Abstract
Hepatitis B virus (HBV) infection is an international public health concern, and chronic infection can lead to the development of cirrhosis, liver failure, or hepatocellular carcinoma as well as the need for liver transplantation. The recurrence of HBV infection following liver transplantation was disproportionately high prior to the introduction of proper prophylactic treatment. Risk factors associated with the recurrence of HBV infection post-transplant include hepatitis B e antigen positivity, high levels of serum HBV DNA, and the presence of an antiviral drug-resistant strain prior to transplantation. The prevention of HBV recurrence began with the introduction of hepatitis B immunoglobulin (HBIG) in the early 1 990s. Nucleos(t)ide analog (NA) antiviral drugs were next to be introduced and, in combination with HBIG, are considered to be extremely effective for the prevention of recurrence. Because of concerns with HBIG, whether HBIG can be used for a short time or discontinued altogether is under debate. All of the NA antiviral drugs have been proven to be effective against HBV, at least in the pretransplant setting, and can be used safely posttransplant. Further investigation is still needed to standardize treatment in the posttransplant setting.
Keywords: Hepatitis B virus, hepatocellular carcinoma, liver transplantation, posttransplantation, risk factors, prevention
Hepatitis B virus (HBV) infection is a serious worldwide public health concern. It is estimated that worldwide, almost 250 million persons have been infected and currently have chronic viral hepatitis due to HBV.1 Within the United States, the number of persons who are estimated to have chronic HBV infection is between 800,000 and 1.4 million, and the number of deaths per year associated with chronic liver disease due to HBV infection is estimated at approximately 3000.2
The clinical impact of HBV infection is primarily related to the high risk of associated hepatic complications. Patients with HBV infection are at increased risk for the development of cirrhosis, liver failure, and hepatocellular carcinoma (HCC). Indeed, the HCC risk alone is thought to be almost 200 times greater in infected patients than in noninfected patients, and estimates of the risk of development of other complications associated with HBV infection are between 15% and 40%.3,4
Despite advances in the antiviral therapies for HBV infection, liver transplantation remains the ultimate treatment for many patients with end-stage liver disease and HCC.5 The risk of reinfection posttransplantation without appropriate prophylaxis is disproportionately high and is associated with severe and rapidly progressive liver disease.6-8 Different combinations of drugs are currently used to prevent and treat patients with HBV infection who have undergone liver transplantation, but the optimal therapy that should be used is still currently under debate.9-12
Unlike hepatitis C virus infection, in which care revolves around treating recurrent disease, the goal in the management of recipients of liver transplants for the treatment of HBV infection is to prevent reinfection.
Risk Factors and the Clinical Course of Hepatitis B Virus Infection Following Liver Transplantation
Risk of Recurrence
The exact mechanism of how HBV infection recurrence occurs posttransplant is still not fully understood but is probably related to the required immunosuppression, particularly in relation to prednisone therapy. For instance, there is a glucocorticoid-responsive enhancer region within the HBV DNA genome.13-15 Another likely contributing factor could be the presence of extrahepatic HBV infection.16,17
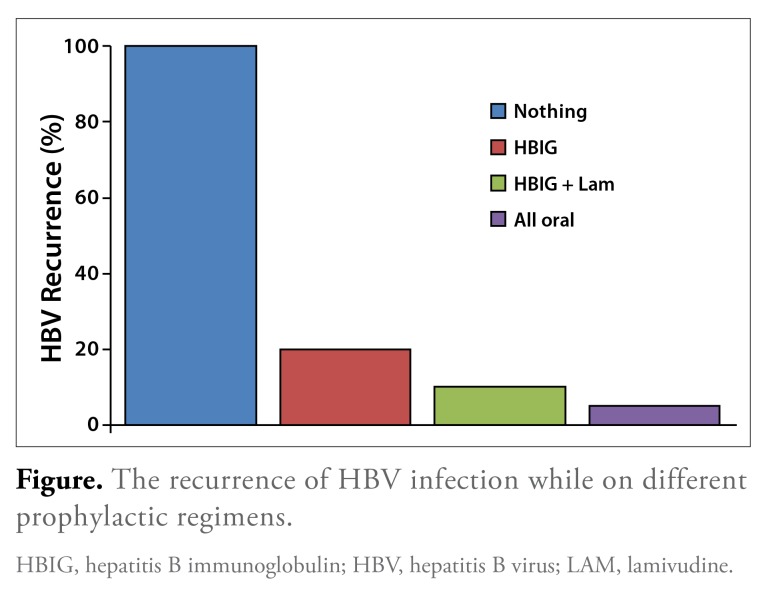
The natural history and management of HBV infection recurrence in liver transplant recipients have evolved significantly over the past few decades. There has been a progressive incremental decrease in the risk of reinfection with the introduction of hepatitis B immunoglobulin (HBIG), oral antiviral agents, and a combination of HBIG and oral antiviral therapies. For instance, prior to the use of HBIG, the risk of reinfection with HBV was reported to be between 75% and 100% (Figure).6,7,18,19 Currently, the risk of reinfection is thought to be less than 10% with the use of combination HBIG and oral therapy.19,20
Figure.
The recurrence of HBV infection while on different prophylactic regimens.
HBIG, hepatitis B immunoglobulin; HBV, hepatitis B virus; LAM, lamivudine.
Multiple risk factors are associated with HBV infection recurrence in liver transplant recipients (Table 1).
Table 1.
Risk Factors for HBV Infection Recurrence Stratified into High-Risk and Low-Risk Groups
| High-Risk Group | Low-Risk Group |
|---|---|
| Pretransplant HCC24,25 | HBeAg-negative patient52 |
| Posttransplant HCC recurrence24,25 | Low serum levels of HBV DNA52 |
| HBeAg-positive patient16 | HDV coinfection52 |
| HBeAg-negative patient with high serum HBV DNA levels16,21,22 | Fulminant HBV infection18 |
| Antiviral drug—resistant strain pretransplant16,21,22 |
HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HDV, hepatitis D virus.
Patients considered at high risk include those with cirrhosis who are hepatitis B e antigen—positive or —negative but with high levels of serum HBV DNA and patients who have an antiviral drug-resistant strain pretransplant.16,20-22 Other associated risk factors that can influence recurrence of HBV infection include no posttransplant prophylaxis or the presence of pretransplant HCC or posttransplant HCC recurrence.20,23-25
Clinical Course and Diagnosis
The reappearance of hepatitis B surface antigen (HBsAg) defines HBV recurrence.26,27 The results of a recent study demonstrated that patients with recurrence of HBV infection, as defined by HBsAg positivity, had varying degrees of hepatic inflammation but trended toward moderate to severe types of hepatic inflammation on biopsy.27 Techniques using polymerase chain reaction (PCR) have demonstrated the ability to detect HBV DNA even before the appearance of HBsAg or aberrations in liver function.28 Indeed, some patients can be seen to have detectable HBV DNA but still remain HBsAg-negative after follow-up periods as long as 10 years.29,30 Whether these patients will ultimately have recurrence of an active HBV infection remains to be seen, but the positive HBV DNA PCR with or without symptoms underscores the necessity of continuing therapy for HBV infection posttransplantation.
The clinical implications of HBV recurrence are significant if untreated because of the rapidity of deterioration associated with recurrence. Cirrhosis of the liver graft has been recorded within 1 to 2 years of recurrence posttransplant.6-8,31
Prevention of Hepatitis B Virus Recurrence
The prevention of reinfection ideally begins in the pretrans-plant period. All patients with decompensated cirrhosis and detectable HBV DNA, regardless of quantitative HBV DNA or liver function test abnormalities, should receive antiviral therapy.16,21,22
Pretransplant
The choice of therapy pretransplant is generally monotherapy with an oral nucleos(t)ide analog (NA). First-line therapeutic agents are entecavir and tenofovir because of their high genetic barrier to drug resistance, potency, and low rate of discontinuation due to adverse effects.32 The rate of resistance limits the utility of lamivudine and telbivudine (Tyzeka, Novartis), and low viral suppression rates prevent widespread use of adefovir.
Posttransplant
Immunoprophylaxis with HBIG and an oral NA should be started at the time of transplant. Strategies using HBIG vary according to the dose administered and may involve intravenous or intramuscular delivery.20,21,33-36 An area of controversy is whether HBIG can eventually be discontinued.20,28,37 A number of studies have addressed the issue of HBIG duration in liver transplant recipients. Limited use of HBIG, defined as 12 months or less of therapy, followed by a switch to an oral regimen of nucleotide/nucleoside combination appears to be effective in preventing recurrent HBV infection (Table 2). However, it is unclear whether HBIG is needed at all after liver transplantation in all patients who have received liver transplants for management of HBV infection.
Table 2.
Immunoprophylaxis for HBV Infection in Liver Transplant Recipients
| Study | Number of Patients | Strategy | Oral Agents (n)* | HBV Recurrence (%) | Median Follow-up (months) |
|---|---|---|---|---|---|
| Fung et al23 | 80 | No HBIG | ETV (80) | 18/80 (23) | 26 |
| Wadhawan et al45 | 75 | No HBIG | LAM + ADV (19) ETV (42) TDF (12) ETV + TDF (2) |
6/75 (8) | 21 |
| Degertekin et al20 | 23 | HBIG for 12 months | LAM ADV ETV TDF LAM + ADV LAM + TDF ETV + ADV TDF + ADV |
3/23 (13) | 53 |
| Nath et al37 | 14 | HBIG for 7 days | LAM + ADV | 0/14 (0) | 14 |
| Teperman et al39 | 16 | HBIG for 6 months | FTC + TDF | 0/16 (0) | 18 |
| Neff et al42 | 10 | HBIG for 7 months | LAM + ADV | 0/10 (0) | 31 |
The specific number of patients on each oral regimen is unknown.
ADV, adefovir; ETV, entecavir; FTC, emtricitabine; HBIG, hepatitis B immunoglobulin; HBV, hepatitis B virus; LAM, lamivudine; TDF, tenofovir.
There are several advantages to using an all-oral therapy over a strategy that still contains HBIG after a limited course of HBIG. One study conducted by Angus and colleagues compared the efficacy of dual therapy with 2 NAs, lamivudine and adefovir, with that of a combination of HBIG and lamivudine in patients who had at least 12 months of HBIG therapy, with the results showing similar efficacy between the 2 groups.38 Another more recent study published by Teperman and colleagues also supports the theory that dual therapy with 2 NAs can be as effective as a combination of HBIG with 1 or 2 NAs after a limited period of HBIG use.39 Many other studies using similar criteria to the ones noted above also support the conclusion that HBIG discontinuation is feasible.25,37,38,40-44 Although a growing wealth of evidence supports the discontinuation of HBIG, most of the patients enrolled in the trials were generally considered to be at low risk, and HBIG was used for at least 12 months following transplant (considered the most vulnerable period) before it was discontinued.38,39
Many institutions are now adopting HBIG-free regimens with oral NA therapy following transplantation if patients are thought to be at low risk of recurrent infection or are discontinuing HBIG after a period of time.
For example, at the University of California, Los Angeles, the combination of HBIG and an oral antiviral therapy is used for the first 12 months after transplantation. After this time, a second agent is introduced, and the HBIG is discontinued.40 This therapy has not only been shown to have similar efficacy to that of HBIG, but the cost benefit also makes the discontinuation of HBIG attractive (Table 3). Although further study with larger populations and longer follow-up periods needs to be conducted for a more standardized posttransplant protocol, the current results appear to be an encouraging step in the right direction.
Table 3.
Comparison of HBIG and Nucleos(t)ides
| HBIG | Nucleos(t)ides | |
|---|---|---|
| Administration | Intravenous or intramuscular | Oral |
| Setting | Office | Home |
| Standardization | No | Yes |
| Frequency | Daily for first week, then monthly | Daily |
| Adverse effects | Injection site reactions | None Renal function? Lactic acidosis? |
| Resistance | Viral breakthrough with monotherapy | Yes, with first generation |
| Mechanism | Neutralize antigen | Inhibit viral replication |
| Monitoring | Anti-HBs titers | Renal function |
| Costs | $$$ | $$ |
Anti-HBs, hepatitis B surface antibody; HBIG, hepatitis B immunoglobulin.
The favorable results for the discontinuation of therapy with HBIG after a finite period of time have raised the question of whether the potency of the newer NA medications has superseded the need for HBIG. A study by Fung and colleagues tried to answer this question.23 In this study, patients were given entecavir in the posttransplant period without any HBIG and underwent follow-up for a mean period of 26 months. The results of the study were that HBV infection recurred in 18 (22.5%) of the 80 patients followed. Somewhat in contradiction to the previous study, Wadhawan and colleagues released a report in which they prospectively followed a cohort of 75 patients in which varying single and dual combinations of NAs were used for prophylaxis instead of HBIG between the years of 2005 and 2012, with encouraging results.45 Recurrence of HBV infection while on therapy was diagnosed in only 6 (8%) of the 75 patients. To be fair, the cohort of 75 patients was deemed to be at low risk for HBV recurrence, evidenced by HBV DNA negativity prior to transplantation. The recent improved results of patients with only combination or singular oral therapy mean that the role of HBIG in the posttransplant setting must be reevaluated to adapt to the changing therapies that are available.
Management of Recurrence
The choice of therapy depends on the prophylactic therapy that the patient was receiving. This is, in part, due to the unfortunate fact that resistance can develop to several of the currently available medications, as seen with lamivudine or adefovir. Generally, if patients are on both HBIG and an oral agent, resistance is likely against HBIG, and the addition of complementary oral agents generally is effective in achieving viral suppression. If patients were taking a nucleoside analogue, then a nucleotide analogue should be added. For instance, the results of a large study demonstrated that the use of adefovir in patients with lamivudine-resistant HBV infection was associated with 95% viral suppression.46
Resistance to adefovir also can develop in patients who were treated with adefovir pretransplantation, and this resistance can carry over into the posttransplantation period.47-50 In the pretransplant setting, studies have indicated that lamivudine and entecavir are potent options in the setting of adefovir resistance.51 Although studies are lacking in the posttransplant phase, it is still prudent to add either lamivudine or entecavir to patients with known adefovir resistance.
Conclusion
The use of prophylactic strategies has reduced the incidence of HBV infection recurrence in liver transplant recipients. Emerging data suggest that the use of HBIG may not necessarily be lifelong. Already, combination oral therapy is effective in treating recurrent HBV infection because of viral breakthrough during HBIG and lamivudine. In addition, the use of all-oral antiviral therapy has been shown to prevent recurrent infection after HBIG has been discontinued. The optimal period of using HBIG is not known, but it is used in most studies for at least 6 to 12 months. A controversial topic is whether HBIG is truly necessary for all patients who have undergone liver transplantation for management of HBV infection. Studies are required to see whether patients at low risk for recurrent infection can receive only all-oral combination therapy.
Footnotes
Dr Manne and Ms Allen have no relevant conflicts of interest to disclose. Dr Saab is a consultant and is on the speakers bureau for Gilead and Bristol-Myers Squibb.
References
- 1.Hepatitis B. World Health Organization. [August 5, 2013]. http://www.who.int/mediacentre/factsheets/fs204/en/ Updated July 2013.
- 2. [August 5, 2013]. http://www.cdc.gov/hepatitis/Statistics/index.htm Viral hepatitis statistics and surveillance. Centers for Disease Control and Prevention. Updated August 2013.
- 3.Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Brooks J, Gelson W, Rushbrook SM. Therapeutic advances in the management of chronic hepatitis B infection. Ther Adv Chronic Dis. 2013;4(4):157–166. doi: 10.1177/2040622313484647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfitzmann R, Nüssler NC, Hippler-Benscheidt M, Neuhaus R, Neuhaus P. Long-term results after liver transplantation. Transpl Int. 2008;21(3):234–246. doi: 10.1111/j.1432-2277.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Grady JG, Smith HM, Davies SE, et al. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol. 1992;l4(1):104–111. doi: 10.1016/0168-8278(92)90138-f. [DOI] [PubMed] [Google Scholar]
- 7.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13(4):619–626. [PMC free article] [PubMed] [Google Scholar]
- 8.Lucey MR, Graham DM, Martin P, et al. Recurrence of hepatitis B and delta hepatitis after orthotopic liver transplantation. Gut. 1992;33(10):1390–1396. doi: 10.1136/gut.33.10.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckebaum S, Sotiropoulos GC, Gerken G, Cicinnati VR. Hepatitis B and liver transplantation: 2008 update. Rev Med Virol. 2009;19(1):7–29. doi: 10.1002/rmv.595. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz N, Shiffman ML, Stravitz TR, et al. Prophylaxsis against recurrance of hepatitis B virus after liver transplantation: a retrospective analysis spanning 20 years. Liver Int. 2008;28(1):72–78. doi: 10.1111/j.1478-3231.2007.01611.x. [DOI] [PubMed] [Google Scholar]
- 11.Coffin CS, Terrault NA. Management of hepatitis B in liver transplant recipients. J Viral Hepat. 2007;l4(suppl 1):37–44. doi: 10.1111/j.1365-2893.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 12.Cholongitas E, Goulis J, Akriviadis E, Papatheodoridis GY. Hepatitis B immunoglobulin and/or nucleos(t)ide analogues for prophylaxis against hepatitis B virus recurrence after liver transplantation: a systematic review. Liver Transpl. 2011;17(10):1176–1190. doi: 10.1002/lt.22354. [DOI] [PubMed] [Google Scholar]
- 13.McMillan JS, Shaw T, Angus PW, Locarnini SA. Effect of immunosuppressive and antiviral agents on hepatitis B virus replication in vitro. Hepatology. 1995;22(1):36–43. [PubMed] [Google Scholar]
- 14.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83(6):1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tur-Kaspa R, Shaul Y, Moore DD, et al. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988;167(2):630–633. [PubMed] [Google Scholar]
- 16.Omata M. Significance of extrahepatic replication of hepatitis B virus. Hepatology. 1990;12(2):364–366. doi: 10.1002/hep.1840120226. [DOI] [PubMed] [Google Scholar]
- 17.Wu LM, Xu X, Zheng SS. Hepatitis B virus reinfection after liver transplantation: related risk factors and perspective. Hepatobiliary Pancreat Dis Int. 2005;4(4):502–508. [PubMed] [Google Scholar]
- 18.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329(25):1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 19.Steinmüller T, Seehofer D, Rayes N, et al. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology. 2002;35(6):1528–1535. doi: 10.1053/jhep.2002.33681. [DOI] [PubMed] [Google Scholar]
- 20.Degertekin B, Han SH, Keeffe EB, et al. NIH HBV-OLT Study Group. Impact of virologic breakthrough and HBIG regimen on hepatitis B recurrence after liver transplantation. Am J Transplant. 2010;10(8):1823–1833. doi: 10.1111/j.1600-6143.2010.03046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGory RW, Ishitani MB, Oliveira WM, et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996;61(9):1358–1364. doi: 10.1097/00007890-199605150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Marzano A, Gaia S, Ghisetti V, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005;11(4):402–409. doi: 10.1002/lt.20402. [DOI] [PubMed] [Google Scholar]
- 23.Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011;l4l(4):l212–1219. doi: 10.1053/j.gastro.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 24.Faria LC, Gigou M, Roque-Afonso AM, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008;134(7):1890–1899. doi: 10.1053/j.gastro.2008.02.064. quiz 2155. [DOI] [PubMed] [Google Scholar]
- 25.Saab S, Yeganeh M, Nguyen K, et al. Recurrence of hepatocellular carcinoma and hepatitis B reinfection in hepatitis B surface antigen-positive patients after liver transplantation. Liver Transpl. 2009;15(11):1525–1534. doi: 10.1002/lt.21882. [DOI] [PubMed] [Google Scholar]
- 26.Demetris AJ, Todo S, Van Thiel DH, et al. Evolution of hepatitis B virus liver disease after hepatic replacement. Practical and theoretical considerations. Am J Pathol. 1990;137(3):667–676. [PMC free article] [PubMed] [Google Scholar]
- 27.Yi NJ, Lee KW, Kong SY, et al. Outcome of various treatments for posttrans-plant hepatitis B virus recurrence. World J Surg. 2013;37(4):812–819. doi: 10.1007/s00268-013-1914-z. [DOI] [PubMed] [Google Scholar]
- 28.Wong SN, Chu CJ, Wai CT, et al. Low risk of hepatitis B virus recurrence after withdrawal of long-term hepatitis B immunoglobulin in patients receiving maintenance nucleos(t)ide analogue therapy. Liver Transpl. 2007;13(3):374–381. doi: 10.1002/lt.21041. [DOI] [PubMed] [Google Scholar]
- 29.Marzano A, Salizzoni M, Debernardi-Venon W, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lami-vudine and passive immunoprophylaxis. J Hepatol. 2001;34(6):903–910. doi: 10.1016/s0168-8278(01)00080-0. [DOI] [PubMed] [Google Scholar]
- 30.Roche B, Feray C, Gigou M, et al. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003;38(1):86–95. doi: 10.1053/jhep.2003.50294. [DOI] [PubMed] [Google Scholar]
- 31.Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation. N Engl J Med. 1989;321(15):1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53(1):62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 33.Terrault NA, Zhou S, Combs C, et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24(6):1327–1333. doi: 10.1002/hep.510240601. [DOI] [PubMed] [Google Scholar]
- 34.Gane EJ, Angus PW, Strasser S, et al. Australasian Liver Transplant Study Group. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132(3):931–937. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RD, Chinnakotla S, Guo L, Perrillo RP, Klintmalm GB, Davis GL. Intramuscular hepatitis B immunoglobulin (HBIG) and nucleosides for prevention of recurrent hepatitis B following liver transplantation: comparison with other HBIG regimens. Clin Transplant. 2007;21(4):510–517. doi: 10.1111/j.1399-0012.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 36.Angus PW, McCaughan GW, Gane EJ, Crawford DH, Harley H. Combination low-dose hepatitis B immune globulin and lamivudine therapy provides effective prophylaxis against posttransplantation hepatitis B. Liver Transpl. 2000;6(4):429–433. doi: 10.1053/jlts.2000.8310. [DOI] [PubMed] [Google Scholar]
- 37.Nath DS, Kalis A, Nelson S, Payne WD, Lake JR, Humar A. Hepatitis B prophylaxis post-liver transplant without maintenance hepatitis B immunoglobulin therapy. Clin Transplant. 2006;20(2):206–210. doi: 10.1111/j.1399-0012.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 38.Angus PW, Patterson SJ, Strasser SI, McCaughan GW, Gane E. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008;48(5):1460–1466. doi: 10.1002/hep.22524. [DOI] [PubMed] [Google Scholar]
- 39.Teperman LW, Poordad F, Bzowej N, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl. 2013;19(6):594–601. doi: 10.1002/lt.23628. [DOI] [PubMed] [Google Scholar]
- 40.Saab S, Desai S, Tsaoi D, et al. Posttransplantation hepatitis B prophylaxis with combination oral nucleoside and nucleotide analog therapy. Am J Transplant. 2011;11(3):511–517. doi: 10.1111/j.1600-6143.2010.03416.x. [DOI] [PubMed] [Google Scholar]
- 41.Choe WH, Kwon SY, Kim BK, et al. Tenofovir plus lamivudine as rescue therapy for adefovir-resistant chronic hepatitis B in hepatitis B e antigen-positive patients with liver cirrhosis. Liver Int. 2008;28(6):8l4–820. doi: 10.1111/j.1478-3231.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 42.Neff GW, Kemmer N, Kaiser TE, et al. Combination therapy in liver transplant recipients with hepatitis B virus without hepatitis B immune globulin. Dig Dis Sci. 2007;52(10):2497–2500. doi: 10.1007/s10620-006-9658-3. [DOI] [PubMed] [Google Scholar]
- 43.Shiffman ML, Stravitz RT, Kimmel M, et al. Tenofovir plus emtricitabine (truvada) prevents recurrence of hepatitis B virus (HBV) in liver transplant (LT) recipients after discontinuing hepatitis B immune globulin (HBIg) [abstract] Hepatology. 2009;50 392A. [Google Scholar]
- 44.Karlas T, Hartmann J, Weimann A, et al. Prevention of lamivudine-resistant hepatitis B recurrence after liver transplantation with entecavir plus tenofovir combination therapy and perioperative hepatitis B immunoglobulin only. Transpl Infect Dis. 2011;13(3):299–302. doi: 10.1111/j.1399-3062.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 45.Wadhawan M, Gupta S, Goyal N, Taneja S, Kumar A. Living related liver transplantation for hepatitis B-related liver disease without hepatitis B immune globulin prophylaxis. Liver Transpl. 2013;19(9):1030–1035. doi: 10.1002/lt.23692. [DOI] [PubMed] [Google Scholar]
- 46.Schiff E, Lai CL, Hadziyannis S, et al. Adefovir Dipivoxil Study 45 International Investigators Group. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13(3):349–360. doi: 10.1002/lt.20981. [DOI] [PubMed] [Google Scholar]
- 47.Angus P, Vaughan R, Xiong S, et al. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003;125(2):292–297. doi: 10.1016/s0016-5085(03)00939-9. [DOI] [PubMed] [Google Scholar]
- 48.Villeneuve JP, Durantel D, Durantel S, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol. 2003;39(6):1085–1089. doi: 10.1016/j.jhep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Lacombe K, Ollivet A, Gozlan J, et al. A novel hepatitis B virus mutation with resistance to adefovir but not to tenofovir in an HIV-hepatitis B virus-co-infected patient. AIDS. 2006;20(17):2229–2231. doi: 10.1097/01.aids.0000252061.35422.84. [DOI] [PubMed] [Google Scholar]
- 50.Borroto-Esoda K, Miller MD, Arterburn S. Pooled analysis of amino acid changes in the HBV polymerase in patients from four major adefovir dipivoxil clinical trials. J Hepatol. 2007;47(4):492–498. doi: 10.1016/j.jhep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Fung SK, Chae HB, Fontana RJ, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44(2):283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Vargas HE, Dodson FS, Rakela J. A concise update on the status of liver transplantation for hepatitis B virus: the challenges in 2002. Liver Transpl. 2002;8(1):2–9. doi: 10.1053/jlts.2002.29765. [DOI] [PubMed] [Google Scholar]