Abstract
Obstructive sleep apnea (OSA) continues to garner widespread attention, mostly because of its health-related consequences such as cardiovascular disorders and associated comorbid symptoms such as daytime sleepiness and snoring. OSA is characterized by repeated episodes of partial or complete upper airway closure during sleep, resulting in frequent arousals and sleep fragmentation, apneas and hypopneas, and intermittent hypoxemia. These perturbations can lead to chronic cardiovascular disorders such as hypertension. Among the many risk factors in the pathogenesis of OSA, obesity is one of the most important.1 Obesity, per se, may also predispose to cardiovascular disorders. In this review, the authors explore the complex interactions between obesity, cardiovascular diseases, and OSA.
Keywords: Obstructive sleep apnea, Obesity, Cardiovascular consequences
OBESITY AND CARDIOVASCULAR DISEASE
Mechanisms
There are various mechanisms by which obesity can lead to cardiovascular disorders. Obesity appears to be a chronic inflammatory state, as evidenced by elevated levels of serum markers of systemic inflammation such as C-reactive protein (CRP) and inter-leukin 6 (IL-6), which can predispose to cardiovascular disorders. Leptin is a novel adipocyte-derived hormone that induces weight loss by decreasing appetite and increasing energy expenditure. Obesity appears to predispose to leptin resistance resulting in elevated plasma leptin levels. Elevated leptin levels are independent risk factors for cardiovascular disorders by promoting platelet aggregation, insulin resistance, and increased sympathetic neural activity.2 Insulin resistance is commonly seen in obesity and is also a predictor for cardiovascular diseases. Insulin stimulates leptin production and the presence of insulin resistance increases the risk for development of diabetes, as well as alterations in lipids, coagulation, fibrinolysis, and inflammation, subsequently predisposing to endothelial dysfunction and atherosclerosis.3 Increased insulin resistance in the peripheral tissues in obesity predisposes to increased sympathetic neural activity.4 Obesity is one of the key variables that constitutes metabolic syndrome.5 Metabolic syndrome confers an increased risk for atherosclerosis and cardiovascular disorders.6 The shift in diet from fiber rich foods to a more Western diet, along with inactivity, has predisposed the general population to obesity. Obesity is also associated with high circulating levels of triglycerides, low high-density lipoprotein (HDL) cholesterol, and plasma concentrations of apolipoprotein B-containing lipoproteins, all being risk factors for increased cardiovascular disorders.7–9
Obesity also has adverse effects on cardiovascular hemodynamics.10 Obesity increases total blood volume and cardiac output, resulting in increased cardiac workload.11 Increased sympathetic neural activity in obesity may conceivably induce an overall increase in heart rate. Combined with the increased cardiac workload, this can lead to hemodynamic consequences. There is an increase in ventricular filling pressures and volumes leading to left ventricular chamber dilation and hypertrophy.11,12 In addition to left ventricular chamber enlargement, obesity leads to left atrial enlargement due to increased blood volume and abnormal diastolic filling.11,13 All these perturbations may result in increased cardiovascular morbidity such as hypertension, congestive heart failure, atrial fibrillation, and arrhythmias.
Cardiovascular Consequences of Obesity
The link between obesity and cardiovascular disease has been well established. The epidemiologic link between obesity and incident hypertension was established by the Framingham study, in which 2027 men and 2267 women were followed up for 8 years.14 In another Framingham study of 5881 participants who were followed up over a 14-year period, every 1 kg/m2 increment in body mass index (BMI; weight in kilograms divided by height in meters squared) led to a 5% to 7% increased risk of congestive heart failure.15 More than 30% of obese patients had heart failure, and the probability increased significantly with increasing duration of obesity.16 Obesity is a major component of the metabolic syndrome, and is an independent risk factor for atherosclerosis and the development of coronary artery disease (CAD).17,18 Due to the hemodynamic effects of obesity as explained here, obesity may also contribute to the higher prevalence of atrial fibrillation (AF). In the prospective Framingham cohort study of 5282 participants who were followed for a mean of 13.7 years, obesity was associated with a 50% increased risk for AF.19 In a recent meta-analysis, obese patients had a 49% increased risk of developing AF that increased in parallel with increasing BMI.20 Obesity increases the risk of sudden cardiac death,21 with substantial evidence supporting the occurrence of frequent complex ventricular arrhythmias even in the absence of heart failure or left ventricular dysfunction.11 The annual rate of sudden cardiac death is nearly 40 times higher than that in the matched nonobese population.22
Because OSA is strongly associated with both obesity and cardiovascular disease, it may plausibly represent an important mediator between obesity and cardiovascular disease themselves. Unfortunately, the presence of OSA is often not assessed in studies examining the relationship between obesity and cardiovascular disorders. In a recent meta-analysis of 40 cohort studies, although obesity led to increased cardiovascular mortality, the presence of OSA was not evaluated in any of these studies.23
OSA AND CARDIOVASCULAR DISEASE
There has recently been increasing evidence from longitudinal cohort studies and a limited number of randomized controlled studies that OSA may play a causal role in the pathophysiology of certain cardiovascular diseases such as systemic hypertension, with increasing evidence shown in others such as heart failure, stroke, and cardiac arrhythmias. However, a few caveats should be noted. First, the OSA literature on cardiovascular diseases has historically been dominated by case series and case-control studies, both of which are vulnerable to hidden biases and have poor discriminating power to determine the cardiovascular risk imparted purely by OSA independent of confounders such as obesity. Second, aside from several small continuous positive airway pressure (CPAP) trials in the setting of systemic hypertension, there remains a paucity of controlled interventional trials assessing the impact of OSA treatment on hard cardiovascular end points and outcomes. Third, the current metric to characterize OSA, the apnea-hypopnea index (AHI; number of apneas and hypopneas per hour of sleep), has its shortcomings. While probably the best composite measurement of OSA severity, the AHI does not quantify other processes operative in the pathophysiology of cardiovascular disease, such as the degree and duration of oxyhemoglobin desaturation or sleep disruption that may occur independently of frank apneas and hypopneas, for example, during snore arousals or periodic limb movements.
Pathophysiology
OSA results in repetitive partial or complete closure of the upper airway during sleep, despite ongoing respiratory efforts. Each such event is associated with oxyhemoglobin desaturation followed by central nervous system arousal to reestablish upper airway patency, followed by reoxygenation. Acute repetitive stressors associated with these cascade of events include nocturnal hypoxemia, increased sympathetic neural activity,24 production of reactive oxygen radicals following reoxygenation,25 and swings in intrathoracic pressure due to respiratory efforts against a collapsed upper airway.26
The swings in intrathoracic pressure during each of these apneic/hypopneic events can be extreme (up to −80 cm of water), resulting in an increase in cardiac afterload (by increasing the left ventricular transmural pressure, and thereby the left ventricular wall stress/tension) and impaired left ventricle relaxation, thereby contributing to decreased stroke volume and cardiac output.26–28
Presence of hypoxemia during these obstructive apneic events results in repetitive bursts of sympathetic neural activity, with increased activity noted even during the day, along with elevated catecholamine levels.24,29 Arousals from OSA also lead to increases in heart rate and blood pressure.30,31 All these factors result in an increase in oxygen demand. Following arousals from apnea and resumption of breathing, there is reoxygenation. This repetitive hypoxemia-reoxygenation has been thought to result in release of free radicals that activates nuclear factor κB, which has been implicated in upregulation of proinflammatory genes and adhesion molecules resulting in endothelial dysfunction, platelet activation, and possibly atherosclerosis.32 This inflammatory pathway could increase blood pressure independently of activation of the sympathetic neural activity.33 Platelet activation and aggregation as well as morning levels of fibrinogen concentration are increased in OSA patients and tend to decrease after one night of CPAP use.34,35 Vibration produced from snoring, which is commonly seen in patients with OSA, may lead to carotid vessel wall damage, perhaps resulting in the formation of atherosclerotic plaques.36 The effects of all these perturbations can potentially result in chronic cardiovascular homeostatic dysregulation resulting in systemic hypertension, congestive heart failure, arrhythmias such as AF, strokes, sudden cardiac death, and pulmonary hypertension. In the next sections, the authors assess the causal role of OSA in the pathophysiology of hypertension, heart failure, and cardiac arrhythmias.
Systemic hypertension
There is strong evidence from both animal models37 and human epidemiologic studies that OSA is one of the causative factors in systemic hypertension. Large prospective longitudinal studies help us understand the link between OSA and hypertension (HTN). The Wisconsin Sleep Cohort Study is a population-based longitudinal study that was initiated in the 1980s, and looked at the natural history of sleep-disordered breathing in adults. Cross-sectional analysis of baseline polysomnograms (PSG) and blood pressure data were collected in 1060 adults in the 30- to 60-year age range. The study showed a linear increase in blood pressure with increasing AHI, and an odds ratio of 1.8 for HTN at an AHI of 15 or more.38 Compelling evidence for the link between OSA and HTN came from their prospective 4-year follow-up data, which showed an increasing odds of developing HTN independent of known confounding factors.39 The Sleep Heart Health Study (SHHS) also demonstrated a dose-response relationship between HTN and indices of OSA severity even after adjustment for confounding factors.40 These associations were evident in both sexes, all age groups, all ethnic groups, and in obese and nonobese subjects. Subsequent analysis showed that AHI was significantly associated with systolic and diastolic HTN for subjects younger than 60 years (odds ratio for AHI between 15 and 29.9/h was 2.38 and odds ratio for AHI more than 30/h was 2.24) but not for those older than 60 years.41
CPAP has been shown to acutely attenuate sympathetic drive and nocturnal blood pressure in patients with OSA.24,42,43 However, the data regarding effects on daytime blood pressure have been more difficult to interpret. Several observational studies, often uncontrolled and from highly select populations, have suggested improvements in daytime blood pressure control with the use of CPAP. Because of these shortcomings, and an apparent true placebo effect realized in the measurement of blood pressure, several randomized, placebo-controlled trials (RCTs) have been performed, yielding variable and sometimes inconsistent results. The generalization of the studies is somewhat limited, because they comprise small sample sizes and the majority of subjects are normotensive at baseline. However, some findings are worth mentioning. With the largest study to date, Pepperell and colleagues44 found a small but significant reduction in blood pressure in a largely normotensive cohort over only 4 weeks of therapy. Becker and colleagues,45 in a controlled trial comparing therapeutic with subtherapeutic CPAP, found fairly dramatic reductions in mean blood pressure (9.9 ± 11.4 mm Hg) in a small cohort with severe OSA (mean AHI >60/h) treated for more than 60 days, the longest trial to date. Potential limitations to the study include a high dropout rate and the fact that about two-thirds were treated with various anti-hypertensive medications. A point that does stand out, suggesting the importance of treatment-dose effect, is that subtherapeutic CPAP reduced the AHI by 50% but did not result in any reduction in blood pressure.
Whereas excessive daytime sleepiness is a common and potentially dangerous consequence of OSA, it is not a universal symptom. There is evidence to suggest that sleepiness may be an important mediator of some of the systemic effects of OSA. That is, in the absence of sleepiness even severe OSA, as quantified by the AHI, does not always translate to reductions in blood pressure after CPAP treatment, regardless of whether normotension or hypertension exists at baseline. In a randomized controlled trial, Barbe and colleagues46 showed that in normotensives with severe sleep apnea by AHI criteria but no daytime sleepiness, CPAP treatment imparted no reductions in blood pressure. Similar findings were recently reported by the Oxford group but in a cohort of hypertensive sleep apneics.47 Even mild subjective sleepiness confers some blood pressure benefit with the use of CPAP.48
Finally, the results of a randomized trial comparing ambulatory blood pressure in moderately severe sleep apneics following treatment with therapeutic CPAP, sham CPAP, or supplemental oxygen are notable.49 Whereas therapeutic CPAP resulted in blood pressure reductions, supplemental oxygen, despite normalizing oxygen saturation, did not. This finding suggests that hypoxia-mediated mechanisms may not fully explain the acute and chronic effects of sleep apnea on the vasculature. It may well be that central nervous system arousals, which are attenuated, if not abolished with CPAP therapy, are equally important, perhaps through effects on sympathetic output or hemodynamics.
A recent meta-analysis of 12 RCTs of blood pressure reduction with CPAP treatment in OSA,50 confirmed by a subsequent article,51 while confirming heterogeneity of study design and population, showed the pooled effect of CPAP treatment in patients with OSA (both normotensive and hypertensive) had a net significant reduction in mean blood pressure of 1.5 to 2 mm Hg. The results also suggest a greater anti-hypertensive effect in those with hypertension and daytime sleepiness at baseline. However, there were differences in the severity of OSA in these treatment trials and differences in inclusion/exclusion criteria.
Because chronic conditions such as OSA-associated hypertension could reasonably lead to vascular remodeling and other structural cardiovascular changes, it is entirely feasible that short-term controlled studies may fail to disclose the true effects of faithful CPAP therapy on hypertension and its consequences. Furthermore, given the prevalence of hypertension and its effects on the development of other cardiovascular diseases, including heart failure and stroke, the results of small changes in blood pressure and decreases in nocturnal blood pressure may be far reaching.
Heart failure
Observational data support the potential importance of OSA in cardiovascular outcomes. Case-control studies suggest that OSA may lead to cardiac structural changes, plausibly explained as an adaptive response to the consequent physiologic stressors.52,53 Incident AF, an important risk factor for heart failure, is associated with the degree of oxyhemoglobin desaturation in OSA.54 A study of mortality in a pure heart failure population with OSA suggested higher death rates in those who were noncompliant with CPAP compared with those who had mild or no OSA.55 Similar inferences might be drawn from a 10-year cohort study by Marin and colleagues,56 who showed an increase in fatal and nonfatal cardiovascular events in patients with severe, untreated OSA.
Given the high rate of coexistence of OSA and heart failure, one cannot exclude the possibility that cardiac function may modulate upper airway function, hence predisposing to OSA. Cardinal features of heart failure include circulatory overload and dependent edema resulting from organ hypoperfusion and associated neuro-hormonal imbalances. There is evidence to suggest that tissue edema, especially position-dependent redistribution, may influence upper airway dimensions and mechanics. Using computed tomography images, Shepard and colleagues57 found that, in men with known OSA, experimental changes in central venous pressure were associated with alterations in upper airway cross-sectional area. The most significant changes were observed with reductions in central venous pressure (by blood pressure cuff inflation of the legs), where increases in airway cross-sectional area were seen at end-inspiratory tidal volume.
Two controlled, short-term interventional trials of CPAP for OSA in the setting of heart failure have been performed, both yielding various positive results.58,59 Using a randomized, parallel comparative design, the control groups were composed of subjects optimally medically managed, though not subjected to placebo. The intervention was applied after a full, second-night in-laboratory polysomnographic titration of CPAP. Kaneko and colleagues58 reported an approximately 9% increase in left ventricular ejection fraction (LVEF) and significant reductions in blood pressure after just 1 month of CPAP therapy. Mansfield and colleagues,59 studying a group of subjects with somewhat less severe degrees of both heart failure and OSA than the subjects of Kaneko and colleagues,58 applied CPAP therapy for 3 months and showed significant improvements in LVEF and reductions in urinary catecholamines, but no changes in blood pressure.
In contrast, a third controlled CPAP interventional trial showed no such changes in any cardiovascular end points, including ejection fraction, blood pressure, and exercise capacity.60 Notwithstanding potential methodological shortcomings that may limit the applicability of the results, including a cross-over design and use of an auto-titrating CPAP machine without confirmatory follow-up PSG, the trial showed significant improvements in daytime symptoms in this group with fairly severe OSA. Unfortunately, with average CPAP use of just over 3 hours per night, it also underscores the well-identified challenges of maintaining adherence to CPAP treatment. It is clear that larger, long-term interventional trials of CPAP treatment, as well as its alternatives, are needed.
Cardiac arrhythmias
Several observational studies have shown an association between OSA and various nocturnal arrhythmias. Recent data from the SHHS, after adjusting for many confounders, showed that, compared with subjects with a respiratory disturbance index (RDI; number of respiratory events per hour) less than 5, those with severe OSA (RDI ≥30) had a higher rate of AF, nonsustained ventricular tachycardia, and ectopic ventricular beats.61 Bradyarrhythmias are commonly encountered in OSA, may correlate with the severity of disordered breathing, can occur with a structurally normal heart, and may be attenuated by effective CPAP therapy.62,63 The aforementioned SHHS data, however, show similar rates of bradycardias and conduction delays between subjects with severe OSA and those without significant OSA.
Mounting data strengthen the association between OSA and AF, 2 disorders that often coexist.64 Continuous cardiac monitoring with an atrial defibrillator showed that the onset of nearly 75% of episodes of persistent atrial fibrillation in OSA patients occurred in the overnight hours (8 PM to 8 AM).65 Retrospective analysis shows that, within 12 months of successful therapeutic electrical cardioversion for atrial fibrillation, untreated sleep apneics were found to have an arrhythmia recurrence rate double that of patients treated with CPAP.66 Recent review of 17 years of PSG data from a population-based cohort suggests that nocturnal hypoxemia associated with OSA influences the incidence of atrial fibrillation.54 Because none of these observational data can convincingly implicate OSA as an independent cause of new-onset atrial fibrillation, further longitudinal cohort studies and outcome-based interventional trials are needed to characterize the relationship between OSA and atrial arrhythmias.
INTERACTION BETWEEN OBESITY AND OSA ON CARDIOVASCULAR CONSEQUENCES
Coexistence of Obesity and OSA
Obesity and OSA often coexist, with more than 40% of obese patients having significant OSA and 70% of OSA patients being obese.67–69 As obesity and OSA tend to commonly coexist, and as each of them have been shown to have similar cardiovascular consequences as previously mentioned, it is important to consider their relative roles and their relative importance of shared common pathways that lead to cardiovascular consequences. Obesity and OSA, independent of each other, can affect similar biologic pathways that predispose to cardiovascular diseases such as predisposing to insulin resistance and oxidative stress.70–73 It is therefore possible that any cardiovascular end point might be explained by obesity without needing to consider OSA, but unfortunately most studies in obese patients assessing the relationship with cardiovascular outcomes did not assess for OSA and exclude it as a possible confounder.
Obesity as a Risk Factor for OSA
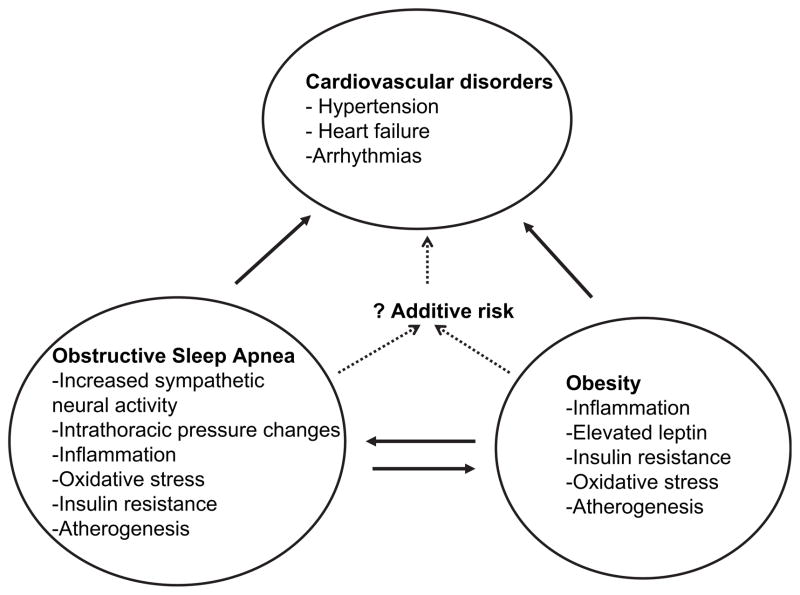
Obesity is one of the main risk factors in the pathogenesis of OSA,1 and the prevalence of OSA is increasing with increasing prevalence of obesity, particularly central (visceral) obesity.74,75 A 10% increase in weight gain is associated with a 6-fold increase in the odds of developing OSA.76 Similarly, another study showed a 4-fold increase in OSA risk with every 6 kg/m2 increment in BMI.77 The causal relationship between obesity and OSA is further supported by some observational studies that show that weight loss leads to a decrease in OSA severity76,78,79 (Fig. 1). The severity of OSA, as estimated by the AHI, increases with obesity.76,80 The severity of OSA is an important predictor of cardiovascular morbidity.39
Fig. 1.
The interaction between obesity and OSA, and its cardiovascular consequences. Note some of the similar pathogenetic mechanisms between OSA and obesity for cardiovascular consequences. Combination of OSA and obesity probably increases the risk for cardiovascular disorders.
OSA as a Risk Factor for Obesity
Patients with OSA may be predisposed to weight gain. In fact there are data to suggest that newly diagnosed OSA patients have difficulty losing weight and may actually be predisposed to excessive weight gain, compared with similarly matched obese subjects without OSA.81,82 The reasons are likely multifactorial. Patients with OSA tend to be hypersomnolent and fatigued, leading to decreased physical activity. Also, obese patients with OSA have leptin resistance so that the weight-reducing effects of leptin are blunted, resulting in a cycle of weight gain and worsening OSA. CPAP use in OSA has been associated with loss of visceral fat.83 Based on the available evidence thus far, it appears that there may be a relation between obesity and OSA that could mutually enhance the progression and severity (see Fig. 1).
Role of Visceral Fat
Visceral fat distribution, irrespective of the overall BMI, appears to be a stronger predictor for cardiovascular disorders.70 Most of the studies assessing the link between OSA and cardiovascular disorders try to control for overall obesity, but not for visceral obesity. Also, visceral fat is a risk factor for OSA and is increased in OSA patients compared with BMI-matched controls.75,84 CPAP therapy has shown to decrease visceral fat accumulation in OSA patients even without an overall decrease in BMI.83 Therefore, visceral fat could be a confounding variable that is not routinely measured in studies assessing OSA and cardiovascular disorders.
Does the Presence of OSA in Obese Patients Place Them at a Higher Risk for Cardiovascular Events than Obese Patients Without OSA?
Some of the basic molecular mechanisms may suggest that OSA in obese patients may place them at higher risk for cardiovascular outcomes (see Fig. 1) than obese patients without OSA, though such assertions await firm clinical evidence. Plasma levels of leptin in OSA patients are even higher than in similarly matched obese but non-OSA subjects,85,86 indicating that the presence of OSA in obese subjects potentiates further leptin resistance and, potentially, cardiovascular disease. Obesity and OSA also predispose to alterations in renal hemodynamics, resulting in sodium retention and volume expansion.87,88 The renin-angiotensin system is activated in obesity and there is a positive correlation between BMI and plasma aldosterone, angiotensinogen, plasma rennin activity, and plasma angiotensin-converting enzyme activity89,90 OSA is also associated with significantly higher levels of angiotensin II and aldosterone compared with healthy controls.90 Therefore it is possible that the activation of the renin-angiotensin-aldosterone system may be augmented by the presence of OSA in obese patients; further studies are needed to confirm this hypothesis. Similarly, oxidative stress has been documented in both OSA and obese patients independent of each other (see Fig. 1). Oxidative stress results in vasoconstriction by blocking nitric oxide synthase enzyme, inactivating nitric oxide, activation of angiotensin II, and a resulting increase of endothelin-1.91,92 Whether OSA exerts a cumulative effect on oxidative stress in obese patients remains to be established, and whether treatment of OSA interrupts these processes to result in tangible improvements in outcomes is unknown.
Does the Presence of OSA in Obese Patients Place Them at a Higher Risk for Cardiovascular Events than OSA Patients Without Obesity?
Although obesity might increase the risk for cardiovascular events in OSA patients compared with OSA without underlying obesity, the authors are unaware of literature to date that specifically compares these 2 groups. The authors propose that experimental models exist that may help answer the question. For example, one might assess cardiovascular outcomes in Asians, who have a lower BMI but have craniofacial features that place them at risk for the development of OSA, with and without obesity for the same degree of OSA severity.
The degree of oxyhemoglobin desaturations and the cumulative burden of nocturnal hypoxia, important predictors of cardiovascular morbidity,93 are not well reflected in the classic AHI metric but may be important variables that may distinguish risk of obesity from OSA. In a recent, novel model of intermittent hypoxia in humans, healthy adults developed significant increases in mean awake blood pressure when exposed to intermittent hypoxia during sleep over a 2-week period.94 As hypoxia appears to be a significant predictor of cardiovascular consequences secondary to OSA, could the presence of obesity augment hypoxia compared with nonobese subjects with similar severity of OSA? In fact, in a recent study obesity was found to be an important predictor on the severity of oxyhemoglobin desaturation during the obstructive apneic and hypopneic events, independent of other confounding variables such as age, gender, event duration, sleep position, and baseline oxyhemoglobin saturation.95 Therefore, obese OSA patients can potentially be at higher risk for cardiovascular morbidity and mortality compared with nonobese OSA patients for the same severity of OSA derived from the AHI. Of course, this will need to be addressed in future prospective studies.
SUMMARY
The current evidence suggests a role for OSA in the development of cardiovascular disorders, though obesity is almost certainly an active confounder in this relationship. OSA and obesity share pathophysiologic mechanisms potentially leading to cardiovascular disorders (see Fig. 1). It may therefore be hypothesized that the presence of OSA in obese patients may further contribute to adverse cardiovascular outcomes when compared with each condition in isolation. These concepts do raise some important questions. Does OSA, in the absence of obesity, lead to adverse cardiovascular outcomes? Are there independent or additive benefits of CPAP therapy and weight loss with regard to cardiovascular outcomes? Similarly, does treatment of OSA in the obese subjects confer the same advantages on cardiovascular function as in nonobese subjects with OSA, or does the presence of obesity attenuate the impact of CPAP on cardiovascular outcomes? Future studies are clearly needed to answer such questions.
Footnotes
Disclosure: Dr Caples supported by research grants from ResMed Foundation, Ventus Medical, Restore Medical and NHLBI HL99534Z-01.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 2001;104:3052. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86:713. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- 4.Abate N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diabetes Complications. 2000;14:154. doi: 10.1016/s1056-8727(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 5.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano JM, Hennekens CH, O’Donnell CJ, et al. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 8.Genest JJ, Jr, Martin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 9.Jeppesen J, Hein HO, Suadicani P, et al. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 10.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Messerli FH, Nunez BD, Ventura HO, et al. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987;147:1725. doi: 10.1001/archinte.147.10.1725. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Milani RV, Ventura HO, et al. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100:1460. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Lavie CJ, Amodeo C, Ventura HO, et al. Left atrial abnormalities indicating diastolic ventricular dysfunction in cardiopathy of obesity. Chest. 1987;92:1042. doi: 10.1378/chest.92.6.1042. [DOI] [PubMed] [Google Scholar]
- 14.Garrison RJ, Kannel WB, Stokes J, 3rd, et al. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 15.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 16.Alpert MA, Terry BE, Mulekar M, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80:736. doi: 10.1016/s0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]
- 17.Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 18.Lavie CJ, Milani RV. Obesity and cardiovascular disease: the Hippocrates paradox? J Am Coll Cardiol. 2003;42:677. doi: 10.1016/s0735-1097(03)00784-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 20.Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155:310. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: patho-physiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113:898. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 24.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 26.Shiomi T, Guilleminault C, Stoohs R, et al. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest. 1991;100:894. doi: 10.1378/chest.100.4.894. [DOI] [PubMed] [Google Scholar]
- 27.Stoohs R, Guilleminault C. Cardiovascular changes associated with obstructive sleep apnea syndrome. J Appl Physiol. 1992;72:583. doi: 10.1152/jappl.1992.72.2.583. [DOI] [PubMed] [Google Scholar]
- 28.Virolainen J, Ventila M, Turto H, et al. Effect of negative intrathoracic pressure on left ventricular pressure dynamics and relaxation. J Appl Physiol. 1995;79:455. doi: 10.1152/jappl.1995.79.2.455. [DOI] [PubMed] [Google Scholar]
- 29.Narkiewicz K, Kato M, Phillips BG, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell CP, Allan L, Atkinson P, et al. The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:965. doi: 10.1164/rccm.2110072. [DOI] [PubMed] [Google Scholar]
- 31.Schneider H, Schaub CD, Chen CA, et al. Effects of arousal and sleep state on systemic and pulmonary hemodynamics in obstructive apnea. J Appl Physiol. 2000;88:1084. doi: 10.1152/jappl.2000.88.3.1084. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 33.Vongpatanasin W, Thomas GD, Schwartz R, et al. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- 34.Bokinsky G, Miller M, Ault K, et al. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. A preliminary investigation. Chest. 1995;108:625. doi: 10.1378/chest.108.3.625. [DOI] [PubMed] [Google Scholar]
- 35.Steiner S, Jax T, Evers S, et al. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104:92. doi: 10.1159/000086729. [DOI] [PubMed] [Google Scholar]
- 36.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207. [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks D, Horner RL, Kozar LF, et al. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746. [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 40.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 1829;283:2000. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 41.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 42.Ali NJ, Davies RJ, Fleetham JA, et al. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 43.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35:144. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 44.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 45.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 46.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134:1015. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 47.Robinson GV, Smith DM, Langford BA, et al. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 48.Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. 2006;61:1083. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 50.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 51.Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 52.Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 53.Usui K, Parker JD, Newton GE, et al. Left ventricular structural adaptations to obstructive sleep apnea in dilated cardiomyopathy. Am J Respir Crit Care Med. 2006;173:1170. doi: 10.1164/rccm.200503-320OC. [DOI] [PubMed] [Google Scholar]
- 54.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 56.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 57.Shepard JW, Jr, Pevernagie DA, Stanson AW, et al. Effects of changes in central venous pressure on upper airway size in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;153:250. doi: 10.1164/ajrccm.153.1.8542124. [DOI] [PubMed] [Google Scholar]
- 58.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 59.Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 60.Smith LA, Vennelle M, Gardner RS, et al. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007;28:1221. doi: 10.1093/eurheartj/ehm131. [DOI] [PubMed] [Google Scholar]
- 61.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm W, Koehler U, Fus E, et al. Outcome of patients with sleep apnea-associated severe bradyarrhythmias after continuous positive airway pressure therapy. Am J Cardiol. 2000;86:688. doi: 10.1016/s0002-9149(00)01055-9. [DOI] [PubMed] [Google Scholar]
- 63.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 64.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell AR, Spurrell PA, Sulke N. Circadian variation of arrhythmia onset patterns in patients with persistent atrial fibrillation. Am Heart J. 2003;146:902. doi: 10.1016/S0002-8703(03)00405-8. [DOI] [PubMed] [Google Scholar]
- 66.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 67.van Boxem TJ, de Groot GH. Prevalence and severity of sleep disordered breathing in a group of morbidly obese patients. Neth J Med. 1999;54:202. doi: 10.1016/s0300-2977(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 68.Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705. [PubMed] [Google Scholar]
- 69.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 70.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 71.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 72.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 73.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Grunstein R, Wilcox I, Yang TS, et al. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533. [PubMed] [Google Scholar]
- 75.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 76.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 77.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 79.Smith PL, Gold AR, Meyers DA, et al. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 80.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 81.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 82.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 83.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 84.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 85.Ip MS, Lam KS, Ho C, et al. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 86.Shimizu K, Chin K, Nakamura T, et al. Plasma leptin levels and cardiac sympathetic function in patients with obstructive sleep apnoea-hypopnoea syndrome. Thorax. 2002;57:429. doi: 10.1136/thorax.57.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S. [PubMed] [Google Scholar]
- 88.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999;892:91. doi: 10.1111/j.1749-6632.1999.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 89.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med. 2001;79:21. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 90.Moller DS, Lind P, Strunge B, et al. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 91.Somers MJ, Harrison DG. Reactive oxygen species and the control of vasomotor tone. Curr Hypertens Rep. 1999;1:102. doi: 10.1007/s11906-999-0080-z. [DOI] [PubMed] [Google Scholar]
- 92.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep. 2002;4:160. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 93.Punjabi NM, Newman AB, Young TB, et al. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tamisier R, Gilmartin GS, Launois SH, et al. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep, and blood pressure. J Appl Physiol. 2009;107:17. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]