovering disease pathways and targets as well as testing new therapeutic approaches (reviewed in [2, 3]).
Ultimately, as a disease of synaptic and cognitive failure (reviewed in [4, 5]), both preclinical hypotheses and translational developments in AD research need to address the crucial therapeutic endpoint – amelioration and/or prevention of cognitive dysfunction. Indeed, the most striking characteristic of Alzheimer’s is the progressive decline of cognitive functioning that is caused by massive loss of neurons and synapses. Most importantly, focusing on the behavioral phenotype offers the advantage of avoiding assumptions on the etiopathogenesis of the disease, ones which may be disproved by future studies. The currently-approved medications for AD, which include acetylcholinesterase inhibitors and a N-methyl-D-aspartate receptor (NMDAR) antagonist, offer only minimal temporary improvements in this regard. In testing new therapeutic targets and compounds, mouse models are key resources for providing access to AD-type pathology in vivo concurrently with behavioral testing options. The mouse models offer the ability to validate molecular targets and screen potential compounds on the translational pathway leading to clinical testing.
Several cognitive assays are available for assessing mice, particularly in their hippocampal-dependent learning and memory abilities. Given that AD pathology initiates and is most severe in the hippocampus and entorhinal cortex of the medial temporal lobe (reviewed in [6]), these murine cognitive assays that are hippocampal-dependent are ideally-suited for AD research. The use of these cognitive assays versus other readouts maximizes the likelihood of selecting a target or compound that is relevant for memory systems in vivo.
Among the cognitive assays that test murine learning and memory, we will highlight and discuss three behavioral tasks that are commonly used to examine associative memory and spatial memory: fear conditioning (FC), radial arm water maze (RAWM), and Morris water maze (MWM). Among their advantages, these tasks are straightforward in implementation and allow for the relatively fast assessment of several batches of mice in a short period of time. However, key parameters need to be well-controlled in order to minimize variability in the results and maximize reproducibility between experiments. In this review, we focus on the practical considerations of these assays – the protocols, guidelines and caveats based on our experience with various AD mouse models.
2. Alzheimer’s disease pathology
In Alzheimer’s disease, there are two primary histopathological features evident upon post-mortem examination of brain tissue – amyloid plaques and neurofibrillary tangles (NFTs) (reviewed in [1, 7]). The plaques consist of insoluble extracellular deposits of the amyloid-β (Aβ) peptide and can be observed throughout the cortex. Neurofibrillary tangles consist of aggregates of hyperphosphorylated tau, a microtubule-binding protein. Evident with both the plaques and NFTs, the misfolding and aberrant aggregation of the constituent protein exemplifies a key pathogenic feature of the disease.
Although amyloid plaques were observed histopathologically in AD brains since Alois Alzheimer’s early descriptions [8], the composition of the plaques remained unknown for decades. In 1985, researchers were finally successful in purifying Aβ and identifying it as the predominant constituent of the plaques [9]. A vast amount of subsequent research implicated Aβ as the main molecular culprit in AD pathogenesis, in what is classically known as the “amyloid cascade hypothesis”. Isolating the peptide then led to the identification and sequencing of the amyloid precursor protein (APP), from which Aβ is produced [10].
APP is a type I transmembrane glycoprotein that is abundantly expressed in the brain, particularly by neurons. APP can undergo a series of cleavages by secretase enzymes, one pathway of which results in the production of Aβ peptides. APP contains α-, β- and γ-secretase cleavage sites. Processing at the α-secretase site releases a large portion of the ectodomain and precludes the formation of Aβ since the cleavage occurs within the Aβ sequence. Alternatively, APP can be cleaved at the β-secretase site, which together with an intramembrane γ-secretase cleavage, produces Aβ peptides. In neurons, the sole β-secretase is β-site APP cleaving enzyme 1 (BACE1), a transmembrane aspartyl protease that generates the N terminus of Aβ. The γ-secretase complex is comprised of four subunits: presenilin 1 or 2 (PS1/PS2), nicastrin, presenilin enhancer 2 (PEN-2) and anterior pharynx-defective 1 (APH-1). There are multiple γ-cleavage site possibilities, which result in the production of Aβ peptides with varying lengths (usually 37–43 amino acids). Approximately 90% of secreted Aβ is 40 amino acids long (Aβ40). However, there is also a smaller proportion of 42 residue-long Aβ peptides (Aβ42) that make up <10% of the total Aβ pool. In a series of studies, Aβ42 was found to have a much higher propensity for aggregation compared to the shorter peptides, leading to a focus on Aβ42 as the main amyloidogenic species in AD [11–14].
A strong body of evidence indicates that soluble oligomers of Aβ, consisting of 2 to 12+ peptides, are a primary neurotoxic culprit in AD pathogenesis (reviewed in [5]). In contrast to amyloid plaques, which do not correlate well with cognitive decline, soluble Aβ species are significantly correlated with disease symptoms and severity [15, 16]. These aggregates can be formed from synthetic or natural Aβ peptides, including those secreted by cells or directly isolated from the brain tissue of AD patients and transgenic mouse models. Many studies have established that Aβ oligomers can exert detrimental effects on neuronal physiology and synaptic transmission. For example, Aβ oligomers appear to preferentially bind to or cluster at synapses, with one study observing that >90% of Aβ oligomer binding in neurons occurs at dendritic spines at sites positive for PSD-95, a marker for post-synaptic compartments [17]. On a structural level, Aβ oligomers have been shown to cause changes in spine morphology and decreases in spine density [18, 19]. This loss of synapses is highly relevant for underlying the cognitive impairments of AD, and indeed, it has been found to be a major structural correlate of dementia [20].
Although the receptor(s)/binding partner(s) and downstream signaling mechanisms induced by Aβ oligomers are not fully elucidated, the functional effects on cognition are well-established. High concentrations of Aβ (> nanomolar) – whether chronically or acutely present, synthetic or naturally-derived – can markedly impair neuronal physiology and synaptic plasticity [e.g. long-term potentiation (LTP)] [21–29], an electrophysiological correlate of memory. More importantly, pathological Aβ exposure can strongly impact behavior, including performance in learning and memory tasks [30–34]. This has been thoroughly demonstrated in various AD mouse models, including both genetic (e.g. transgenic) and non-genetic (e.g. acute exposure) models.
Although the predominant focus in AD research and drug discovery has been on Aβ, accumulating evidence indicates that the other major protein that undergoes misfolding and accumulation – tau – plays a key mediating role in the pathogenesis of AD as well as other tauopathies. Tau is a natively unfolded microtubule-associated protein that functions in regulating microtubule stability and therefore, axonal development and transport [35]. As a result of alternative splicing, there are multiple tau isoforms. A primary differentiating feature of these isoforms is the number of microtubule-binding repeats in the C-terminal region, with three (3R) and four (4R) repeat-containing tau as the predominant isoforms in neurons. Both 3R- and 4R-tau are included in the NFTs of AD [36]. However, similar to Aβ, there may be isoform-specific characteristics in the aggregation and fibril-forming capacity of tau [37]. In general, with AD and other tauopathies, the dysregulation of tau phosphorylation and its subsequent aggregation into NFT structures impairs neuronal functioning and ultimately leads to cell death. In fact, recent research points to tau as a requisite mechanistic factor in Aβ-induced pathology – lowering of tau can prevent neurotoxicity in Aβ-treated cultures and AD mouse models [38–41].
2.1 Genetically modified mouse models of AD
Serving as the foundational rationale for many genetically modified AD mouse models as well as strengthening support for the amyloid hypothesis, a large number of mutations in the genes for APP or PS1/2 have been discovered in families with early onset familial AD (FAD) [42–46]. All of these mutations were found to increase total Aβ production, enhance aggregate formation or increase the ratio of Aβ42:Aβ40, resulting in a higher proportion of aggregation-prone Aβ [47–52]. So far, over 30 APP mutations and almost 200 PS1/PS2 mutations have been identified and linked with FAD [53]. Although FAD accounts for < 5% of all AD cases, there is a very high degree of phenotypic similarity between FAD and sporadic late-onset AD (LOAD), suggesting that mechanistic information obtained about FAD would also be directly relevant for LOAD [54].
Following upon the discoveries of the various FAD mutations, the creation of transgenic mouse models which express the mutated genes further emphasized the pathogenic link between Aβ and AD (Table 1; reviewed in [2, 3]). These mouse models include ones which overexpress mutant human APP, PS1 and/or microtubule-associated protein tau (MAPT). The first such model to be developed was the PDAPP transgenic mouse that overexpresses APP with a FAD-associated mutation (V171F) [55]. Located at the γ-secretase cleavage site, this mutation shifts processing to longer Aβ peptide lengths, thereby increasing the ratio of Aβ42:Aβ40. Another widely-used model is the Tg2576 mouse line, which overexpresses human APP with two point mutations (K670N, M671L) that were originally identified in a Swedish family with FAD [43, 56]. These mutations are located by the β-secretase site and bias APP processing towards the amyloidogenic pathway, leading to higher overall levels of Aβ.
Table 1.
Transgenic mouse models of Alzheimer’s disease.
| Model | Description | Outcome | Plaques | NFTs | Neuron loss |
Synaptic deficits |
Memory deficits |
Notes | Example | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Single transgenic | ||||||||||
| APP (Swedish) | Mutations at β-secretase cleavage site (aa 670/1) | Enhanced cleavage by β-secretase; overall more Aβ (all forms) | YES: 9–12mo | NO | NO | YES | YES | Mostly dense cored plaques, some tau hyperphosphorylation. Synaptic and memory defects generally precede amyloid deposits. | Tg2576 (Swedish) | Hsiao et al. 1996 Science [56] |
| APP (Indiana, London) | Mutations at gamma-secretase cleavage site (aa 717) | Enhanced cleavage by gamma-secretase; increased Aβ 42:40 ratio | YES: 9–12mo | NO | NO | YES | YES | Higher levels of diffuse amyloid deposits. | PDAPP (Indiana) | Games et al. 1995 Nature [55] |
| APP (Arctic, Dutch, Flemish, Italian) | Mutations within Aβ sequence (aa 692/3) | Enhanced Aβ oligomerization, formation of protofibrils | YES: 9–12mo | NO | NO | YES | YES | Pronounced cerebral amyloid angiopathy. | TgAPParc, APPDutch | Ronnback et al. 2011 Neurobiol Aging; Herzig 2004 et al. Nat Neurosci [57,58] |
| APP (Japanese) | Mutation within Aβ sequence (aa E693delta - deletion of glutamate 22) | Enhanced Aβ oligomerization with no fibrillization or plaques | NO | NO | YES (>24mo) | YES | YES | Intracellular accumulation of Aβ oligomers from 8mo; includes tau hyperphosphorylatoin, gliosis. | TgAPP (E693) | Tomiyama et al. 2010 J Neurosci [59] |
| multiple APP mutations | Combination of single APP FAD mutations | Combination of effects on APP processing/Aβ | YES | NO | NO | YES | YES | Increased amyloid pathology over single APP mutation models. | TgCRND8, J20 | Chishti et al. 2001 J Biol Chem; Davis et al. 2004 J Biol Chem [60,61] |
| Amyloid-β (Aβ) | Human Aβ40 or 42 fusion protein with BRI | Overexpression and secretion of only Aβ, no extra APP cleavage products | YES: 3mo | NO | NO | YES | YES | Reactive gliosis and amyloid pathology only with Aβ42 model; similar overexpression level as Tg2576 | BRI-Aβ42 | McGowan et al. 2005 Neuron [62] |
| Presenilin-1 | FAD point mutations or exon 9 deletion in human PS1 | Altered APP cleavage; enhanced Aβ 42:40 ratio | NO | NO | NO | YES | NO | Accelerated neurodegeneration in older mice >13mo; PS2 model also available with rare mutation, not commonly used | PS1 (M146V/L), PS1(dE9) | Duff et al. 1996 Nature [51] |
| Tau | Point mutations in human MAPT (FTD mutations) | Increased tau phosphorylation/aggregation | NO | YES (>6mo) | YES | YES | YES | Significant motor defects, brainstem and spinal cord pathology in some strains. Inducible promoter models may be more appropriate for cognitive behavioral studies. |
JNPL3, MAPT (P301L), MAPT(VLW) | Ramsden et al. 2005 J Neurosci; Lim et al. 2001 Mol Cell Neurosci; Terwel et al. 2005 J Biochem [63–65] |
| Multi-transgenic | ||||||||||
| APP/PS1 | Double transgenic (APP FAD mutant overexpression, PS FAD mutant expression or knock-in) | Accelerated phenotype and pathology but minimal neurodegeneration | YES: 3–6mo | NO | NO | YES | YES | Significant hippocampal neuron loss in some subtypes (APP(swe+lon)/PS1) | APP(swe)/PS1(M146L), APP(swe)/PS1(A246E) | Holcomb et al. 1998 Nat Med; Borchelt et al. 1997 Neuron [66,67] |
| APP/Tau | Double transgenic (APP FAD mutant overexpression, tau FTD mutant overexpression) | Accelerated phenotype and pathology but minimal neurodegeneration | YES: 9mo | YES | YES | YES | YES | Increased amyloid deposition compared to Tg2576/reports of high death rate | APP(swe)/tau (P301L), APP (swe)/tau (VLW) | Lewis et al. 2001 Science; Ribe et al. 2005 Neurobio Dis [68, 69] |
| APP/PS1/Tau | Triple transgenic; FAD APP and FTD tau transgenes in PS1 FAD knockin | Accelerated phenotype and pathology including NFTs | YES: 3–6mo | YES | YES | YES | YES | Early intraneuronal deposits; plaques precede tangles | 3xTgAPP [APP(swe)/PS1(M146V)/MAPT(P301L)] | Oddo et al. 2003 Neuron [70] |
Transgenic mouse models have demonstrated that overexpression of APP and/or Aβ is sufficient to recapitulate many important characteristics of AD. In general, the mutant APP-overexpressing mice develop amyloid plaque pathology, loss of synapses, impaired synaptic plasticity and cognitive deficits. Co-expression of a mutant PS1 transgene accelerates the pathology to a significantly younger age, as evidenced by the double transgenic APP/PS1 model [66, 67]. A caveat with some of the AD mouse models is that there is minimal widespread neurofibrillary tangles and neurodegeneration, a defining characteristic of AD. However, a triple transgenic mouse model (3xTgAPP) that expresses mutant tau along with the APP and PS1 transgenes has been reported exhibit significant neurodegeneration along with neurofibrillary tangles [70]. In addition, the removal of nitric oxide synthase (NOS) 2 in mice expressing mutated APP results in the development of a much larger spectrum of AD-like pathology, including amyloid plaques, tau pathology, neuronal loss and behavioral impairments [76, 77].
In AD research efforts, transgenic mouse models have been valuable tools in elucidating disease mechanisms as well as testing potential therapeutic strategies. In particular, behavioral testing with cognitive assays are a primary component of their utility and value. The transgenic APP-overexpressing mouse models develop cognitive dysfunction at ages ranging from 2 to 12 months, depending on the specific transgenes. In the PDAPP mouse model, significant memory impairments are evident after 6 months of age in several behavioral tasks including FC, RAWM and MWM. In the Tg2576 mouse model, the onset of the performance impairments in these tasks varies depending upon the task, from approximately 4 months with FC [78–82] to more than 6 months with the RAWM and MWM [83, 84]. In general, deficits at the earlier ages tend to be more subtle but usually progress to marked impairments at older ages. Significantly, behavioral impairments can start prior to the accumulation of Aβ plaques, a fact that highlights the central importance of soluble Aβ species in AD pathogenesis.
Mutations in the MAPT gene are associated with familial fronto-temporal dementia (FTD) and parkinsonism linked to chromosome 17 (FTDP-17). These mutations generally decrease tau binding to microtubules or increase the proportion of 4R-tau, which enhances susceptibility to aggregation. Various transgenic mouse models expressing mutant human MAPT have been developed and do recapitulate tau pathology characterized by hyperphosphorylation and accumulation into NFTs [64, 65]. However, due to significant pathology in the brainstem and spinal cord in many of these strains, there can be age-dependent motor defects that prevent their use in behavioral tasks such as FC, RAWM and MWM. However, newer models with inducible tau transgene expression [63, 85] as well as the 3xTgAPP model [70] are reported to exhibit less motor-related pathology and have been characterized in behavioral cognitive assays.
Although no current model exhibits all of the signs, symptoms and anatomopathological hallmarks of AD, the use of mouse models has greatly contributed to a better understanding of the pathophysiology of the disease and enabled the development of novel therapeutic strategies. In general, in picking an AD mouse model, the mice should present an increase in Aβ levels and/or hyperphosphorylated tau along with synaptic and memory deficits as well as synaptic and neuronal loss (i.e. a human AD-like phenotype).
2.2 Non-genetic mouse models of AD
Although genetically modified models of AD have been very valuable resources for investigating the mechanisms underlying disease pathogenesis and progression, the overexpression of AD-related genes might mislead the interpretation of the findings derived from these studies. For instance, the APP and PS1 transgenes could affect neuronal function through a variety of mechanisms that are not necessarily related to Aβ and/or AD-like pathology [86, 87]. Indeed, the trafficking and signaling properties of the full-length form of APP and its natural cleavage products, which include Aβ, soluble APPα (sAPPα), sAPPβ and amyloid precursor protein intracellular domain (AICD), are likely different in non-disease physiology and could therefore impact CNS development, synaptic function and memory with overexpression. To separate Aβ-specific effects from the other effects of APP and PS1 overexpression, we and others have often used models of the disease in which Aβ per se would be responsible for observed deficits (Table 2). Given that natural oligomers of human Aβ as well as synthetically produced human Aβ oligomers impair LTP [21–29], oligomeric Aβ directly infused into the ventricles or hippocampi of adult animals provides an acute Aβ pathology model. The Aβ can be infused via pre-implanted cannulas, which facilitate direct delivery and help avoid issues related to the blood brain barrier [30–34]. These experiments have shown that Aβ per se is capable of impairing different types of memory including associative and reference memory, suggesting that the peptide plays a direct role in memory impairment in general.
Table 2.
Non-transgenic mouse models of Alzheimer’s disease.
| Model | Description | Outcome | Plaques | NFTs | Neuron loss | Synaptic deficits | Memory deficits | Notes | Example | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-transgenic | ||||||||||
| Acute Aβ injection | Direct infusion of Aβ into the brain (e.g. dorsal hippocampi, lateral ventricles) | Acute local Aβ elevation | NO | NO | NO | YES | YES | Aβ source/preparation/conformation is crucial | Cell-derived Aβ, Aβ dimers from human brain, 200 nM synthetic Aβ oligomers | Walsh et al. 2002 Nature; Shankar et al. 2008 Nat Med; Puzzo et al. 2012 Neurobiol Aging; Watterson et al. 2013 PLOS One; Fiorito et al. 2013 Eur J Med Chem [21, 30, 32–34] |
| Acute tau injection | Direct infusion of tau into the brain (e.g. dorsal hippocampi) | Acute local tau elevation | NO | NO | NO | YES | YES | Different tau isoforms can be used, as well as naturally-derived tau from brain extracts | Tau oligomers from human brain, recombinant tau oligomers | Moe et al. 2008, 2009, 2010, 2010; Lasagna-Reeves et al. 2012 Sci Reports [71, 101–104] |
| Aged animal models | Aged mice, rats, dogs, non-human primates | Natural age-related deficits | YES (dogs, non-human primates) | NO | NO | YES | YES | Exhibit cognitive deficits, brain hypermetabolism, cholinergic defects, altered calcium homeostasis, oxidative stress, neophobia. | Aged mice (>18–20 months old) | Gallagher et al. 1993 Behav Brain Res; Gower et al. 1993 Behav Brain Res [72, 73] |
| Senescence-accelerated prone mice (SAMP8) | Spontaneously mutated inbred mouse strain | Shortened lifespan and accelerated aging phenotype; elevated levels of endogenous APP and Aβ | NO | NO | NO | YES | YES | Some tau hyperphosphorylation along with decreased spine density and synaptic proteins; increased gliosis and oxidative stress. | SAMP8 | Yagi et al. 1988 Brain Res; Okuma et al. 1998 Jpn J Pharmacol [74, 75] |
Another advantage of using the Aβ-infusion model is the possibility of using animals other than mice. For instance, intracerebroventricular infusion of Aβ in rats induced an impairment of MWM performance [88–93], an effect that was worsened by a concomitant cerebral hypoxia [94]. Additional advantages of using this model include the low costs and significantly less labor/time requirements compared with developing a transgenic mouse colony. However, disadvantages of using the Aβ infusion model are: i) it does not reproduce the characteristic hallmarks of AD represented by senile plaques, neurofibrillary tangles and neuronal loss; ii) it requires a very careful methodological standardization of Aβ preparation and efficacy testing in interleaved experiments; iii) it does not allow testing of a treatment on disease progression; iv) the animals are cannulated, which can be an issue for behavioral studies since cannulas can fall out, and multiple injections can stress animals, making it difficult to follow animals over time.
Following upon the discovery of extracellular soluble tau in the cerebrospinal fluid (CSF) of both healthy individuals and, in higher amounts, in AD patients [95–100], our group first tested the effect of tau oligomers on synaptic function and demonstrated that, similar to Aβ, tau oligomers are capable of impairing LTP [101, 102]. These experiments led to investigations on whether tau oligomers infused onto the dorsal hippocampi are capable of affecting memory. Consistent with the LTP results, it was found that memory was impaired in oligomeric-tau infused mice [71, 103, 104]. Taken all together, these studies demonstrate the potential of using alternative, acute exposure models for investigating AD pathogenesis and drug testing (Table 2).
3. Behavioral Studies
The medial temporal lobe (MTL) has been recognized as a critical region for explicit or declarative memory since the famous case studies of memory impairment resulting from hippocampal damage in H.M. and other patients [105]. The MTL belongs to the limbic system, which supports a variety of functions such as learning, memory and emotional behavior via the integration of multiple structures including the hippocampus and the regions surrounding it (perirhinal, parahippocampal, and entorhinal cortex), the amygdala, the cingulate gyrus, the fornix, the mammillary body, the septal area, the piriform cortex, the anterior thalamic nuclei, the hypothalamus, the epithalamus, and other structures. The hippocampus, in particular, has been recognized to be critical for spatial memory and is therefore the focus of this review [106–108]. Because of its vulnerability to damage at the earliest stages of AD, the hippocampus is considered fundamental for understanding the disease pathophysiology [8, 109–114]. Indeed, most of the behavioral protocols aim to measure hippocampal-dependent memory, and many efforts have been made to develop models of human memory in monkeys and rodents [115, 116]. However, differences across species should be considered; for example, the hippocampus codes spatial and episodic verbal memory in humans [117–119], whereas in rodents it is mainly associated with spatial navigation and a highly represented olfactory behavior [120–125].
Among the various types of behavioral paradigms, we will mainly consider behavioral tasks that might reflect defects in humans affected by AD. For instance, the FC task studies associative memory that is affected in AD patients. The RAWM task studies short-term memory (STM), a type of memory that is affected early in the disease [126]. The MWM task studies long-term memory (LTM) which is affected at late stages of the disease. Regardless of the experimental paradigm, it is crucial that the behavioral task is designed and conducted appropriately by taking into account the species-specific behavioral characteristics, the correct interpretation of the results, and the biological mechanisms underlying the type of memory studied, other than strain, age, sex, and the peculiar features of genetic modified animal models.
3.1 Fear Conditioning (FC)
Memory can be distinguished as explicit and implicit; the latter can be further differentiated as non-associative (habituation and sensitization) and associative learning (classical and operant conditioning). FC is a form of associative learning based on the classical or Pavlovian conditioning, which involves the learning of relationships between two stimuli: a neutral stimulus, named conditioned stimulus (CS), causing a weak responses that typically has no relation with the task to be learned (e.g. light or sound); and a stimulus able to evoke a typical behavior response (e.g. salivation) known as unconditioned stimulus (US). The Russian physiologist Ivan Pavlov (Nobel Prize for Physiology or Medicine in 1904) was the first to demonstrate the phenomenon of classical conditioning. Having noticed that the mere sight of the plate with food induced salivation in dogs (unconditioned response - UR), he decided to investigate whether salivation could be induced in response to a neutral stimulus, such as the sound of a bell, associated with the meal presentation. While the sound of the bell itself did not evoke any response, after a few trials in which the sound was coupled to the food, dogs began to salivate before receiving the food in response to the simple sound of the bell (conditioned response - CR). This kind of conditioning was called “classical reward conditioning”, or appetitive conditioning since the US was followed by a pleasant event such as food. In contrast, aversive or defensive FC occurs when the US was followed by an unpleasant event (such as an electrical shock) triggering a series of automatic responses e.g. freezing. The important variables in the phenomenon of Pavlovian conditioning, and thus of FC, are: i) the CS should be administered before the US; ii) the interval between CS and US is critical for most of the examples of conditioning; iii) CS and US should be contiguous; iv) if the CS is repetitively presented in the absence of the US, the CR will decrease in intensity until it disappears according to the phenomenon of extinction, which also involves the learning of new information and is therefore not an equivalent of forgetting. Classical conditioning has the advantage of being long-lasting and quickly-acquired, thus allowing the additional testing of memory persistence [127]. FC can be investigated both in animal models and humans, e.g. as a model for post-traumatic stress disorder [128]. It is a useful tool for investigating both emotional and contextual memory, and by extension, the integrity of the main brain structures involved: the amygdala and the hippocampus, respectively. Furthermore, FC has the advantage of being much faster than other behavioral tasks since it requires only one day of training. Finally, it offers the advantage of precisely confining to a very short time (we use 2 seconds) the moment at which contextual learning occurs (an advantage that turns very useful for biochemical experiments).
3.1.1 Methodology
FC has been deeply investigated by LeDoux’s and Fanselow’s research groups (for reviews see [129–132]. In the most commonly used procedures, a one-trial learning of the CS, represented by a light or a sound, is paired to an electric foot shock (aversive US). Rodents will learn to associate: i) the light or the sound with the aversive stimulus, which is evident when the CS is presented again to stimulate remembrance of the foot shock (Cued FC); ii) the place or context where they received the aversive stimulus, which is demonstrated when they are later placed in the same context (e.g. the conditioning chamber) to stimulate memory of the foot shock (Contextual FC). Following an aversive stimulus, the CR will be represented by fear [133], which is physiologically accompanied by a series of behavioral responses evolved for escaping from the source of danger [134]. The fight-or-flight sympathetic response [135] involves an increase of catecholamine, ACTH and cortisol secretion along with a consequent increase of function in cardiovascular, respiratory systems, and an inhibition of parasympathetic functions such as digestion. In rodents, several studies have demonstrated that the defensive behavior associated with fear is represented by freezing [136]: a total absence of movements except for those necessary for breathing. This is thought to be an innate defense mechanism aimed at feigning death in front of a predator.
Although several protocols have been proposed [see for example [137], here we will describe a procedure that has been successfully used in our laboratories to investigate both contextual and cued conditioning in animal models of AD [33, 34, 138–144] (see Fig. 1 for a picture of the apparatus). Mice are tested individually in a conditioning chamber that is located in a sound-attenuating box with a clear Plexiglas window that allows video recording of the experiment. The conditioning chamber has a 36-bar insulated shock grid floor. The floor is removable, and after each experimental subject, it is cleaned with 75% ethanol and then again with water. To provide background white noise (72 dB), a single computer fan is installed in one side of the sound-attenuating chamber. Mice are handled once a day for 3 days before behavioral experiments. Only one animal at a time is present in the experimentation room. It is placed in the conditioning chamber for 2 minutes, following which a discrete tone (CS) at 2800 Hz and 85 dB is delivered for 30 sec. In the last 2 sec of the tone, the mouse receives a foot shock (US) through the bars of the floor (0.50–0.80 mA for 2 sec). The experimenter might regulate the intensity of foot shock; for example it is possible to elicit stronger memory by using a 0.75 mA foot shock. After the CS/US pairing, the mice are left in the conditioning chamber for another 30 sec and are then placed back in their home cages. Freezing behavior can be scored by using dedicated software. In our personal experience, double-checking by manually scoring freezing time has been very useful. Twenty-four hours after training, the mouse is placed back in the conditioning chamber. This allows the evaluation of contextual fear learning, since a mouse with intact contextual memory should be able to recognize the context where it received the foot shock the day before. Freezing is measured for 5 consecutive minutes. To evaluate cued fear learning, we replace the mouse in the conditioning chamber 36 hours after training. In this case, we create a novel context by inserting a triangular cage with smooth flat floor and with vanilla odorant. After 2 min (pre-CS test), the mouse is exposed to a tone with the same characteristic of the tone used during training for 3 min (CS test), and freezing is measured. To obtain a sample appropriate for statistical analyses, we usually use 15–20 animals per condition.
Figure 1.
Picture of the fear memory apparatus used in our laboratories.
An important control that needs to be performed during testing of fear memory is the sensory threshold assessment. Changes in the sensory perception of the shock might be due to the experimental manipulation leading to a false attribution to memory of the observed phenotype. A sequence of single foot shocks is delivered to animals placed on the same electrified grid used for FC. Initially, a 0.1 mA shock is delivered for 1 sec, and the animal’s behavior is evaluated for flinching, jumping, and vocalization. At 30 sec intervals, the shock intensity is increased by 0.1 mA to 0.7 mA and then returned to 0 mA in 0.1 mA increments at 30 sec intervals. Threshold to vocalization, flinching, and then jumping is quantified for each animal by averaging the shock intensity at which each animal manifests a behavioral response to the foot shock.
3.1.2 Brain regions involved in FC
Whereas the amygdala is the main brain structure in the fear circuit that is involved in the formation and storage of emotional memories, the hippocampus participates in learning the context that triggers fear [129–132, 145–150]. Lesion studies performed in rodents have demonstrated that the amygdala is involved in the fear responses triggered by both the cue and the context; when the hippocampus is damaged, both pre- and post-training, only contextual conditioning is impaired [146, 151, 152]. Thus, the amygdala has a primary associative role in the processing of fear, whereas the hippocampus communicates the sensorial representation of the context that informs the amygdala of the environment of the dangerous event. It acts as a sensory relay for visuo-spatial, auditory, olfactory, or other sensory input, thus requiring the integrity of thalamus [153] (reviewed in [131]), and as a motor relay, since hippocampal lesions can also result in increased locomotor activity that affects freezing [151]. Contextual conditioning occurs also in the absence of the cue -the impairment of contextual conditioning by lesions of the hippocampus has been related with loss of a conjunctive representation of the features of the context [154]. Once the hippocampus has recognized and consolidated these features, contextual information would presumably be stored in the neocortex.
At a molecular level, contextual FC is based on a form of synaptic plasticity sharing mechanisms with NMDA-dependent LTP. Actually, administration of an NMDA antagonist into the dorsal or ventral hippocampus selectively impairs contextual FC [151, 155]. Also NMDA modulation in other brain areas may affect contextual memory. For example, at the dorsolateral periaqueductal grey, inhibition of NMDA receptors, neuronal NOS, and soluble guanylyl cyclase attenuate contextual FC in rats [156], whereas activation of NMDA receptors in the medial prefrontal cortex has been found to be needed for the acquisition of both trace and contextual fear memories [157, 158]. The involvement of cAMP/PKA and nitric oxide/cGMP/PKG pathways in contextual fear learning and consolidation has been widely demonstrated by pharmacological and genetic approaches in hippocampus, amygdala and prefrontal cortex [159–165], and has the role of the transcription factor c-AMP-Responsive-Element binding [CREB; [166–168]. Cholinergic, serotoninergic and GABAergic signals have been also demonstrated to be crucial in contextual FC [160, 169–179].
3.1.3 FC in AD models
Conditioned fear responses have been demonstrated to be impaired in patients with mild to moderate AD [180, 181]. Consistent with these findings, the Tg2576 mouse model showed an impairment of contextual fear learning at 4–6 months [78–82] when plaques are not yet detectable, but the Aβ42:Aβ40 ratio is modified [80]. This defect worsens with age, [78, 182] and becomes profound at 12–14 months [183, 184]. It should be also noted that the same animals have provided different results in different studies assessing cued learning which was found to be either normal [183, 184] or altered [185].
Double transgenic APP/PS1 mice present an impairment of contextual FC starting at about 3 months, whereas cued FC is normal, indicating that the learning deficits is based upon the hippocampal dysfunction [78, 138, 140, 186]. In these mice the behavioral changes are concomitant with the increase of Aβ and early plaque deposition. Some authors have shown a reduction of auditory FC in aged APP/PS1 mice at 12–14 months, notwithstanding a very low presence of plaques in the amygdala [187].
The APPswe/PS1dE9 mouse, another double transgenic model of AD, shows early contextual memory impairment [188, 189], even if some authors have detected it at older age [14-months; [190], and others have found normal spatial learning and memory, FC, and sensorimotor gating at 7 months, with abnormal social recognition memory [191]. Additional transgenic models that present abnormal contextual fear memory before the onset of amyloid plaques are the PS1M146V knockin mice [192], the APP-SL 7-5 transgenic mice overexpressing human APP695 harboring the double Swedish and London mutations [193], the APP J20 carrying the APP Indiana (V717F) and Swedish (K670M) mutations [194], the 5XFAD mice (Tg6799 line) that co-overexpress FAD mutant forms of human APP (the Swedish mutation: K670N, M671L; the Florida mutation: I716V; the London mutation: V717I) and presenilin 1 (PS1) (M146L; L286V) [195]. Finally, it has been demonstrated that contextual memory is impaired in acute models of AD induced by i.c.v. or hippocampal [33] injections of oligomeric Aβ42 [34, 196].
3.2 Radial Arm Water Maze (RAWM)
The RAWM was designed to measure spatial learning and memory performances in rodents [197–199], as a combination of dry radial arm mazes (RAM; [121, 200] and MWM [201]. Dry RAM consists of a platform equipped with a variable number of spaced arms radiating from the center. Rodents should enter one of the arms, using spatial cues in the room, to reach food or water. This kind of task requires both working memory (WM) when retaining information for a very short time and reference memory when retaining memory for longer times. The limitations of RAM include the deprivation of food or water and the presence of confounding olfactory signals. Moreover, dry RAM is less sensitive in detection of search strategies than MWM, because once the rodent has chosen an arm, it goes to the end of it expecting the reward. Thus, RAM is more useful for investigating changes of error patterns than searching patterns. Another difference between RAM and MWM entails the acquisition time. Indeed, spatial learning can be achieved in about 6–10 trials in MWM, whereas for RAM acquisition rate depends upon the protocol used ranging from 2 days to 3–4 weeks [for a review on RAM and MWM comparison, see [202]].
Thus, the advantages of RAWM result from the ability: i) to combine a complex spatial environment with an easy way of measuring animal performances by counting errors, which does not require a video tracking system or computers; ii) to give animals a strong motivation (to escape from water) without requiring food deprivation or foot shock; iii) to possibly test both WM and reference memory.
3.2.1 Methodology
Several RAWM protocols have been used, with differences in the apparatus and animal acquisition phase [83, 198, 199, 203, 204]. Here, we will describe two different protocols that have been used in our laboratories, one requiring three weeks, mostly testing WM [83, 138, 140, 205], and another one requiring two days, testing reference memory [33, 34, 204], respectively.
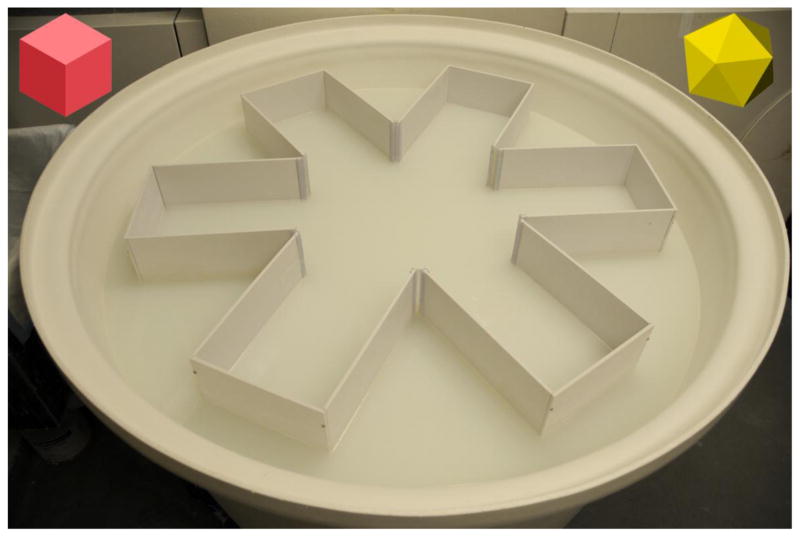
The RAWM apparatus is the same described for MWM (see below), except for the addition of arms [from 4 to 16, depending on the pool size and the chosen procedure; [206]], that are equidistant and radiating from the center. Arms can be made of aluminum, Plexiglas or plastic. In our apparatus, we have used a 110 cm plastic large circular pool in which we inserted 6 arms (height 50 cm) made of white-glazed aluminum and fixed one to each other to form a 60° angle at the center between two adjacent arms (see Fig. 2). Each arm is 35 cm long, and the central free area has a diameter of 40 cm. Each swim alley is 25 cm wide. We label each alley with a number (1–6) by placing a label on the pool edge where mouse cannot see it (it should not be a cue). The pool is filled until 25 cm of the arms are submerged by water and 25 cm are outside. We add non-toxic paint to increase water opacity.
Figure 2.
Picture of the RAWM apparatus used in our laboratories.
As in MWM, rodents are tested individually and the goal of the mouse is to escape from water by finding a submerged platform positioned at the end of one of six arms. In the three week “long” protocol, the platform remains in the same location for each day of trial; this location is changed randomly day by day. On each day, the mouse undergoes 4 consecutive acquisition trials (named A1–A4). For each trial it starts the test from a different randomly chosen arm, so that it has to rely on its STM of the platform location based on spatial cues present in the room, and not on its LTM of the platform location from previous days. For each trial (lasting up to 60 seconds), the experimenter counts the errors made by the mouse each time it enters the wrong arm or when the animal needs more than 15 seconds to make a decision. Indeed, as in MWM, thigmotaxis or passivity can occur, whereby a mouse floats or swims in the central area without choosing an arm. After each error, the mouse is gently pulled back to the starting arm for that trial. After four consecutive acquisition trials, the mouse is placed in its home cage for 30 minutes and then returned to the maze and administered a fifth retention trial (R), which is an indicator of STM. Animals are trained for 3 weeks, i.e. until the wild type (WT) control mice reach asymptotic performance (an asymptotic level of one to three errors on A4 and R). The scores for each mouse on the last 3 days of testing are averaged and used for statistical analysis.
In the “short” 2-days RAWM version of the task, the goal arm does not vary from day by day but is kept constant for all trials, with a different starting arm on successive trials. This protocol allows reaching of the learning criterion in only 2 days. During the first day, the mouse is trained in 15 trials (T1–T15) to identify the platform location in a goal arm by alternating between a visible and a hidden platform from T1 to T12 (beginning with the visible platform in the assigned arm). In the last four trials (T13–T15) only a hidden platform is utilized. During the second day the same procedure is repeated by using only the hidden platform from T1 to T15. An entrance into an arm with no platform, or failure to select an arm after 15 sec is counted as an error and the mouse is returned to the start arm. Each trial lasts up to 60 sec, and at the end of each trial, the mouse is kept on the platform for 15 sec. The number of errors and time to complete the trial is recorded. The average of errors made in 3 consecutive trials for each mouse is calculated and used for statistical analyses.
We have found this procedure highly suitable for rapidly testing STM. A confounding factor could be the physical fatigue that results from 15 consecutive trials, which may interfere with the learning process. For this reason, spaced practice training might be established by running the mice in cohorts of 4 and alternating different cohorts through the 15 training trials over 3 hour testing periods each day. The goal platform location will be different for each mouse. After all of the mice in cohort 1 have performed a trial to locate a visible platform, this is switched from visible to hidden. After each mouse from cohort 1 completes six alternating visible-hidden trials, they are left to rest under a heating source, and mice from cohort 2 are tested in the same way. When all the cohorts have performed the T1–T6 trials, all of the cohorts are tested again to complete the T7–T12 trials in a similar alternating fashion. At this point, all mice have to perform the last 3 hidden platform trials (T13–T15).
In conclusion, in the “long” RAWM protocol, mice are required to learn that the platform is maintained in the same position on each trial within a day, but it is moved to a different arm each day; in contrast, in the “short” RAWM protocol, the goal arm does not change day by day.
As for all experiments, one must be assured of the validity of the controls. Thus, if a large number of WT mice have not reached the learning criterion in the final three trials, more days of training can be added.
In both versions of the RAWM, on the day after the last test, the mice undergo a visible test to control for possible motivational, visual and motor deficits. This is very important especially when using genetically modified animals; for example, certain strains can inherit a retinal degeneration mutation leading to blindness [207, 208]. Following testing with the RAWM, animals undergo two daily sessions (each consisting of three 1 min trials) for two days, and the time taken to reach a visible platform (marked with a green flag) randomly positioned in a different place each time is measured. Moreover, both during the hidden and the visible test it is possible to measure swimming speed and distance travelled to reach the platform in order to analyze possible motor effects on the performance. Indeed, an animal can reach the platform faster or slower, not because of an alteration of its spatial memory skills but because of potential effects on its swimming or sensory abilities. An impairment of the RAWM test with normal performance on the visible test indicates that the mouse has a memory impairment that is not related to motivational, visual and motor skills.
Finally, when assessing performance with the RAWM, the following factors should also be taken into account: i) differences between rats and mice, especially in correlation with the different strains of mice (see below); ii) differences between males and females, even if the literature is contradictory regarding better WM performances in males [209] or females [206, 210]; for this, we suggest performing behavioral tests in sex-balanced groups of mice; iii) methodological factors such as size of the pool, lightening, procedures; iv) animal handling. Another essential factor is the age of the animals, which will be discussed in detail in the next section.
3.2.2 RAWM and Working memory
The definition of WM is still an issue of debate in the neuroscience community. Another issue is the difference between rodent and human WM. Memory can be classified by a temporal or qualitative criterion: the first takes into account the time that the memory of the information remains effective, whereas the second distinguishes memory according to the type of information retained. Thus, depending of the “length” of memory, we can distinguish: i) iconic memory and echoic memory, two forms of very short sensory memory for visual or auditory stimuli; ii) STM, which is able to hold information only for few seconds if it is not transferred to iii) LTM, which is a form of permanent memory theoretically able to store an indefinite amount of information for a life-time. Conversely, memory classification according to a quality criterion differentiates declarative or explicit memory and non-declarative or implicit or procedural memory. Declarative memory refers to specific personal events characterized by “warmth and intimacy”, as described by William James in 1890 [211]. It is autobiographical, and events are recalled and placed in relation to the time of life of the individual in which they occurred [see [212]]. Conversely, non-declarative memory is a typical memory process aimed at recollecting how to perform an action by identifying or using a stimulus that had already been presented. Non-declarative memory is characterized by unconscious influences of past experiences, which are manifested by changes in the speed or the ways in which the same task is performed. It includes motor learning, perceptual representation system (to identify words and objects on the basis of their shape and structure), procedural memory (to acquire skills and habits through repeated exercises), and WM. In particular, the latter is considered a memory system entrusted with the task of manipulating the information temporarily in order to maintain it for basic cognitive activities such as comprehension, reasoning and problem solving. The term “working memory” was coined in 1960 in relation to the new theories comparing the mind with computer functioning [213]. Before, neuroscientific debate focused on STM as the ability to remember information for a short time (on the order of seconds) as opposite to the more durable LTM.
What is WM in rodents? How is it possible to differentiate WM from STM by using behavioral testing in animals? The first attempt was made in the late seventies [120, 121, 214] by using behavioral tasks such as the RAM in which WM was considered the capacity to remember which arm a rodent had visited in a session. Indeed, this form of memory is transient and it serves to carry out a specific task in a given time. In an interesting review, Dudchenko defines WM as “a short term memory for an object, stimulus, or location that is used within a testing session, but not typically between sessions and it is distinguishable from reference memory, which is a memory that would typically be acquired with repeated training, and would persist from days to months” [215]. Particular emphasis is also given to the concept of forgetting because WM, once used, is immediately forgotten. This confirms that WM is a type of STM typically used within a testing session, but not between testing sessions, because the information will be deleted after its use [215].
As mentioned before, WM temporarily retains information for manipulation in carrying out complex cognitive tasks [216]. Thus, it requires both spatial and procedural memory and relies upon the same brain structures described for MWM, particularly the hippocampus and medial prefrontal cortex. This cortical region is mainly involved in the temporary storage and processing of information lasting up to seconds, whereas the hippocampus is more critical when a longer storage is required [217].
3.2.3 RAWM in Alzheimer’s disease models
WM performance is sensitive not only to damage of the above-mentioned brain areas, but also to normal aging [218], and neurodegeneration such as AD neuropathology [219]. Indeed, testing WM deficits is essential in Tg mouse models of AD to establish the progression of the disease and the effectiveness of potential new drugs. Although WM might also be tested by a modified version of MWM [220], we have found RAWM to be the most reliable task for investigating WM in AD animal models. RAWM was first used in 1985 [197] by inserting an 8-arm radial maze into a water tank where rats were forced to swim and escape onto a bench in one of the maze arms, with the position of the submerged platform changing for each trial. Since then, several protocols have been utilized and the RAWM task has also been modified to study reference spatial memory in a short term paradigm that lasts only 2 days, particularly suitable for AD models [33, 34, 204].
Several studies have been performed using RAWM to detect an impairment of WM in animal models of AD, but results are controversial especially with respect to the age at which RAWM detects a WM deficit [221]. In single Tg2576 transgenic mice the impairment of WM has been detected at 4.5 [222] or 8–9 months of age [223, 224]. Wilcock et al. [225, 226], conversely, found a RAWM impairment in APP mice at old age (from 24 months), but results are not available for younger animals that were not tested for RAWM. Results are also contradictory in APP/PS1 mice. In APPswe/PS1dE9 some studies demonstrated an impairment of RAWM performances starting at 6 months of age [227], whereas in other papers transgenic mouse performance was normal at 7 months of age and reduced at 13 months [228] or at 10–15 months [229]. The group of Morgan also showed that RAWM was impaired starting at about 15 months of age in both APP and APP/PS1(line 5.1) mice, whereas they learned and remembered the platform location at 6 and 11.5 months of age [230, 231]. Recently, Webster et al [232] have studied RAWM in four different age groups (7, 11, 15, and 24 months) in APP/PS1 knock-in mice characterized by the introduction of a Swedish FAD K670N/M671L point mutations, the humanization of the mouse β-amyloid sequence, and the introduction of a P264L mutation in the PS1 gene. These mice performed worse than WT controls in the RAWM task starting at 15 months of age, and the increase of errors paralleled the increase of age and the disease progression. In our laboratory, we have also demonstrated that the worsening of WM increased with age, in parallel with the accumulation of Aβ. However, WM was affected as early as 3 months of age in APP/PS1 (line 6.2) mice [83, 138, 140] as demonstrated by the increased number of errors at A4 and R compared with WT littermates. This impairment worsened in older animals (6–8 months). Conversely, reference memory tested by MWM started to be impaired at 6–8-months in Tg mice [83, 138, 140].
The choice of the experimental samples groups is fundamental. For example, if we aim to demonstrate the positive effect of a drug on AD onset and progression, we need to use 4 groups of animals: AD model without treatment; AD model with treatment; WT littermates without treatment; WT littermates with treatment. If littermate controls are not available, WT mice of the same sex, age and with the proper genetic background should be used. This simple experimental scheme provides important information: i) AD model without treatment should present synaptic or cognitive deficits at the appropriate age when such dysfunction is known to be present; ii) AD model with treatment, as the primary experimental group, may demonstrate a change in synaptic plasticity and memory measures due to the treatment; iii) WT without treatment should not present deficits, and synaptic and memory measures should be consistent with the known data on the strain; iv) WT with treatment usually do not present a change in synaptic plasticity and memory when the treatment selectively acts on a disease-related pathway; however, in some cases, an alteration of synaptic plasticity and memory is possible if the drug affects the physiological mechanisms at the basis of learning and memory. Moreover, if the treatment is expected to slow the onset of the disease, it should be started earlier than a drug designed to treat the overt disease.
In conclusion, despite the possible differences due to the protocol and the strain used, we believe that RAWM is a useful task to investigate early STM impairment in animal models of AD.
3.3 Morris Water Maze (MWM)
The MWM was first recognized by Richard G. Morris in 1981 [201] as a behavioral test for hippocampal-dependent spatial learning and long-term spatial memory in rodents. It is now one of the most used tools in behavioral neuroscience due to a number of advantages [233–236]: i) simple and fast execution, no pre-training period, and relatively low number of animals required; ii) possibility of differentiating between spatial learning and long-term spatial memory, and testing of non-spatial abilities such as visual and motor performance; iii) reduction of olfactory interferences, which are known to rely on the hippocampus; iv) absence of unpleasant procedures based on the delivery of an electric shock or food deprivation; v) low costs since the apparatus can be easily made. Along with many other research groups, we have successfully used MWM to test spatial memory impairment in models of AD [83, 138, 140] and aging [237], and to evaluate the effects of drugs with enhancing or deleterious effects on cognition.
3.3.1 Methodology
Several papers have described the MWM methodology (reviewed in [233, 236, 238, 239]) since the apparatus and/or the training procedures can exert significant influences on spatial performance. Here, we will describe the most commonly used apparatus and protocol that we have personally tested in recent decades with good results and a high degree of reproducibility in both normal, aged and genetically modified mice models of AD [32, 83, 138, 140–142, 237].
Briefly, the apparatus consists of: i) a plastic large circular pool (height: 75–80 cm; diameter 150–200 cm for rats and 90–120 cm for mice) filled with water (up to about 40 cm below the edge to prevent the animal jumps out), maintained at about 25°Cand made opaque to hide the submerged platform, e.g., by the addition of nontoxic white paint, powdered milk or synthetic opacifiers; 2) a submerged platform (1 cm under the surface of the water) measuring 10 × 10 cm, preferably made of Plexiglas, non visible to the swimming animal (hence the name “hidden test”); 3) stationary geometric cues made by different objects usually placed on the 4 cardinal points of the maze (we have found this to be more efficacious) or on the walls of the room but visible by animals during swimming; it is important that cues are not moved during the test; 4) a camera mounted on the ceiling and connected to a video tracking system and software for motion detection. If this system is based on the color difference between the maze and the animal, one should use a white animal on a black background or, in our case, a black mouse (e.g. of the common C57Bl/6 genetic background) in a white maze (see Fig. 3).
Figure 3.
Picture of the MWM apparatus used in our laboratories.
During the entire protocol the role of the experimenter should be carefully considered. It is preferable for the experiment to be conducted after a period of handling by only one experimenter. Moreover, the experimenter should remain stationary in a constant location (or leave the room) because he/she represents a distal cue for the swimming animals. Finally, it is important to perform the experiment in at least three cohorts, possibly by different experimenters (we adopt a similar precaution also for FC and RAWM).
Rodents are tested individually. First, animals undergo a training period that can last from 2 to 10 days; this training reveals the ability of the animal to learn in relation to spatial cues. Animals are placed into the pool where they learn to locate the hidden platform beneath the surface of the water. In our protocol, mice are trained for 3 days in 2 daily sessions each consisting of three 1 min trials. Conventionally, the maze is divided in 4 quadrants by a cross whose vertices represent the 4 cardinal points: North (N), South (S), East (E) and West (W). For each trial the animal is placed in the water starting from a different randomly chosen quadrant (that does not contain the platform), whereas the platform is always positioned in the same place (SW). If the animal fails to find the platform within the given time (60 sec), the trial is considered over and the animal is placed on the platform for 15 seconds before starting with the second trial. Sessions are held 4 hrs apart, so that the mice have to rely on LTM of the platform location. Typically, the time taken to reach the hidden submerged platform (escape latency) is recorded; this time measure progressively decreases, which produces a characteristic learning curve. However, since the ability to reach the platform can be based on a praxis strategy (animals learn sequence of movements), a taxon strategy (animals recognize proximal cues), or a spatial navigation strategy (animals map the spatial environment and recognize distal cues) [240], the video tracking system should also allow the measurement of non-mnemonic behaviors or strategies (length of the swimming path, path directionality, or cumulative distance to platform [241]).
After this acquisition training, on the 4th day (or in any case, 24 h after training) a probe trial is usually performed. The platform is removed from the pool and mice are allowed to freely swim for a certain period of time (we perform four 1-min trials, for each animal starting from a different cardinal point). For the probe analyses, the maze is virtually divided into 4 quadrants, the target quadrant (TQ; i.e. the one previously containing the platform), and 3 non-target quadrants (AL = adjacent left, AR = adjacent right, and OQ = opposite quadrant). The percent time spent in each quadrant seeking the platform is analyzed (usually, a normal mouse will spend about 35–40% or more of the time in the TQ). Moreover, it is possible to examine the number of times it crosses the platform area and the travelled trajectory.
As with the RAWM, a visible test is used to assess motivational, visual and motor abilities. We usually perform this test with the same protocol as the hidden acquisition training during the 5th and 6th day. An impairment of the hidden acquisition test with normal performance on the visible test indicates that the mouse has a spatial memory impairment that is not related to motivational, visual and motor skills.
3.3.2 Factors that may influence MWM performance
MWM was originally designed to study spatial memory in rats [201, 233]. However, mice have recently become the most used animals for studying the pathophysiology and therapeutic targeting of diseases due to the availability of transgenic models [242]. In this regard, it should be noted that mice perform more poorly than rats in MWM [243], partially due to the fact that their performance can be affected by thigmotaxis (the tendency to swim near the wall of the maze) and passivity (mice tend to float more compared to rats) [236, 244]. In these cases, the experimenter could intervene by prodding the mouse with an object. Alternatively, the mouse can be left in the water until the end of the trial and then tested again on the same day or the day after. If it continues to fail, the mouse should be removed from the data analysis. Maintaining a low water temperature increases the aversive environment and thus lessens the potential impact of thigmotaxis and passivity. In any case, it should be noted if an entire experimental group consistently exhibits atypical behavioral tendencies that can influence MWM results, especially when testing transgenic mice [242]. Among WT mice, C57Bl/6 animals are considered the better performer of MWM experiments [208, 245, 246], whereas albino mice, due to their visual impairment, tend to show impairments in MWM [247].
In general, lighting is another factor to be considered when performing tasks that depend upon the ability to process visuo–spatial information. Light should be soft and diffuse, and there should be no light reflections in the water. It is important to keep in mind that rodents are nocturnal animals that usually rely on olfactory capabilities rather than visual acuity [123, 248, 249], with the former not being involved in MWM.
Steroid hormones also affect hippocampal function and influence animal responses to stress [250–252]. Indeed, it is important to minimize the amount of stress with a correct period of pre-training handling, soft lighting, and an appropriate manipulation during the test (i.e. lift mice out of the water on the hand rather than by the tail). If very high-anxiety behavior is noticed, anxiety-specific tests [253, 254] can be conducted, especially in transgenic mouse models, to exclude anxiety-related effects on performance in the memory tests.
Behavior can be also influenced by viral, bacterial and nematode infections [255–258]; thus, a veterinary health control must be up to date.
Another important factor is age. MWM performance declines with increasing age of the animals [237, 259, 260]. Part of this decline may be due to age-related changes in swimming abilities, locomotion and exploration. On the other hand, it is very difficult to perform MWM in very young mice (<1 month) due to their tendency to jump out of the pool.
3.3.3 Brain regions involved in MWM
It is well known that the integrity of the hippocampus is necessary for spatial memory; however other brain structures might influence spatial memory by influencing spatial navigation, motor performances or movement organization [reviewed in [239]]. First, integrity of input and output hippocampal connections (i.e. fimbria-fornix, entorhinal and perirhinal cortices) is required [261–267]. Other critical brain structures include: thalamic structures [92, 268–274], mammillary body [275–280], amygdala [281–284], locus coeruleus [285–288], cerebellum [289–293], prefrontal cortex [294–300], anterior cingulate cortex [301–303], and the striatum [304–307]. The cholinergic basal forebrain, and in particular the nucleus basalis of Meynert, provide the major cholinergic projections to the cerebral cortex and hippocampus and has been linked to memory (reviewed in [308]). Lesions or degeneration of these structures are considered central during aging and AD (reviewed in [309, 310]); indeed, lesions of the forebrain cholinergic system do induce MWM deficits [311]. These should be taken into account when designing a protocol, especially with drug manipulations or the use of genetically modified mice that can present impairments of several brain structures.
3.3.4 MWM in Alzheimer’s disease models
MWM has been often utilized to investigate the presence of rodent cognitive impairment that can be correlated with the cognitive decline in AD patients. However, given that LTM deficits start late in the disease process in AD, MWM deficits can similarly manifest later in AD animal models (see below).
It is possible to use non-transgenic, single transgenic or multi-transgenic models of the disease and each model has its strengths and weakness. Among the most commonly used transgenic mouse models, many manifest an increase of Aβ with plaque formation that parallels the onset of LTM deficits (reviewed in [312]). In our laboratories, we have often used the double transgenic APP(swe)/PS1(M146L) line 6.2 [83]. These animals have the advantage of presenting synaptic and memory deficits at 3–6 months of age together with amyloid increase and deposition. However, it is important to note that while RAWM impairment is already present at 3–5 months of age, MWM starts to be deficient at 6–8 months of age [83, 138, 140]. Thus, it is important to choose the appropriate age in relation with the behavioral test to be performed. Unfortunately, due to commercial issues, it is very difficult to acquire this strain, especially in Europe, so many research groups are using more readily available models such as 3xtg-AD [70]. These models show all of the anatomopathological hallmarks of the disease (senile plaques, neurofibrillary tangles, neuronal loss), together with synaptic and cognitive deficits. In these mice, we detected a MWM impairment, especially in the hidden test, at 4 months of age (manuscript in preparation).
4. Conclusions
Animal behavior evaluation has become a fundamental tool in multiple areas of translational neuroscience and is useful for studying i) the physiological mechanisms underlying neurological disorders, ii) the functional modifications induced by genetic manipulation or chemical treatment, and iii) the efficacy of novel drugs in reversing phenotypes in disease models. This is particularly useful in the AD field, as a clinical hallmark of the disease is memory loss. Furthermore, the “primum movens” of AD is still unknown. Thus, using cognition-based behavior as a readout can help avoid assumptions about the disease pathogenesis that might be disproved by future studies. Towards this end, Tables 3 and 4 list some AD approved drugs and experimental compounds, that have provided positive results in various behavioral assays performed in our laboratories and might therefore be used as positive controls.
Table 3.
Approved Alzheimer’s disease drugs found to ameliorate cognitive deficits in Alzheimer’s disease mouse models
| Compound | Action | Behavioral Task(s) | Model(s) Tested | Reference |
|---|---|---|---|---|
| Memantine | NMDAR antagonist | FC, MWM | 3xTg-AD | Martinez-Coria et al., 2010 [313] |
| MWM | APP/PS1 | Minkeviciene et al., 2004 [314] | ||
| MWM | APP (APP23) | Van Dam et al., 2005; Van Dam and De deyn, 2006 [315,316] | ||
| MWM | APP (Tg2576) | Chen et al., 2010 [317] | ||
| FC | Ts65Dn (Down syndrome mouse) | Costa et al., 2008 [318] | ||
| Donepezil | AChE inhibitor | FC | intracerebroventricular Aβ injection | Tsunekawa et al., 2008 [319] |
| FC | APP (Tg2576) | Dong et al., 2005 [185] | ||
| MWM | APP (APP23) | Van Dam et al., 2005; Van Dam et al., 2008 [315,320] | ||
| Donepezil, Memantine | MWM | APP/PS1 | Nagakura et al., 2013 [321] | |
| Galantamine | AChE inhibitor, nAChR modulator | FC | intracerebroventricular Aβ injection | Wang et al., 2007 [322] |
| MWM | intracerebroventricular Aβ injection | Takeda et al., 2009 [323] | ||
| MWM | APP (APP23) | Van Dam et al., 2005; Van Dam and De deyn, 2006 [315,316] |
Table 4.
Example experimental compounds found to ameliorate cognitive deficits in Alzheimer’s disease mouse models
| Compound | Action | Behavioral Task(s) | Model(s) Tested | Reference |
|---|---|---|---|---|
| Ciproxifan | H3 antagonist | MWM | APP (Tg2576) | Bardgett et al., 2011 [324] |
| AF267B | M1 muscarinic agonist | MWM | 3xTg-AD | Caccamo et al., 2006 [325] |
| Nicotine | nicotinic receptor agonist | RAWM MWM |
chronic Aβ infusion APP/PS1 |
Srivareerat et al., 2011 [326] Inestrosa et al., 2013 [327] |
| A-582941 | α7-nAChR agonist | FC, MWM | 3xTg-AD | Medeiros et al., 2013 [328] |
| CHF5074 | γ-secretase modulator | FC | APP (Tg2576) | Imbimbo et al., 2010 [329] |
| TAK-070 | β-secretase inhibitor | MWM | APP (Tg2576) | Fukumoto et al, 2010 [330] |
| GRL-8234 | β-secretase inhibitor | MWM | APP (Tg2576) | Chang et al., 2010 [331] |
| PBT2 | Aβ aggregation inhibitor | MWM | APP/PS1 | Adlard et al., 2008 [332] |
| Bexarotene | retinoid receptor X agonist | FC, MWM | APP/PS1 | Cramer et al., 2012 [333] |
| Rolipram | PDE4 inhibitor | FC, RAWM, MWM | APP/PS1 | Gong et al., 2004 [140] |
| Sildenafil | PDE5 inhibitor | FC, RAWM, MWM | APP/PS1 | Puzzo et al., 2009 [138] |
| Compound 7a | PDE5 inhibitor | FC, RAWM | APP/PS1; Aβ42 intrahippocampal infusion | Fiorito et al., 2013 [33] |
| E64 | cysteine protease inhibitor | FC, RAWM | APP/PS1 | Trinchese et al., 2008 [143] |
| BDA-410 | calpain inhibitor | FC, RAWM | APP/PS1 | Trinchese et al., 2008 [143] |
| Trichostatin A | histone deacetylase inhibitor | FC | APP/PS1 | Francis et al., 2009 [144] |
| MW108, MW181 | p38αMAPK inhibitor | FC, RAWM | Aβ42 intrahippocampal infusion | Watterson et al., 2013 [34] |
Although cognitive assays of mouse models can provide important information on the validity and efficacy of targets and compounds, it is important to keep in mind that these tasks are incomplete analogs of human cognition. A key limitation is that many cognitive functions are unique to humans or cannot be adequately measured in experimental models (e.g. WM related to language or math). Indeed, these differences might explain, at least in part, the recent high-profile clinical trial failures with drugs that showed promising efficacy in preclinical behavioral tasks. However, in the absence of practical alternatives, mouse models will continue to be essential for accessing AD-type pathology in vivo and testing therapeutic strategies. Cognitive assays will provide the most utility in combination with other experimental arms that support a strong mechanistic correspondence between behavior and molecular target.
Acknowledgments
We thank Walter Gulisano (School of Medicine, University of Catania, Italy) for helpful comments and suggestions. This work was supported by NIH grant NS049442 and AG034248 to O.A. and Neuroscience Program - Compagnia di San Paolo (#2008.2363) and Alzheimer’s Association (IIRG-09-134220) to D.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2.Laferla FM, Green KN. Animal Models of Alzheimer Disease. Cold Spring Harbor perspectives in medicine. 2012 doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–45. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 5.Mucke L, Selkoe DJ. Neurotoxicity of Amyloid beta-Protein: Synaptic and Network Dysfunction. Cold Spring Harbor perspectives in medicine. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clinical anatomy. 1995;8:429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 9.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–6. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 11.Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, et al. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J Biol Chem. 1992;267:546–54. [PubMed] [Google Scholar]
- 12.Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer’s disease. J Mol Biol. 1991;218:149–63. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–7. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 14.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–8. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 15.Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–13. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 21.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 22.Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–7. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 23.Freir DB, Holscher C, Herron CE. Blockade of long-term potentiation by beta-amyloid peptides in the CA1 region of the rat hippocampus in vivo. J Neurophysiol. 2001;85:708–13. doi: 10.1152/jn.2001.85.2.708. [DOI] [PubMed] [Google Scholar]
- 24.Itoh A, Akaike T, Sokabe M, Nitta A, Iida R, Olariu A, et al. Impairments of long-term potentiation in hippocampal slices of beta-amyloid-infused rats. Eur J Pharmacol. 1999;382:167–75. doi: 10.1016/s0014-2999(99)00601-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–33. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci. 2005;25:6887–97. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–14. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–21. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 32.Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiology of aging. 2012;33:1484, e15–24. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Fiorito J, Saeed F, Zhang H, Staniszewski A, Feng Y, Francis YI, et al. Synthesis of quinoline derivatives: discovery of a potent and selective phosphodiesterase 5 inhibitor for the treatment of Alzheimer’s disease. Eur J Med Chem. 2013;60:285–94. doi: 10.1016/j.ejmech.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watterson DM, Grum-Tokars VL, Roy SM, Schavocky JP, Bradaric BD, Bachstetter AD, et al. Development of Novel Chemical Probes to Address CNS Protein Kinase Involvement in Synaptic Dysfunction. PloS one. 2013;8:e66226. doi: 10.1371/journal.pone.0066226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedert M, Spillantini MG, Crowther RA. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A. 1992;89:1983–7. doi: 10.1073/pnas.89.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqua A, Luo Y, Meyer V, Swanson MA, Yu X, Wei G, et al. Conformational basis for asymmetric seeding barrier in filaments of three-and four-repeat tau. Journal of the American Chemical Society. 2012;134:10271–8. doi: 10.1021/ja303498q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–9. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 40.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 43.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, et al. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–7. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 44.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–8. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 45.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 46.Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–6. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 47.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360:672–4. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 48.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 49.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, et al. The Swedish mutation causes early-onset Alzheimer’s disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1:1291–6. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 50.Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 51.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–3. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 52.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–13. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 53.Cruts M, Theuns J, Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Human mutation. 2012;33:1340–4. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 55.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–7. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 56.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 57.Ronnback A, Zhu S, Dillner K, Aoki M, Lilius L, Naslund J, et al. Progressive neuropathology and cognitive decline in a single Arctic APP transgenic mouse model. Neurobiology of aging. 2011;32:280–92. doi: 10.1016/j.neurobiolaging.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–60. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 59.Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–56. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–70. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 61.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 62.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–9. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–47. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Molecular and cellular neurosciences. 2001;18:702–14. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- 65.Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, et al. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem. 2005;280:3963–73. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- 66.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 67.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 68.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 69.Ribe EM, Perez M, Puig B, Gich I, Lim F, Cuadrado M, et al. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiology of disease. 2005;20:814–22. doi: 10.1016/j.nbd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 70.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 71.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–62. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 73.Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–73. doi: 10.1016/0166-4328(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 74.Yagi H, Katoh S, Akiguchi I, Takeda T. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res. 1988;474:86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- 75.Okuma Y, Nomura Y. Senescence-accelerated mouse (SAM) as an animal model of senile dementia: pharmacological, neurochemical and molecular biological approach. Japanese journal of pharmacology. 1998;78:399–404. doi: 10.1254/jjp.78.399. [DOI] [PubMed] [Google Scholar]
- 76.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, et al. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1537–45. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, et al. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:12867–72. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dineley KT, Xia X, Bui D, Sweatt JD, Zheng H. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem. 2002;277:22768–80. doi: 10.1074/jbc.M200164200. [DOI] [PubMed] [Google Scholar]
- 79.Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2005;25:8898–902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–6. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Molecular and cellular neurosciences. 2007;34:621–8. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Saydoff JA, Olariu A, Sheng J, Hu Z, Li Q, Garcia R, et al. Uridine prodrug improves memory in Tg2576 and TAPP mice and reduces pathological factors associated with Alzheimer’s disease in related models. J Alzheimers Dis. 2013;36:637–57. doi: 10.3233/JAD-130059. [DOI] [PubMed] [Google Scholar]
- 83.Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–14. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 84.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao X, Sudhof T. A transcriptionally active complex of APP with Fe65 and histone acyltransferase Tip60. Science. 2001;293:115–20. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 87.Kamal A, Almenar-Queralt A, LeBlanc J, Roberts E, Goldstein L. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–8. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- 88.Nitta A, Itoh A, Hasegawa T, Nabeshima T. beta-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci Lett. 1994;170:63–6. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 89.Chen SY, Wright JW, Barnes CD. The neurochemical and behavioral effects of beta-amyloid peptide(25–35) Brain Res. 1996;720:54–60. doi: 10.1016/0006-8993(96)00136-9. [DOI] [PubMed] [Google Scholar]
- 90.Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–93. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 91.Cheng YF, Wang C, Lin HB, Li YF, Huang Y, Xu JP, et al. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25–35 or Abeta1–40 peptide in rats. Psychopharmacology. 2010;212:181–91. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- 92.Han M, Liu Y, Tan Q, Zhang B, Wang W, Liu J, et al. Therapeutic efficacy of stemazole in a beta-amyloid injection rat model of Alzheimer’s disease. Eur J Pharmacol. 2011;657:104–10. doi: 10.1016/j.ejphar.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 93.Asle-Rousta M, Kolahdooz Z, Oryan S, Ahmadiani A, Dargahi L. FTY720 (fingolimod) attenuates beta-amyloid peptide (Abeta42)-induced impairment of spatial learning and memory in rats. Journal of molecular neuroscience: MN. 2013;50:524–32. doi: 10.1007/s12031-013-9979-6. [DOI] [PubMed] [Google Scholar]
- 94.Choi BR, Lee SR, Han JS, Woo SK, Kim KM, Choi DH, et al. Synergistic memory impairment through the interaction of chronic cerebral hypoperfusion and amlyloid toxicity in a rat model. Stroke; a journal of cerebral circulation. 2011;42:2595–604. doi: 10.1161/STROKEAHA.111.620179. [DOI] [PubMed] [Google Scholar]
- 95.Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 96.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18:413–7. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 97.Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 98.Schonknecht P, Pantel J, Hunt A, Volkmann M, Buerger K, Hampel H, et al. Levels of total tau and tau protein phosphorylated at threonine 181 in patients with incipient and manifest Alzheimer’s disease. Neurosci Lett. 2003;339:172–4. doi: 10.1016/s0304-3940(02)01481-7. [DOI] [PubMed] [Google Scholar]
- 99.Haense C, Buerger K, Kalbe E, Drzezga A, Teipel SJ, Markiewicz P, et al. CSF total and phosphorylated tau protein, regional glucose metabolism and dementia severity in Alzheimer’s disease. Eur J Neurol. 2008;15:1155–62. doi: 10.1111/j.1468-1331.2008.02274.x. [DOI] [PubMed] [Google Scholar]
- 100.Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–62. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moe JG, Chatterjee I, Davidowitz EJ, Arancio O. Modulation of synaptic function by extracellular tau enriched in oligomers. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2009:499. [Google Scholar]
- 102.Moe JG, Puzzo D, Chatterjee I, Davidowitz E, Arancio O. Evaluation of the effect of extracellular tau oligomers on synaptic function. Society for Neuroscience, 38th Annual Meeting; Washington, DC. 2008. p. 543.17/R12. [Google Scholar]
- 103.Moe JG, Chatterjee I, Puzzo D, Staniszewski A, Fa’ M, Davidowitz E, et al. Validation of extracellular tau oligomer target for drug discovery in a novel animal model. Society for Neuroscience, 40th Annual Meeting; San Diego. 2010. p. 527.8. [Google Scholar]
- 104.Moe JG, Chatterjee I, Puzzo D, Staniszewski A, Fa’ M, Davidowitz EJ, et al. Extracellular oligomeric tau inhibits memory formation in mice. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2010:S277. [Google Scholar]
- 105.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Claredon; 1978. [Google Scholar]
- 107.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 108.Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual review of psychology. 1993;44:453–95. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- 109.Probst A, Basler V, Bron B, Ulrich J. Neuritic plaques in senile dementia of Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res. 1983;268:249–54. doi: 10.1016/0006-8993(83)90490-0. [DOI] [PubMed] [Google Scholar]
- 110.Bussiere T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, et al. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience. 2002;112:75–91. doi: 10.1016/s0306-4522(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 111.Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, et al. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuro Image. 2003;20:667–82. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- 112.Rekart JL, Quinn B, Mesulam MM, Routtenberg A. Subfield-specific increase in brain growth protein in postmortem hippocampus of Alzheimer’s patients. Neuroscience. 2004;126:579–84. doi: 10.1016/j.neuroscience.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, Miller JP, Gado MH, McKeel DW, Rothermich M, Miller MI, et al. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuro Image. 2006;30:52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiology of aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Clark RE, Squire LR. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proc Natl Acad Sci U S A. 2013;110 (Suppl 2):10365–70. doi: 10.1073/pnas.1301225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23:656–61. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milner B. The memory defect in bilateral hippocampal lesions. Psychiatric research reports. 1959;11:43–58. [PubMed] [Google Scholar]
- 118.Sass KJ, Sass A, Westerveld M, Lencz T, Novelly RA, Kim JH, et al. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. Journal of clinical and experimental neuropsychology. 1992;14:662–72. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- 119.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 120.Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- 121.Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1976:2. doi: 10.1037/0097-7403.33.3.213. [DOI] [PubMed] [Google Scholar]
- 122.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 123.Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav Neurosci. 1988;102:331–9. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- 124.Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–42. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wood ER, Dudchenko PA. Aging, spatial behavior and the cognitive map. Nat Neurosci. 2003;6:546–8. doi: 10.1038/nn0603-546. [DOI] [PubMed] [Google Scholar]
- 126.Association As. Memory loss and other symptoms of dementia. 2013 [Google Scholar]
- 127.Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–77. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 128.Layton B, Krikorian R. Memory mechanisms in posttraumatic stress disorder. The Journal of neuropsychiatry and clinical neurosciences. 2002;14:254–61. doi: 10.1176/jnp.14.3.254. [DOI] [PubMed] [Google Scholar]
- 129.LeDoux J. The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–34. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 131.Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- 132.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 134.Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues in clinical neuroscience. 2002;4:231–49. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cannon WB. Bodily changes in pain, hunger, fear and rage. New York, NY: 1915. [Google Scholar]
- 136.Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of comparative and physiological psychology. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 137.Wehner JM, Radcliffe RA. Cued and contextual fear conditioning in mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 8. Chapter 8. 2004. p. 5C. [DOI] [PubMed] [Google Scholar]
- 138.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29:8075–86. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, et al. Ubiquitin Hydrolase Uch-L1 Rescues beta-Amyloid-Induced Decreases in Synaptic Function and Contextual Memory. Cell. 2006;126:775–88. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 140.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–34. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Puzzo D, Privitera L, Fa M, Staniszewski A, Hashimoto G, Aziz F, et al. Endogenous amyloid-beta is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–30. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–45. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Francis YI, Fa M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, et al. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J Alzheimers Dis. 2009;18:131–9. doi: 10.3233/JAD-2009-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 146.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 147.Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- 148.Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 149.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–74. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 150.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–14. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 152.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 153.Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–8. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–80. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 155.Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–74. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- 156.Aguiar DC, Hott SC, Deolindo MV, Guimaraes FS, Resstel LB. The dorsolateral periaqueductal grey N-methyl-D-aspartate/nitric oxide/cyclic guanosine monophosphate pathway modulates the expression of contextual fear conditioning in rats. Journal of psychopharmacology. 2013 doi: 10.1177/0269881113504012. [DOI] [PubMed] [Google Scholar]
- 157.Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–96. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–4. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 160.Wallenstein GV, Vago DR, Walberer AM. Time-dependent involvement of PKA/PKC in contextual memory consolidation. Behav Brain Res. 2002;133:159–64. doi: 10.1016/s0166-4328(01)00476-4. [DOI] [PubMed] [Google Scholar]
- 161.Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–8. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- 162.Shan Q, Chan GC, Storm DR. Type 1 adenylyl cyclase is essential for maintenance of remote contextual fear memory. J Neurosci. 2008;28:12864–7. doi: 10.1523/JNEUROSCI.2413-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kelley JB, Anderson KL, Itzhak Y. Pharmacological modulators of nitric oxide signaling and contextual fear conditioning in mice. Psychopharmacology. 2010;210:65–74. doi: 10.1007/s00213-010-1817-8. [DOI] [PubMed] [Google Scholar]
- 164.Zoubovsky SP, Pogorelov VM, Taniguchi Y, Kim SH, Yoon P, Nwulia E, et al. Working memory deficits in neuronal nitric oxide synthase knockout mice: potential impairments in prefrontal cortex mediated cognitive function. Biochemical and biophysical research communications. 2011;408:707–12. doi: 10.1016/j.bbrc.2011.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nassireslami E, Nikbin P, Payandemehr B, Amini E, Mohammadi M, Vakilzadeh G, et al. A cAMP analog reverses contextual and tone memory deficits induced by a PKA inhibitor in Pavlovian fear conditioning. Pharmacology, biochemistry, and behavior. 2013;105:177–82. doi: 10.1016/j.pbb.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 166.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 167.Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- 168.Viosca J, Lopez de Armentia M, Jancic D, Barco A. Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem. 2009;16:193–7. doi: 10.1101/lm.1254209. [DOI] [PubMed] [Google Scholar]
- 169.Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–6. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- 170.Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000;20:8515–27. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Misane I, Kruis A, Pieneman AW, Ogren SO, Stiedl O. GABA(A) receptor activation in the CA1 area of the dorsal hippocampus impairs consolidation of conditioned contextual fear in C57BL/6J mice. Behav Brain Res. 2013;238:160–9. doi: 10.1016/j.bbr.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 172.Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- 173.Santos JM, Martinez RC, Brandao ML. Effects of acute and subchronic treatments with fluoxetine and desipramine on the memory of fear in moderate and high-intensity contextual conditioning. Eur J Pharmacol. 2006;542:121–8. doi: 10.1016/j.ejphar.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 174.Figueredo LZ, Moreira KM, Ferreira TL, Fornari RV, Oliveira MG. Interaction between glutamatergic-NMDA and cholinergic-muscarinic systems in classical fear conditioning. Brain research bulletin. 2008;77:71–6. doi: 10.1016/j.brainresbull.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 175.Chang SD, Liang KC. Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study. Neurobiology of learning and memory. 2012;98:17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 176.Zhang WN, Bast T, Xu Y, Feldon J. Temporary inhibition of dorsal or ventral hippocampus by muscimol: Distinct effects on measures of innate anxiety on the elevated plus maze, but similar disruption of contextual fear conditioning. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 177.Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn Mem. 2004;11:102–7. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Almada RC, Albrechet-Souza L, Brandao ML. Further evidence for involvement of the dorsal hippocampus serotonergic and gamma-aminobutyric acid (GABA)ergic pathways in the expression of contextual fear conditioning in rats. Journal of psychopharmacology. 2013;27:1160–8. doi: 10.1177/0269881113482840. [DOI] [PubMed] [Google Scholar]
- 179.Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, et al. Cholinergic blockade frees fear extinction from its contextual dependency. Biological psychiatry. 2013;73:345–52. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer’s disease. Neuropsychologia. 2002;40:1187–95. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 181.Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain: a journal of neurology. 2008;131:1646–57. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dobarro M, Gerenu G, Ramirez MJ. Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:2245–57. doi: 10.1017/S1461145713000631. [DOI] [PubMed] [Google Scholar]
- 183.Corcoran KA, Lu Y, Turner RS, Maren S. Overexpression of hAPPswe impairs rewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer’s disease. Learn Mem. 2002;9:243–52. doi: 10.1101/lm.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer’s disease-like pathology. Nat Med. 2008;14:1106–11. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- 185.Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG. Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology. 2005;181:145–52. doi: 10.1007/s00213-005-2230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hsiao YH, Kuo JR, Chen SH, Gean PW. Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiology of disease. 2012;45:1111–20. doi: 10.1016/j.nbd.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 187.Knafo S, Venero C, Merino-Serrais P, Fernaud-Espinosa I, Gonzalez-Soriano J, Ferrer I, et al. Morphological alterations to neurons of the amygdala and impaired fear conditioning in a transgenic mouse model of Alzheimer’s disease. The Journal of pathology. 2009;219:41–51. doi: 10.1002/path.2565. [DOI] [PubMed] [Google Scholar]
- 188.Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:870–80. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bonardi C, de Pulford F, Jennings D, Pardon MC. A detailed analysis of the early context extinction deficits seen in APPswe/PS1dE9 female mice and their relevance to preclinical Alzheimer’s disease. Behav Brain Res. 2011;222:89–97. doi: 10.1016/j.bbr.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 190.Liu B, Frost JL, Sun J, Fu H, Grimes S, Blackburn P, et al. MER5101, a novel Abeta1–15:DT conjugate vaccine, generates a robust anti-Abeta antibody response and attenuates Abeta pathology and cognitive deficits in APPswe/PS1DeltaE9 transgenic mice. J Neurosci. 2013;33:7027–37. doi: 10.1523/JNEUROSCI.5924-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cheng D, Logge W, Low JK, Garner B, Karl T. Novel behavioural characteristics of the APP(Swe)/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Behav Brain Res. 2013;245:120–7. doi: 10.1016/j.bbr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–12. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 193.Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. The Journal of pharmacology and experimental therapeutics. 2008;326:739–44. doi: 10.1124/jpet.108.140293. [DOI] [PubMed] [Google Scholar]
- 194.Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, et al. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci. 2013;33:15596–602. doi: 10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Devi L, Ohno M. Effects of levetiracetam, an antiepileptic drug, on memory impairments associated with aging and Alzheimer’s disease in mice. Neurobiology of learning and memory. 2013;102:7–11. doi: 10.1016/j.nlm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 196.Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, et al. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. Journal of neuroscience research. 2010;88:2923–32. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Buresova O, Bures J, Oitzl MS, Zahalka A. Radial maze in the water tank: an aversively motivated spatial working memory task. Physiology & behavior. 1985;34:1003–5. doi: 10.1016/0031-9384(85)90028-9. [DOI] [PubMed] [Google Scholar]
- 198.Shear DA, Dong J, Haik-Creguer KL, Bazzett TJ, Albin RL, Dunbar GL. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Experimental neurology. 1998;150:305–11. doi: 10.1006/exnr.1998.6767. [DOI] [PubMed] [Google Scholar]
- 199.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–52. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 200.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and neural biology. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 201.Morris RGM. Spatial localization does not require the presence of local cues. Learning and Motivation. 1981;12:239–60. [Google Scholar]
- 202.Hodges H. Maze procedures: the radial-arm and water maze compared. Brain research Cognitive brain research. 1996;3:167–81. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 203.Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA. Effect of age on the radial arm water maze-a test of spatial learning and memory. Neurobiology of aging. 2004;25:223–9. doi: 10.1016/s0197-4580(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 204.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–9. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 205.Petrone A, Battaglia F, Wang F, Susa A, Su J, Zagzag D, et al. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long term potentiation. EMBO J. 2003;22:4121–31. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiology & behavior. 2000;70:311–7. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- 207.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision research. 2002;42:517–25. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 208.Clapcote SJ, Lazar NL, Bechard AR, Wood GA, Roder JC. NIH Swiss and Black Swiss mice have retinal degeneration and performance deficits in cognitive tests. Comparative medicine. 2005;55:310–6. [PubMed] [Google Scholar]
- 209.Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- 210.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 211.James W. Principles of psychology. I. New York: Dover Publications; 1890. [Google Scholar]
- 212.Eichenbaum H. The Cognitive Neuroscience of Memory: An Introduction. Oxford University Press; 2011. [Google Scholar]
- 213.Pribam KH, Miller GA, Galanter E. Plans and the structure of behavior. New York: 1960. [Google Scholar]
- 214.Honig WK. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum Associates; 1978. Studies of working memory in the pigeon. [Google Scholar]
- 215.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience and biobehavioral reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 216.Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 217.Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Frontiers in aging neuroscience. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, et al. In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci U S A. 2003;100:14193–8. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. The Quarterly journal of experimental psychology B, Comparative and physiological psychology. 1986;38:365–95. [PubMed] [Google Scholar]
- 221.Stewart S, Cacucci F, Lever C. Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer’s mouse model. J Alzheimers Dis. 2011;26:105–26. doi: 10.3233/JAD-2011-101827. [DOI] [PubMed] [Google Scholar]
- 222.Golub MS, Germann SL, Mercer M, Gordon MN, Morgan DG, Mayer LP, et al. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiology of aging. 2008;29:1512–23. doi: 10.1016/j.neurobiolaging.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–52. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 224.Rees TM, Berson A, Sklan EH, Younkin L, Younkin S, Brimijoin S, et al. Memory deficits correlating with acetylcholinesterase splice shift and amyloid burden in doubly transgenic mice. Current Alzheimer research. 2005;2:291–300. doi: 10.2174/1567205054367847. [DOI] [PubMed] [Google Scholar]
- 225.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, et al. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5340–6. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. Journal of neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xiong H, Callaghan D, Wodzinska J, Xu J, Premyslova M, Liu QY, et al. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer’s disease. Neuroscience bulletin. 2011;27:221–32. doi: 10.1007/s12264-011-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Volianskis A, Kostner R, Molgaard M, Hass S, Jensen MS. Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1deltaE9-deleted transgenic mice model of ss-amyloidosis. Neurobiology of aging. 2010;31:1173–87. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 229.Sood A, Warren Beach J, Webster SJ, Terry AV, Buccafusco JJ. The effects of JWB1-84-1 on memory-related task performance by amyloid Abeta transgenic mice and by young and aged monkeys. Neuropharmacology. 2007;53:588–600. doi: 10.1016/j.neuropharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 230.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 231.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, et al. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 232.Webster SJ, Bachstetter AD, Van Eldik LJ. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimer’s research & therapy. 2013;5:28. doi: 10.1186/alzrt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 234.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain research Brain research reviews. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 235.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 236.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Palmeri A, Privitera L, Giunta S, Loreto C, Puzzo D. Inhibition of phosphodiesterase-5 rescues age-related impairment of synaptic plasticity and memory. Behav Brain Res. 2013;240:11–20. doi: 10.1016/j.bbr.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 238.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. The International journal of neuroscience. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 239.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain research Brain research reviews. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 240.Terry Alvin VJ. National Institutes of Health. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): CRC Press; 2009. Spatial Navigation (Water Maze) Tasks. NCBI Bookshelf. A service of the National Library of Medicine. [Google Scholar]
- 241.Dalm S, Grootendorst J, de Kloet ER, Oitzl MS. Quantification of swim patterns in the Morris water maze. Behavior research methods, instruments, & computers: a journal of the Psychonomic Society, Inc. 2000;32:134–9. doi: 10.3758/bf03200795. [DOI] [PubMed] [Google Scholar]
- 242.Lipp HP, Wolfer DP. Genetically modified mice and cognition. Current opinion in neurobiology. 1998;8:272–80. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 243.Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends in neurosciences. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- 244.Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiology & behavior. 1996;60:1191–7. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- 245.Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance. Behavior genetics. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- 246.Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 247.Adams B, Fitch T, Chaney S, Gerlai R. Altered performance characteristics in cognitive tasks: comparison of the albino ICR and CD1 mouse strains. Behav Brain Res. 2002;133:351–61. doi: 10.1016/s0166-4328(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 248.Reid IC, Morris RG. The enigma of olfactory learning. Trends in neurosciences. 1993;16:17–20. doi: 10.1016/0166-2236(93)90043-l. [DOI] [PubMed] [Google Scholar]
- 249.Lavenex P, Schenk F. Integration of olfactory information in a spatial representation enabling accurate arm choice in the radial arm maze. Learn Mem. 1996;2:299–319. doi: 10.1101/lm.2.6.299. [DOI] [PubMed] [Google Scholar]
- 250.Luine VN. Steroid Hormone Modulation of Hippocampal Dependent Spatial Memory. Stress. 1997;2:21–36. doi: 10.3109/10253899709014735. [DOI] [PubMed] [Google Scholar]
- 251.Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, et al. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Canadian journal of experimental psychology = Revue canadienne de psychologie experimentale. 2008;62:247–60. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- 252.Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. Journal of neuroendocrinology. 2013 doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 253.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gould TD. Mood and anxiety related phenotypes in mice; characterization using behavioral tests. Humana Press Inc; 2009. [Google Scholar]
- 255.Yayou K, Takeda M, Tsubone H, Sugano S, Doi K. The disturbance of water-maze task performance in mice with EMC-D virus infection. The Journal of veterinary medical science/the Japanese Society of Veterinary Science. 1993;55:341–2. doi: 10.1292/jvms.55.341. [DOI] [PubMed] [Google Scholar]
- 256.Kavaliers M, Colwell DD. Reduced spatial learning in mice infected with the nematode, Heligmosomoides polygyrus. Parasitology. 1995;110 (Pt 5):591–7. doi: 10.1017/s0031182000065318. [DOI] [PubMed] [Google Scholar]
- 257.Braithwaite VA, Salkeld DJ, McAdam HM, Hockings CG, Ludlow AM, Read AF. Spatial and discrimination learning in rodents infected with the nematode Strongyloides ratti. Parasitology. 1998;117 (Pt 2):145–54. doi: 10.1017/s003118209800290x. [DOI] [PubMed] [Google Scholar]
- 258.Espinoza JA, Bohmwald K, Cespedes PF, Gomez RS, Riquelme SA, Cortes CM, et al. Impaired learning resulting from respiratory syncytial virus infection. Proc Natl Acad Sci U S A. 2013;110:9112–7. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, et al. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–15. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 260.Bergado JA, Almaguer W, Rojas Y, Capdevila V, Frey JU. Spatial and emotional memory in aged rats: a behavioral-statistical analysis. Neuroscience. 2011;172:256–69. doi: 10.1016/j.neuroscience.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 261.Nilsson OG, Shapiro ML, Gage FH, Olton DS, Bjorklund A. Spatial learning and memory following fimbria-fornix transection and grafting of fetal septal neurons to the hippocampus. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1987;67:195–215. doi: 10.1007/BF00269466. [DOI] [PubMed] [Google Scholar]
- 262.Roof RL, Zhang Q, Glasier MM, Stein DG. Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res. 1993;57:47–51. doi: 10.1016/0166-4328(93)90060-4. [DOI] [PubMed] [Google Scholar]
- 263.Whishaw IQ, Jarrard LE. Similarities vs. differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- 264.Liu P, Bilkey DK. Perirhinal cortex contributions to performance in the Morris water maze. Behav Neurosci. 1998;112:304–15. doi: 10.1037//0735-7044.112.2.304. [DOI] [PubMed] [Google Scholar]
- 265.Buckley MJ, Wilson CR, Gaffan D. Fornix transection impairs visuospatial memory acquisition more than retrieval. Behav Neurosci. 2008;122:44–53. doi: 10.1037/0735-7044.122.1.44. [DOI] [PubMed] [Google Scholar]
- 266.Vann SD, Erichsen JT, O’Mara SM, Aggleton JP. Selective disconnection of the hippocampal formation projections to the mammillary bodies produces only mild deficits on spatial memory tasks: implications for fornix function. Hippocampus. 2011;21:945–57. doi: 10.1002/hipo.20796. [DOI] [PubMed] [Google Scholar]
- 267.Mala H, Rodriguez Castro M, Pearce H, Kingod SC, Nedergaard SK, Scharff Z, et al. Delayed intensive acquisition training alleviates the lesion-induced place learning deficits after fimbria-fornix transection in the rat. Brain Res. 2012;1445:40–51. doi: 10.1016/j.brainres.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 268.Mumby DG, Cameli L, Glenn MJ. Impaired allocentric spatial working memory and intact retrograde memory after thalamic damage caused by thiamine deficiency in rats. Behav Neurosci. 1999;113:42–50. [PubMed] [Google Scholar]
- 269.Savage LM, Sweet AJ, Castillo R, Langlais PJ. The effects of lesions to thalamic lateral internal medullary lamina and posterior nuclei on learning, memory and habituation in the rat. Behav Brain Res. 1997;82:133–47. doi: 10.1016/s0166-4328(97)80983-7. [DOI] [PubMed] [Google Scholar]
- 270.Hamani C, Dubiela FP, Soares JC, Shin D, Bittencourt S, Covolan L, et al. Anterior thalamus deep brain stimulation at high current impairs memory in rats. Experimental neurology. 2010;225:154–62. doi: 10.1016/j.expneurol.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 271.Bor J, Brunelin J, Sappey-Marinier D, Ibarrola D, d’Amato T, Suaud-Chagny MF, et al. Thalamus abnormalities during working memory in schizophrenia. An fMRI study Schizophrenia research. 2011;125:49–53. doi: 10.1016/j.schres.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 272.Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, et al. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci. 2012;32:9947–59. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, et al. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci. 2013;33:8772–83. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Frontiers in systems neuroscience. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Santin LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–50. doi: 10.1016/s0166-4328(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 276.Conejo NM, Gonzalez-Pardo H, Vallejo G, Arias JL. Involvement of the mammillary bodies in spatial working memory revealed by cytochrome oxidase activity. Brain Res. 2004;1011:107–14. doi: 10.1016/j.brainres.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 277.Radyushkin K, Anokhin K, Meyer BI, Jiang Q, Alvarez-Bolado G, Gruss P. Genetic ablation of the mammillary bodies in the Foxb1 mutant mouse leads to selective deficit of spatial working memory. The European journal of neuroscience. 2005;21:219–29. doi: 10.1111/j.1460-9568.2004.03844.x. [DOI] [PubMed] [Google Scholar]
- 278.Aranda L, Begega A, Sanchez-Lopez J, Aguirre JA, Arias JL, Santin LJ. Temporary inactivation of the supramammillary area impairs spatial working memory and spatial reference memory retrieval. Physiology & behavior. 2008;94:322–30. doi: 10.1016/j.physbeh.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 279.Mendez M, Mendez-Lopez M, Lopez L, Aller MA, Arias J, Arias JL. Mammillary body alterations and spatial memory impairment in Wistar rats with thioacetamide-induced cirrhosis. Brain Res. 2008;1233:185–95. doi: 10.1016/j.brainres.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 280.Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48:2316–27. doi: 10.1016/j.neuropsychologia.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 281.Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiology of learning and memory. 1995;64:156–68. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- 282.Spanis CW, Bianchin MM, Izquierdo I, McGaugh JL. Excitotoxic basolateral amygdala lesions potentiate the memory impairment effect of muscimol injected into the medial septal area. Brain Res. 1999;816:329–36. doi: 10.1016/s0006-8993(98)01104-4. [DOI] [PubMed] [Google Scholar]
- 283.Galliot E, Levaillant M, Beard E, Millot JL, Pourie G. Enhancement of spatial learning by predator odor in mice: involvement of amygdala and hippocampus. Neurobiology of learning and memory. 2010;93:196–202. doi: 10.1016/j.nlm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 284.Gaskin S, White NM. Parallel processing of information about location in the amygdala, entorhinal cortex and hippocampus. Hippocampus. 2013;23:1075–83. doi: 10.1002/hipo.22179. [DOI] [PubMed] [Google Scholar]
- 285.Riekkinen P, Jr, Sirvio J, Riekkinen P. Interaction between raphe dorsalis and nucleus basalis magnocellularis in spatial learning. Brain Res. 1990;527:342–5. doi: 10.1016/0006-8993(90)91156-b. [DOI] [PubMed] [Google Scholar]
- 286.Compton DM, Dietrich KL, Smith JS, Davis BK. Spatial and non-spatial learning in the rat following lesions to the nucleus locus coeruleus. Neuroreport. 1995;7:177–82. [PubMed] [Google Scholar]
- 287.Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on beta-adrenergic receptor activation. Cerebral cortex. 2009;19:2827–37. doi: 10.1093/cercor/bhp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Khakpour-Taleghani B, Lashgari R, Motamedi F, Naghdi N. Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience. 2009;158:1284–91. doi: 10.1016/j.neuroscience.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 289.Lalonde R. Cerebellar contributions to instrumental learning. Neuroscience and biobehavioral reviews. 1994;18:161–70. doi: 10.1016/0149-7634(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 290.Petrosini L, Leggio MG, Molinari M. The cerebellum in the spatial problem solving: a co-star or a guest star? Progress in neurobiology. 1998;56:191–210. doi: 10.1016/s0301-0082(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 291.Petrosini L, Molinari M, Dell’Anna ME. Cerebellar contribution to spatial event processing: Morris water maze and T-maze. The European journal of neuroscience. 1996;8:1882–96. doi: 10.1111/j.1460-9568.1996.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 292.Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–9. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- 293.Passot JB, Sheynikhovich D, Duvelle E, Arleo A. Contribution of cerebellar sensorimotor adaptation to hippocampal spatial memory. PloS one. 2012;7:e32560. doi: 10.1371/journal.pone.0032560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mogensen J, Pedersen TK, Holm S, Bang LE. Prefrontal cortical mediation of rats’ place learning in a modified water maze. Brain research bulletin. 1995;38:425–34. doi: 10.1016/0361-9230(95)02009-g. [DOI] [PubMed] [Google Scholar]
- 295.Granon S, Poucet B. Medial prefrontal lesions in the rat and spatial navigation: evidence for impaired planning. Behav Neurosci. 1995;109:474–84. doi: 10.1037//0735-7044.109.3.474. [DOI] [PubMed] [Google Scholar]
- 296.Chiba AA, Kesner RP, Reynolds AM. Memory for spatial location as a function of temporal lag in rats: role of hippocampus and medial prefrontal cortex. Behavioral and neural biology. 1994;61:123–31. doi: 10.1016/s0163-1047(05)80065-2. [DOI] [PubMed] [Google Scholar]
- 297.Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behav Brain Res. 1997;84:203–23. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- 298.McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral cortex. 1996;6:600–11. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 299.Romanides AJ, Duffy P, Kalivas PW. Glutamatergic and dopaminergic afferents to the prefrontal cortex regulate spatial working memory in rats. Neuroscience. 1999;92:97–106. doi: 10.1016/s0306-4522(98)00747-7. [DOI] [PubMed] [Google Scholar]
- 300.Hannesson DK, Vacca G, Howland JG, Phillips AG. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res. 2004;153:273–85. doi: 10.1016/j.bbr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 301.Warburton EC, Aggleton JP, Muir JL. Comparing the effects of selective cingulate cortex lesions and cingulum bundle lesions on water maze performance by rats. The European journal of neuroscience. 1998;10:622–34. doi: 10.1046/j.1460-9568.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- 302.Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–64. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.St-Laurent M, Petrides M, Sziklas V. Does the cingulate cortex contribute to spatial conditional associative learning in the rat? Hippocampus. 2009;19:612–22. doi: 10.1002/hipo.20539. [DOI] [PubMed] [Google Scholar]
- 304.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–38. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 305.Block F, Kunkel M, Schwarz M. Quinolinic acid lesion of the striatum induces impairment in spatial learning and motor performance in rats. Neurosci Lett. 1993;149:126–8. doi: 10.1016/0304-3940(93)90752-7. [DOI] [PubMed] [Google Scholar]
- 306.Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 307.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–98. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gonzalez CL, Miranda MI, Gutierrez H, Ormsby C, Bermudez-Rattoni F. Differential participation of the NBM in the acquisition and retrieval of conditioned taste aversion and Morris water maze. Behav Brain Res. 2000;116:89–98. doi: 10.1016/s0166-4328(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 309.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 310.Gratwicke J, Kahan J, Zrinzo L, Hariz M, Limousin P, Foltynie T, et al. The nucleus basalis of Meynert: A new target for deep brain stimulation in dementia? Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 311.Frielingsdorf H, Thal LJ, Pizzo DP. The septohippocampal cholinergic system and spatial working memory in the Morris water maze. Behav Brain Res. 2006;168:37–46. doi: 10.1016/j.bbr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 312.Shineman DW, Basi GS, Bizon JL, Colton CA, Greenberg BD, Hollister BA, et al. Accelerating drug discovery for Alzheimer’s disease: best practices for preclinical animal studies. Alzheimers Res Ther. 2011;3:28. doi: 10.1186/alzrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, et al. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. The American journal of pathology. 2010;176:870–80. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer’s disease. The Journal of pharmacology and experimental therapeutics. 2004;311:677–82. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 315.Van Dam D, Abramowski D, Staufenbiel M, De Deyn PP. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology. 2005;180:177–90. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- 316.Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2006;16:59–69. doi: 10.1016/j.euroneuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 317.Chen TF, Huang RF, Lin SE, Lu JF, Tang MC, Chiu MJ. Folic Acid potentiates the effect of memantine on spatial learning and neuronal protection in an Alzheimer’s disease transgenic model. J Alzheimers Dis. 2010;20:607–15. doi: 10.3233/JAD-2010-1396. [DOI] [PubMed] [Google Scholar]
- 318.Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1624–32. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- 319.Tsunekawa H, Noda Y, Mouri A, Yoneda F, Nabeshima T. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25–35) Behav Brain Res. 2008;190:224–32. doi: 10.1016/j.bbr.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 320.Van Dam D, Coen K, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of donepezil in the APP23 mouse model for Alzheimer’s disease. Psychopharmacology. 2008;197:37–43. doi: 10.1007/s00213-007-1010-x. [DOI] [PubMed] [Google Scholar]
- 321.Nagakura A, Shitaka Y, Yarimizu J, Matsuoka N. Characterization of cognitive deficits in a transgenic mouse model of Alzheimer’s disease and effects of donepezil and memantine. Eur J Pharmacol. 2013;703:53–61. doi: 10.1016/j.ejphar.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 322.Wang D, Noda Y, Zhou Y, Mouri A, Mizoguchi H, Nitta A, et al. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine ameliorates the cognitive dysfunction in beta amyloid25–35 i.c.v-injected mice: involvement of dopaminergic systems. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1261–71. doi: 10.1038/sj.npp.1301256. [DOI] [PubMed] [Google Scholar]
- 323.Takeda S, Sato N, Niisato K, Takeuchi D, Kurinami H, Shinohara M, et al. Validation of Abeta1–40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res. 2009;1280:137–47. doi: 10.1016/j.brainres.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 324.Bardgett ME, Davis NN, Schultheis PJ, Griffith MS. Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP Tg2576 mouse model of Alzheimer’s disease. Neurobiology of learning and memory. 2011;95:64–72. doi: 10.1016/j.nlm.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–82. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 326.Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA. Chronic nicotine restores normal Abeta levels and prevents short-term memory and E-LTP impairment in Abeta rat model of Alzheimer’s disease. Neurobiology of aging. 2011;32:834–44. doi: 10.1016/j.neurobiolaging.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 327.Inestrosa NC, Godoy JA, Vargas JY, Arrazola MS, Rios JA, Carvajal FJ, et al. Nicotine prevents synaptic impairment induced by amyloid-beta oligomers through alpha7-nicotinic acetylcholine receptor activation. Neuromolecular medicine. 2013;15:549–69. doi: 10.1007/s12017-013-8242-1. [DOI] [PubMed] [Google Scholar]
- 328.Medeiros R, Castello NA, Cheng D, Kitazawa M, Baglietto-Vargas D, Green KN, et al. alpha Nicotinic Receptor Agonist Enhances Cognition in Aged 3xTg-AD Mice with Robust Plaques and Tangles. The American journal of pathology. 2013 doi: 10.1016/j.ajpath.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 329.Imbimbo BP, Giardino L, Sivilia S, Giuliani A, Gusciglio M, Pietrini V, et al. CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20:159–73. doi: 10.3233/JAD-2010-1366. [DOI] [PubMed] [Google Scholar]
- 330.Fukumoto H, Takahashi H, Tarui N, Matsui J, Tomita T, Hirode M, et al. A noncompetitive BACE1 inhibitor TAK-070 ameliorates Abeta pathology and behavioral deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30:11157–66. doi: 10.1523/JNEUROSCI.2884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chang WP, Huang X, Downs D, Cirrito JR, Koelsch G, Holtzman DM, et al. Beta-secretase inhibitor GRL-8234 rescues age-related cognitive decline in APP transgenic mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:775–84. doi: 10.1096/fj.10-167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 333.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]