Abstract
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, and can be further classified as nonsquamous carcinoma (including adenocarcinoma, which accounts for 50% of NSCLCs) and squamous NSCLC, which makes up 30% of NSCLC cases. The emergence of inhibitors of epidermal growth factor receptors, anaplastic lymphoma kinase, and vascular endothelial growth factors (VEGF) in the last decade has resulted in steady improvement in clinical outcome for patients with advanced lung adenocarcinoma. However, improvements in the survival of patients with squamous NSCLC have remained elusive, presenting an urgent need for understanding and investigating therapeutically relevant molecular targets specifically in squamous NSCLC. Although anti-VEGF therapy has been studied in squamous NSCLC, progress has been slow, in part due to issues related to pulmonary hemorrhage. In addition to these safety concerns, several phase III trials that initially included patients with squamous NSCLC failed to demonstrate improved overall survival (primary endpoint) with the addition of antiangiogenic therapy to chemotherapy compared with chemotherapy alone. Angiogenesis is an established hallmark of tumor progression and metastasis, and the role of VEGF signaling in angiogenesis is well established. However, some studies suggest that while inhibiting VEGF signaling may be beneficial, prolonged exposure to VEGF/VEGF receptor (VEGFR) inhibitors may allow tumor cells to utilize alternative angiogenic mechanisms and become resistant. As a result, agents that target multiple angiogenic pathways simultaneously are also under evaluation. This review focuses on current and investigational antiangiogenic targets in squamous NSCLC, including VEGF/VEGFRs, fibroblast growth factor receptors, platelet-derived growth factor receptors, and angiopoietin. Additionally, clinical trials investigating VEGF- and multi-targeted antiangiogenic therapies are discussed.
1. Introduction
Lung cancer is the leading cause of cancer deaths in the United States [1], with a 5-year survival rate of approximately 16% [2,3]. The World Health Organization (WHO) classifies lung cancer into 2 major classes based on its biology, therapy and prognosis: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) [3]. Representing more than 85% of lung cancer cases, NSCLC is the most common of these [3]. The 2 major NSCLC histologies are nonsquamous carcinoma (can be further classified as adenocarcinoma, which accounts for approximately 40% of NSCLCs, large cell carcinoma, and other cell types), and squamous NSCLC, which makes up 30% of NSCLC cases [4].
The survival of patients with advanced lung adenocarcinoma improved in the early 2000s, most likely due to the emergence of inhibitors of epidermal growth factor receptors (EGFRs), anaplastic lymphoma kinase (ALK), and vascular endothelial growth factor (VEGF) [5]. However, similar improvements in the survival of patients with squamous NSCLC have not been observed; this may be attributed to the fact that most of the treatment advances in NSCLC in the past decade have improved outcomes for adenocarcinoma, but demonstrated modest if any benefit in squamous NSCLC [6]. Therefore, understanding and investigating potential molecular targets specifically in squamous NSCLC could help transform the treatment of this class of lung cancer.
Given that angiogenesis is an established hallmark of progressively detrimental tumors, inhibiting proangiogenic factors represents a potential avenue for therapeutic development [7]. While the role of VEGF in angiogenesis is well-established [8–10], it is also known that additional signaling molecules and pathways contribute to aberrant blood vessel formation [11]. Notably, some studies postulate that inhibiting VEGF and its receptors (VEGFRs) in early treatment settings may be beneficial, but that prolonged exposure to VEGF/VEGFR inhibitors may allow tumor cells to utilize alternative mechanisms to find oxygen and nutrients to sustain their growth [12]. As a result, in addition to VEGF-targeted therapy, studies are also exploring additional antiangiogenic pathways as potential targets in squamous NSCLC.
This review article discusses current and investigational antiangiogenic pathways in squamous NSCLC, including VEGF/VEGFR, fibroblast growth factor receptors (FGFRs), platelet-derived growth factor receptors (PDGFRs), and angiopoietin. Clinical trials investigating VEGF- and multi-targeted antiangiogenic therapies are also discussed. Relevant clinical trials and other published evidence were identified using PubMed and ClinicalTrials.gov; however, no specific search terms were used.
2. Preclinical Studies of Potential Antiangiogenic Targets in Squamous NSCLC
2.1 VEGF Signaling
VEGF, also referred to as VEGF-A, is a member of a family of growth factors that also includes VEGF-B, VEGF-C, VEGF-D, VEGF-E (found only in viruses), VEGF-F (identified from snake venom), and placenta growth factor (PlGF) [13,14]. As the prototype member of the family, VEGF is secreted by tumor cells and tumor-associated stromal tissues [15], and is also the most extensively studied proangiogenic signaling factor [11,16]. VEGF and VEGF-B are commonly expressed in NSCLC (usually at higher levels in adenocarcinoma than in squamous NSCLC), and have established roles in tumor cell proliferation, metastasis, and angiogenesis [17]. VEGF activation of VEGFR-1, VEGFR-2, and downstream signaling pathways (eg, phosphoinositide 3 kinase [PI3K], phospholipase C-γ, and v-src sarcoma viral oncogene homolog [src]) is a well established initial step in promoting angiogenesis [10,14]. Activation of these receptors triggers downstream signaling by the mitogen-activated protein kinase (MAPK) pathway, among others [18].
Multiple preclinical studies and systematic reviews have evaluated the role of VEGF and VEGFR in NSCLC cases. For example, a study conducted in 1996 reported that 5-year survival rates for patients with low versus high levels of VEGF mRNA were 77.9% and 16.7%, respectively, suggesting a potential prognostic role for VEGF in NSCLC [19]. Eventually, VEGF was linked to angiogenesis in studies of resected NSCLC tumors specimens, in which VEGF expression levels were significantly associated with new vessel formation (r = 0.44; P <0.0001) [20].
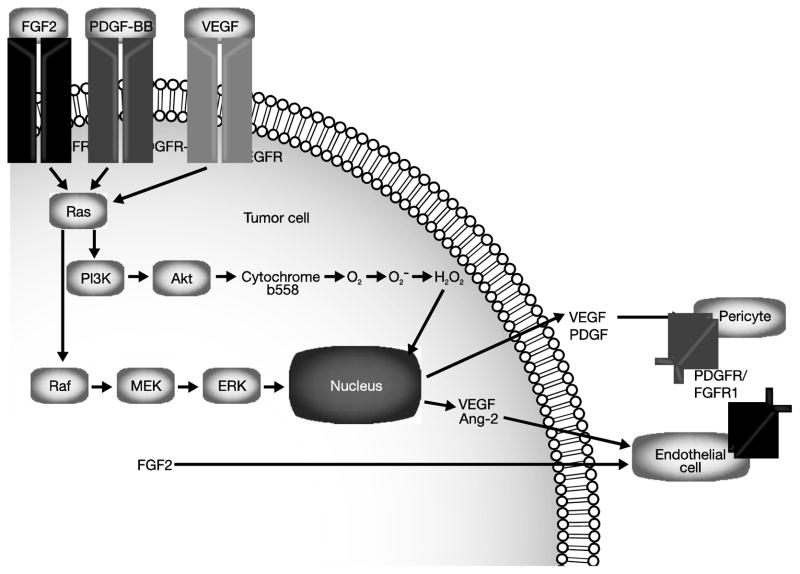
Once the underlying mechanisms of angiogenesis were better characterized, the presence of VEGF and VEGFR in squamous NSCLC was associated with disease spread and nodal metastases, according to an early study of 91 NSCLC tumor specimens [21]. Although VEGF was the focus of early studies on tumor angiogenesis, it is now known that other signaling pathways also contribute to angiogenesis, probably in concert with VEGF and VEGFR. Key examples of such cross-talk are illustrated in Figure 1.
Figure 1. Cross-talk between VEGFR, FGFR, and PDGFR signaling pathways.
In addition to VEGFR signaling, other pathways (eg, FGFR, PDGFR) have been shown to contribute to angiogenesis, possibly in concert with VEGFR. Republished with permission of the American Society for Clinical Investigation from Arbiser JL [83] © 2007.
Abbreviations: Akt, protein kinase B; Ang-2, angiopoietin-2; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor, FGFR, fibroblast growth factor receptor; MEK, mitogen activated protein kinase kinase; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol-3-kinase; Raf, v-raf 1 murine leukemia viral oncogene homolog 1; Ras, retrovirus-associated DNA sequences; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
2.2 Platelet-derived Growth Factor Signaling
PDGFs are a family of 5 ligands (ie, PDGF-AA, -AB, -BB, -CC, and -DD) that bind and activate 2 tyrosine kinase receptors, PDGF-α and –β, with varying affinities [22]. Activation of these receptors triggers signaling through various downstream pathways [18]. In NSCLC, PDGF has been shown to be an autocrine regulator of VEGF expression, suggesting a role for PDGF in tumor progression via angiogenesis [23]. In addition, according to a single-center study of surgical sections of NSCLC tumors and cell lines, PDGF influences tumor size and patient prognosis more so than VEGF [23]. While most studies have focused on the role of PDGFR in NSCLC, one study reported that PDGFRA gene amplification observed in an adenosquamous NSCLC cell line was also present in a subset of squamous cell NSCLC clinical samples, but in none of the adenocarcinoma samples that were screened by fluorescence in situ hybridization. This finding suggests that the PDGFRA pathway might be an oncogenic mechanism unique to the squamous histologic subtype of NSCLC, and warrants further study [24].
Further establishing the role of PDGF in NSCLC, elevated levels of PDGFs have been associated with poorer outcomes, comprised of a shortened disease-specific survival in NSCLC (including squamous NSCLC histology), independent of tumor stage or degree of differentiation [23,25,26]. For example, PDGF-B was found to be a prognostic factor in patients with NSCLC; in the study, its presence was associated with a significantly shorter disease-specific survival in a study of 55 patients with stage I to IIIA NSCLC treated with postoperative radiotherapy (hazard ratio [HR], 5.42; P = 0.002) [27].
In addition to being involved in hemangiogenesis, PDGFs are also involved in lymphangiogenesis [28]. In 335 tumor samples from patients with resected stage I to IIIA NSCLC, elevated levels of PDGF-A were independently associated with lymph node metastasis, and coexpression of PDGF-B and VEGFR-3 was associated with decreased survival among patients with NSCLC [18]. One explanation for this association is that the metastatic process may be attributable to PDGF activation of VEGF-mediated pathways [18]. Alternatively, the role of PDGF-B in lymphangiogenesis may be more direct, via its activation of PDGF-α and –β [29], or through a combination of PDGFR- and VEGFR-mediated pathways.
Some tumors with increased PDGF levels are resistant to anti-VEGF therapies, supporting a role for complementary or co-dependent effects of PDGF and VEGF on angiogenesis (Figure 1). Preclinical studies in cells and mouse tumor models have shown increased efficacy of dual blockade of VEGF and PDGF compared with VEGF inhibition alone [30,31].
2.3 Angiopoietin
Angiopoietins (ie, Ang-1, Ang-2, and Ang-4), along with their receptor, endothelial TEK tyrosine kinase (Tie-2), have been implicated in angiogenic processes, including vascular permeability and vessel maturation [32]. Ang-1-mediated activation of Tie-2 has been linked to decreased vascular permeability, leading to angiogenesis in cultured endothelial cells [32]. When treated with an Ang-1 agonist, mice with subcutaneously implanted tumors developed tumor progression and lung metastases [33]. In other cases, high levels of Ang-2 expression in tumor cells was associated with poor prognosis, including an increased likelihood of angiogenesis [34] and decreased overall survival (OS).
Although exclusive studies exploring the role of angiopoietins in squamous NSCLC are limited, Ang-1 and Ang-2 have been identified as potent angiogenic factors, functioning in concert with VEGF [35]. Similar to interactions between PDGF/PDGFR and FGF/FGFR pathways, angiopoietins and Tie-2 also work with VEGF/VEGFR to mediate angiogenesis [32,33,36]. Studies have demonstrated that patients with tumors that co-overexpress Ang-2 and VEGF have decreased survival compared with those with overexpression of either factor alone, or with various expression levels of Ang-4 [36].
2.4 Fibroblast Growth Factor Signaling
FGFRs are a family of 4 cell surface receptors that stimulate cell proliferation and survival in many cell types through their activation of the MAPK and PI3K/Akt pathways [37,38]. Over 20 members of the FGF family of growth factors have been identified; FGF signaling has been associated with a wide variety of developmental and normal signaling processes, and several family members (eg, FGF-1, FGF-2, FGF-4, FGF-5, and FGF-8) have also been implicated in angiogenesis [37].
Several studies have indicated that FGFR may be a valid target for antiangiogenic therapy for squamous cell histologies of NSCLC. Molecular silencing and pharmacological studies of FGFR have revealed that among NSCLC cell lines, FGFR-dependent autocrine signaling was observed in H226, H520 and H1703 squamous cell carcinomas [39]. Serum basic FGF (bFGF) levels have also been shown to be increased in both squamous and adenocarcinoma histologies, relative to healthy controls [40]. In addition, several studies have demonstrated that FGFR1 is specifically upregulated in squamous NSCLC. One of these that reported FGFR1 amplification in 21% of NSCLC squamous tumors versus 3% of adenocarcinomas also found that cell lines amplified for FGFR1 were dependent on its signaling to maintain cell growth. Subsequently, inhibition of FGFR1 by short hairpin RNAs (shRNAs) or by small molecules blocked cell growth [41]. Additionally, a study of 232 NSCLC cases confirmed these findings and reported FGFR1 amplification in 22% of squamous cell tumors, but not tumors with other histologies, and also reported tumor shrinkage after applying an FGFR1-targeted small molecule to the specimens [42]. Further, findings from another study indicated that the presence of FGFR1 amplification in squamous NSCLC is even higher, at 41.5%; the same study also reported FGFR1 amplification in a higher percentage of nonsquamous cell NSCLCs (14.3%) [43].
Similar to the aforementioned relationship between VEGF and PDGFR, VEGF and FGFR have demonstrated co-expression in NSCLC tumors, and may have synergistic roles in angiogenesis (Figure 1) [44]. A study of 58 tumor samples from patients with surgically resected NSCLC specimens identified a significant correlation between bFGF levels and VEGF levels (r = 0.44; P <0.001) [45]. Some evidence also indicates that the relationship between VEGF and FGF/FGFR may be prognostic in NSCLC. For example, a study of 71 patients with NSCLC of adenocarcinoma or squamous histology showed that bFGF levels were most strongly correlated with poor prognosis in patients whose tumors also exhibited high VEGF levels (P <0.0001), regardless of histologic subtype [46].
As previously mentioned, inhibitors of the FGF/FGFR pathway have been shown to block tumor growth and shrink tumors in some preclinical studies. One of these studies showed that blocking bFGF activity in 3 human NSCLC cell lines using antisense oligonucleotides, a bFGF antisense cDNA-expressing vector, or a neutralizing monoclonal antibody halted cell growth [47]. By using genetic (dominant-negative FGFR1 [dnFGFR1]) and pharmacological (FGFR small molecule inhibitors) means, NSCLC cell line growth was severely limited, and dnFGFR1 expression also inhibited tumor growth in xenograft tumor models [48]. The FGF/FGFR pathway has also identified as a potential mechanism of resistance to VEGF-targeted therapy in preclinical models of pancreatic islets [12].
3. History of Trials Investigating VEGF-targeted agents in NSCLC
Most of the studies in NSCLC agents have so far focused on anti-VEGF therapy, with limited studies in squamous NSCLC due to concerns regarding life-threatening pulmonary hemorrhage. Bevacizumab (Avastin®, Genentech; South San Francisco, CA, US), a monoclonal antibody (mAb) directed against VEGF, was the first VEGF-targeted therapy to prolong survival when combined with carboplatin and paclitaxel in a selected population of patients with NSCLC [49]. Despite the efficacy demonstrated by bevacizumab in phase II and III trials in patients with NSCLC [49,50], clinically significant bleeding events, including major hemoptysis, delayed further evaluation of bevacizumab in patients with squamous NSCLC [50]. Thus, bevacizumab is indicated only for patients with nonsquamous NSCLC [51]. Questions regarding the mechanisms by which anti-VEGF agents induce pulmonary hemorrhage remain unanswered, with some evidence suggesting an association with the inhibition of the physiological endothelial repair processes mediated by VEGF [52], tumor erosion of vessels [53], and the central location of tumors close to major blood vessels [53,54].
Some studies have also indicated that severe pulmonary hemorrhage observed with bevacizumab therapy was associated with squamous histology (resulted in the subsequent exclusion of patients with predominantly squamous tumors from several trials), the presence or development of tumor cavitation, and the central location of tumors adjacent to major blood vessels [50]. Squamous cell tumors are more likely thought to be centrally located and cavitated compared with adenocarcinoma tumors. Thus, it is still not clear whether squamous histology is the central independent risk factor for pulmonary hemorrhage with anti-VEGF therapy or whether there are other contributing factors such as central location and cavitation [50].
In an early phase II trial of bevacizumab in NSCLC, life-threatening pulmonary hemorrhage was reported in 6 patients, 4 of whom had squamous NSCLC; 4 of the 6 cases were fatal and 5 of the 6 occurred in the low-dose bevacizumab arm [50]. Of note, all 6 patients had tumors that were centrally located close to major blood vessels and 5 had cavitation or necrosis (either observed at baseline or developed during therapy). Only a small increased risk of serious bleeding (approximately 4%) was reported in patients with nonsquamous tumors [50]. Median time to progression (TTP) was longer with high-dose bevacizumab compared with chemotherapy alone (7.4 vs 4.2 months, respectively; P = 0.023), but TTP in the low-dose bevacizumab arm and OS in both bevacizumab dose groups were similar to the control arm [50].
More recently, using strategies to reduce and closely monitor toxicity, a number of studies have revisited the potential of bevacizumab in squamous NSCLC. In an attempt to minimize the toxicity of bevacizumab in squamous NSCLC, the BRIDGE trial evaluated a more sequential administration of chemotherapy and bevacizumab. In the study, patients received carboplatin and paclitaxel for 2 cycles, followed by carboplatin and paclitaxel with bevacizumab in Cycles 3 to 6, and lastly bevacizumab, until progression or unacceptable toxicity. The incidence of grade 3 pulmonary hemorrhage was 3.2% (1 of 31; 90% confidence interval [CI], 0.3%–13.5%); median progression-free survival (PFS) was 6.2 months [55]. While the incidence of pulmonary hemorrhage was lower than that reported in the phase II study described above [50], the use of bevacizumab therapy in squamous NSCLC remains investigational.
A recently reported pilot trial (SWOG-S0533) [56] evaluated the feasibility of incorporating bevacizumab into standard chemo-radiation for patients with inoperable, locally advanced, stage III NSCLC. Patients in the trial were treated with cisplatin, etoposide, and radiotherapy, followed by consolidation docetaxel and bevacizumab. In addition, patients were stratified into a high risk group if they had squamous histology, hemoptysis, or tumor with cavitation or that was near a major vessel. During safety analysis, the major adverse events (AEs) associated with consolidation docetaxel and bevacizumab in the high risk group were incidences of grade 3 pneumonitis and fatal hemoptysis (2 of 12 for each), which resulted in termination of evaluation of the high risk group [56]. Median OS was 23 months in the low risk group and 17 months in the high risk group [56].
A recent phase I/II trial specific to stage III NSCLC that investigated the incorporation of bevacizumab and erlotinib with induction therapy (carboplatin, paclitaxel, and bevacizumab) followed by concurrent chemoradiation (carboplatin, paclitaxel, bevacizumab, and thoracic conformal radiation with either no erlotinib or 1 of 2 doses of erlotinib) reported no cases of pulmonary hemorrhage among 12 patients with squamous histology treated with 2 cycles of induction therapy [57]. However, 2 delayed grade 5 pulmonary hemorrhage events occurred after concurrent therapy, leading to the exclusion of patients with squamous histology from the study. Another grade 3 pulmonary hemorrhage event occurred during concurrent therapy; however the patient was able to complete treatment (to 74 Gy) with discontinuation of bevacizumab. Median PFS and OS were similar for patients with squamous (10 and 17.1 months, respectively) and nonsquamous histology (10 and 18.7 months, respectively) [57].
Data in squamous NSCLC on the safety of motesanib (Amgen; Thousand Oaks, CA, US), an oral inhibitor of VEGFR-1, -2, and -3, PDGFR, and stem cell factor receptor (c-kit), have also been reported [58]. The phase III MONET 1 trial combined first-line carboplatin and paclitaxel with motesanib in patients with NSCLC. This trial was suspended after increased mortality and hemoptysis were reported in patients with squamous cell histology, and the MONET 1 protocol was subsequently amended to exclude further enrollment of patients with squamous cell tumors [58,59]. The study did not meet its primary endpoint of OS in the overall nonsquamous population or in the subset analysis of patients with adenocarcinoma [58]. Median OS for the overall nonsquamous population was 13.0 months with motesanib plus chemotherapy versus 11.0 months with chemotherapy alone (HR, 0.90; 95% CI, 0.78–1.04; P = 0.14). Clinically significant bleeding events in squamous NSCLC have also been reported in clinical trials of other agents, including single-agent sunitinib (Sutent®, Pfizer; New London, CT, US) [60] and sorafenib (Nexavar®, Bayer; Leverkusen, Germany) [61,62]. A phase II trial of sunitinib in chemotherapy-pretreated patients with advanced NSCLC revealed that 2 of 3 cases of fatal hemorrhage occurred in patients with squamous NSCLC [60]. Of 7 (11.1%) patients achieving confirmed objective response, 1 had squamous NSCLC [60]. A phase II study of pemetrexed or sunitinib or pemetrexed plus sunitinib as second-line therapy for advanced NSCLC reported generally greater toxicity in the sunitinib arms, which each included 15% of patients with squamous histology, and no improvement in OS with sunitinib (7.0 vs 6.7 vs 10.5 months for sunitinib alone vs sunitinib + pemetrexed vs pemetrexed alone; 2-sided P = 0.0179) [63]. The phase III ESCAPE trial of sorafenib in combination with first-line carboplatin/paclitaxel, which enrolled 223 (24%) patients with squamous NSCLC, demonstrated an increased mortality risk among sorafenib-treated patients with squamous cell tumors (HR, 1.85; 95% CI, 1.22–2.81) [61]. The study did not reach its primary endpoint, with a median OS of 10.7 months with sorafenib plus chemotherapy versus 10.6 months with chemotherapy alone (HR, 1.15; 95% CI, 0.94–1.41; P = 0.915) in the overall population; median OS in patients with squamous NSCLC was 8.9 versus 13.6 months, respectively (HR, 1.85; 95% CI, 1.22–2.81). Safety concerns from the ESCAPE trial resulted in a protocol amendment to exclude patients with squamous NSCLC from a similar phase III trial (NExUS) of sorafenib plus gemcitabine/cisplatin [62,64].
Of note, however, several clinical trials of antiangiogenic agents in NSCLC have reported more favorable safety profiles with inclusion of patients with squamous histology. In a phase III study of sunitinib plus erlotinib versus placebo plus erlotinib in previously treated advanced NSCLC (28% squamous histology in both arms), the incidences of treatment-related pulmonary hemorrhage (0.4% in both arms) and hemoptysis (4.0% with sunitinib/erlotinib vs 2.5% with erlotinib/placebo) were similar between groups [65]. Median OS was similar between treatment groups (9.0 months for sunitinib + erlotinib vs 8.5 months for placebo + erlotinib [HR, 0.922; P = 0.1388]); on multivariate analysis, nonsquamous histology was not identified as a favorable prognostic factor for OS [65]. A phase III study of carboplatin/paclitaxel plus the vascular disrupting agent vadimezan (ASA404), a flavone acetic acid analog, or placebo in patients with previously untreated advanced NSCLC reported no increase in vascular toxicity for the squamous histology cohort (n = 265) compared with the nonsquamous subset (n = 978). Hemoptysis (all grades) was observed in 6.4% and 6.2% of patients in the vadimezan and placebo arms, respectively; only 1 patient per arm experienced a grade 4 hemoptysis event [66]. The study failed to meet its primary endpoint of OS: median OS was 13.4 versus 12.7 months in the vadimezan and placebo arms, respectively (HR, 1.01; 95% CI, 0.85–1.19; P = 0.535). There were also no differences in OS between treatment groups when stratified by histology (nonsquamous NSCLC: HR, 0.98; 95% CI, 0.80–1.19; squamous NSCLC: HR, 1.10; 95% CI, 0.79–1.52) [66].
A summary of data available from clinical trials evaluating antiangiogenic agents in squamous NSCLC is provided in Table 1.
Table 1.
Clinical Trials Evaluating VEGF-targeted Therapies With Data Available in Squamous NSCLC
| Trial | Patient population | Regimen | RR | PFS | OS | Fatal hemorrhagic events |
|---|---|---|---|---|---|---|
| Johnson et al [50] | Advanced or recurrent NSCLC (N = 99); 20% squamous histology | Bevacizumab 15 mg/kg + carboplatin/paclitaxel vs carboplatin/paclitaxel alone | 31.5% vs 18.8% (overall) | TTP: 7.4 vs 4.2 mo; P = 0.023 (overall) | 17.7 vs 14.9 mo; P = 0.63 (overall) | Bevacizumab arm, n = 4 |
| BRIDGE [55] | Squamous NSCLC (N = 31) | Bevacizumab 15 mg/kg | NR | 6.2 mo (overall) | NR | N/A |
| SWOG-S0533 pilot study [56] | Unresectable advanced NSCLC (N = 29); 41% squamous histology | Bevacizumab 15 mg/kg + docetaxel 75 mg/m2 | NR | NR | 23 mo (nonsquamous) 17 mo (squamous) |
Bevacizumab arm, n = 2 (squamous, n = 2) |
| Socinski et al [57] | Stage III NSCLC (N = 45); 27% squamous histology | Induction carboplatin/paclitaxel + bevacizumab 15 mg/kg followed by concurrent carboplatin/paclitaxel + bevacizumab 10 mg/kg + TCRT (with or without erlotinib) | 60% (overall) | 10.2 mo (overall) 10 mo (squamous) |
18.4 mo (overall) 17.1 mo (squamous) |
N/Aa |
|
| ||||||
|
Sunitinib
| ||||||
| Socinski et al [60] | Chemotherapy-pretreated, advanced NSCLC (N = 63); 22% squamous histology | Sunitinib 50 mg/day | 11.1% (overall); squamous, n = 1 | 12.0 wk (overall) | 23.4 wk (overall) | Sunitinib arm, n = 3 (squamous, n = 2) |
| Scagliotti et al [65] | Chemotherapy-pretreated advanced refractory NSCLC (N = 960); sunitinib arm, 28% squamous histology | Sunitinib 37.5 mg/day + erlotinib 150 mg/day vs placebo + erlotinib 150 mg/day | 10.6% vs 6.9% (overall) | 3.6 vs 2.0 mo; P = 0.0023 (overall) | 9.0 vs 8.5 mo; P = 0.1388 (overall) | Sunitinib arm, n = 1 (hemoptysis) |
| Heist et al [63] | Chemotherapy-pretreated advanced NSCLC (N = 128); sunitinib arms, 15% squamous histology each | Sunitinib 37.5 mg/day vs pemetrexed 500 mg/m2 vs sunitinib 37.5 mg/day + pemetrexed 500 mg/m2 | NR | 3.3 vs 4.4 vs 3.7 mo; P = 0.3 (overall) | 7.0 vs 10.5 vs 6.7 mo; P = 0.0179 (overall) | N/A |
|
| ||||||
|
Sorafenib
| ||||||
| ESCAPE [61] | Untreated advanced NSCLC (N = 926); sorafenib arm, 23% squamous histology | Sorafenib 400 mg bid + carboplatin/paclitaxel vs carboplatin/paclitaxel alone | 27.4% vs 24.0% (overall) | 4.6 vs 5.4 mo (overall) 4.3 vs 5.8 mo (squamous) |
10.7 vs 10.6 mo (overall) 8.9 vs 13.6 mo (squamous) |
Sorafenib arm, n = 4 (squamous, n = 2) |
|
| ||||||
| Vadimezan | ||||||
|
| ||||||
| Lara et al [66] | Previously untreated advanced NSCLC (N = 1,299); 20% squamous histology | Vadimezan 1,800 mg/m2 + carboplatin/paclitaxel vs placebo + carboplatin/paclitaxel | 24.7% vs 24.6% (overall) | 5.5 vs 5.5 mo (overall) | 13.4 vs 12.7 mo (overall) | N/A |
VEGF, vascular endothelial growth factor; NSCLC, non-small cell lung cancer; RR, response rate; PFS, progression-free survival; OS, overall survival; TTP, time to progression; NR, not reported; N/A; not applicable; TCRT, thoracic conformal radiation therapy.
Two cases of delayed grade 5 pulmonary hemorrhage were observed in patients with squamous histology 53 and 151 days following TCRT.
4. Ongoing Trials Evaluating Antiangiogenic Agents in Squamous NSCLC
Newer investigational agents with the potential for improved safety profiles (without any major issues with life-threatening hemoptysis), and a wider range of targets, including FGFR, are being studied. Given the safety concerns arising from VEGF-targeted therapies in squamous NSCLC, the vast majority of contemporary clinical trials exclude participants with squamous histology. However, several ongoing trials do include squamous participants, and these studies are summarized in Table 2. The antiangiogenic agents currently being evaluated in studies involving squamous NSCLC participants include the following: axitinib (AG-013736, Pfizer; New London, CT, USA), a VEGFR/PDGFR/c-kit tyrosine kinase inhibitor (TKI) [67,68]; nintedanib (BIBF 1120; Boehringer Ingelheim; Ingelheim, Germany), a VEGFR-1, -2, and -3, PDFGR-α/-β, and FGFR-1, -2, and -3 TKI that also has activity against the src family and fms-like tyrosine kinase 3 (FLT3) [69]; cediranib (Recentin™, AstraZeneca; Wilmington, DE, US), a TKI targeting VEGFR-1, -2, and -3, PDGFR-a/-β, FGFR-1, and c-kit [70]; pazopanib (Votrient™; GlaxoSmithKline; London, UK), a TKI targeting VEGFR-1, -2, and -3, PDGFR-a/-β, FGFR-1 and -3, and c-kit [71,72]; and ramucirumab (IMC-1121B; ImClone LLC, a subsidiary of Lilly; Bridgewater, NJ, US), a recombinant, human anti-VEGFR-2 mAb [73].
Table 2.
Clinical Trials Evaluating Antiangiogenic Agents in Squamous NSCLC
| Study description | Primary endpoint(s) | Target accrual, n | Current statusa |
|---|---|---|---|
| Axitinib + first-line gemcitabine/cisplatin (NCT00735904; phase II) | RR | 38 | Completed |
| Nintedanib + first-line gemcitabine/cisplatin (LUME-Lung 3, NCT01346540; phase I/II) | Phase I: frequency, intensity, and duration of AEs and DLTs Phase II: PFS |
165 | Recruiting |
| Nintedanib or placebo + second-line docetaxel (LUME-Lung 1, NCT00805194; phase III) | PFS | 1,300 | Active, no longer recruiting |
| Cediranib or placebo + first-line carboplatin/paclitaxel (CAN-NCIC-BR29, NCT00795340; phase III) | OS | 306 | Active, no longer recruiting |
| Pazopanib (NCT01208064; phase II/III) | OS | 600 | Recruiting |
| Pazopanib + erlotinib or placebo + erlotinib (NCT01027598; phase II) | PFS | 201 | Active, no longer recruiting |
| Ramucirumab + pemetrexed and carboplatin/cisplatin or + gemcitabine and carboplatin/cisplatin (NCT01160744; phase II) | PFS | 280 | Recruiting |
NSCLC, non-small cell lung cancer; RR, response rate; AEs, adverse events; DLTs, dose-limiting toxicities; PFS, progression-free survival; OS, overall survival.
Per ClinicalTrials.gov, accessed November 2013.
One of the ongoing studies, a phase II study of axitinib in patients with squamous NSCLC (NCT00735904), was completed at the end of 2011 and results are forthcoming. In addition, based on phase I results [74], a phase I/II trial (LUME-Lung 3; NCT01346540) evaluating nintedanib as first-line therapy in combination with gemcitabine and cisplatin in patients with squamous NSCLC is currently recruiting. The LUME-Lung 3 study will be investigated in 2 parts, the first of which will be an open label, phase I study that aims to identify the maximum tolerated dose of nintedanib to be used for standard first-line treatment with 3 weekly schedules of gemcitabine/cisplatin. Part 2 is a phase II placebo- controlled efficacy study that will evaluate nintedanib in combination with standard 3 weekly cycles of gemcitabine/cisplatin therapy in patients with at least stable disease after 2 previous courses of the chemotherapy.
In addition to the squamous NSCLC-specific clinical trials described above, several NSCLC studies that do not exclude squamous histology have also been initiated to evaluate antiangiogenic agents in NSCLC. The LUME-Lung 1 phase III trial of nintedanib or placebo plus second-line docetaxel in patients with stage IIIB/IV or recurrent NSCLC (NCT00805194) has been initiated and does not exclude participants with squamous histology. Preliminary results showed that nintedanib plus docetaxel significantly improved median PFS for all patients compared with the placebo arm (3.4 vs 2.7 months; HR, 0.79; 95% CI, 0.68–0.92; P = 0.0019), with similar median PFS observed between treatment groups when stratified by histology (adenocarcinoma NSCLC: 4.0 vs 2.8 months; HR, 0.77; 95% CI 0.62–0.96; P = 0.0193; squamous NSCLC: 2.9 vs 2.6 months; HR, 0.77; 95% CI 0.62–0.96; P = 0.02). Median OS was similar between treatment groups for all patients (10.1 vs 9.1 months; HR, 0.94; 95% CI, 0.83–1.05; P = 0.272), and significantly improved in the nintedanib arm among patients with adenocarcinoma histology (12.6 vs 10.3 months; HR, 0.83; 95% CI, 0.70–0.99; P = 0.0359) [75]. Similarly, pazopanib is currently under investigation in a phase II/III clinical trial that is not excluding patients with squamous NSCLC (NCT01208064). In the study, pazopanib is being compared with a placebo as maintenance therapy for patients with NSCLC who have received first-line chemotherapy. A phase II study of ramucirumab in combination with platinum-based chemotherapy versus platinum-based chemotherapy alone as first-line treatment for patients with recurrent or advanced NSCLC, including those with squamous histology, is currently in progress (NCT01160744).
5. Genomic Sequencing of Squamous NSCLC
Recent genomic sequencing studies have identified several genes that are altered in squamous NSCLC, suggesting the potential for targeted therapies against these molecular abnormalities. For example, Hammerman and colleagues reported discoidin domain receptor 2 (DDR2) mutations in approximately 4% of squamous NSCLC samples, which were associated with sensitivity to dasatinib (Sprycel®, Bristol-Myers Squibb; Princeton, NJ, US), a multi-target kinase inhibitor, in xenograft models [76]. An ongoing phase II trial is evaluating dasatinib in subjects with advanced cancers (including NSCLC) harboring DDR2 mutation (NCT01514864). PIK3CA mutations have been reported in approximately 7% of squamous NSCLC cell lines [77,78], and preclinical evidence suggests efficacy with PI3K pathway inhibitors in NSCLC tumors with PI3KCA mutations [79]. FGFR1 gene amplification has been observed in approximately 20% of patients with squamous NSCLC, which suggests that FGFR1 may represent a promising therapeutic target in this subset of patients [41,42]. The Cancer Genome Atlas (TCGA) project, which is the first comprehensive sequencing effort in squamous NSCLC to date, reported high mutation and genomic alteration rates in squamous NSCLC [80]. TP53 mutations were observed in more than 80% of squamous NSCLCs; other frequent genetic alterations included amplification of FGFR1, SOX2, and PDGFR- and mutated genes affecting PI3K signaling. The results from this comprehensive sequencing study may help to identify new targeted therapeutic strategies for the treatment of squamous NSCLC.
6. Conclusions
Given the survival statistics for advanced lung cancer, the development of more effective therapies for NSCLC, particularly patients with squamous histology, should be a key focus for clinical evaluation. Some advances in NSCLC have been made with the emergence of targeted therapies. However, because of safety concerns (eg, pulmonary hemorrhage with VEGF-targeted therapy) and a higher prevalence of relevant therapeutic targets in other histologies (eg, relatively low frequency of EGFR mutations in patients with squamous histology), patients with squamous histology have been excluded from many of these breakthroughs [3]. While EGFR mutations and ALK rearrangements are less common, other molecular abnormalities have been recently discovered in squamous NSCLC, including DDR2 mutations, PIK3CA mutations, and FGFR amplifications/mutations [81,82]. Moreover, the potential of angiogenesis as a therapeutic target in squamous NSCLC has been revisited with the evaluation of modified administration of therapies that were previously considered unsafe for patients with squamous histology. In addition, studies of preclinical models suggest that there is substantial cross-talk between VEGF signaling and other pathways involved in angiogenesis, suggesting that inhibition of several of these pathways at once may provide a more comprehensive blockade of angiogenesis in NSCLC. Some multi-targeted, antiangiogenic agents have shown preclinical and early clinical activity in NSCLC, and results are awaited for these agents specifically in squamous NSCLC.
Acknowledgments
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). Writing and editorial assistance was provided by Janetricks Chebukati, PhD, of MedErgy, which was contracted by BIPI for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The authors received no compensation related to the development of the manuscript.
Footnotes
Conflict of Interest Statement
Dr. Perez-Soler has served as a consultant/speaker for Genetech/Roche, Lilly, Pfizer, and Boehringer Ingelheim. Dr. Piperdi has served on Speaker’s Bureaus for Pfizer and Genentech/Roche and as an advisory/consultant for Genentech/Roche and Amgen. Dr. Merla has no potential conflicts of interest to disclose.
Contributor Information
Bilal Piperdi, Email: bpiperdi@montefiore.org, Associate Professor of Medicine, Montefiore Medical Center, 1300 Morris Park Avenue, Mazur Building, Room 616, Bronx, NY 10461, Telephone: (718) 904-2488, Fax: (718) 904-2830.
Amartej Merla, Montefiore Medical Center, 111 East 210th Street, Hofheimer Main, Room 100, Bronx, NY 10467.
Roman Perez-Soler, Professor, Department of Medicine; Chief, Division of Oncology, Montefiore Medical Center, 111 East 210th Street, Hofheimer Main, Room 100, Bronx, NY 10461.
References
- 1.American Cancer Society. Cancer Facts & Figures, 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. [Accessed 17 Jan 2013];Non-Small Cell Lung Cancer. V.2.2013. http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
- 4.American Cancer Society. Detailed Guide: Lung Cancer - Non-Small Cell. [Accessed 8 Jul 2010];What is non-small cell lung cancer? http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/lung-cancer--non-small-cell--non-small-cell-lung-cancer.
- 5.Morgensztern D, Waqar S, Subramanian J, et al. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–9. doi: 10.1097/JTO.0b013e3181ba3634. [DOI] [PubMed] [Google Scholar]
- 6.Gold KA, Wistuba II, Kim ES. New strategies in squamous cell carcinoma of the lung: identification of tumor drivers to personalize therapy. Clin Cancer Res. 2012;18:3002–7. doi: 10.1158/1078-0432.CCR-11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF, Sioussat TM, Brown LF, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275–8. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 10.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–73. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J, Klagsbrun M. Vascular physiology. A family of angiogenic peptides. Nature. 1987;329:671–2. doi: 10.1038/329671a0. [DOI] [PubMed] [Google Scholar]
- 12.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Lohela M, Bry M, Tammela T, et al. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–65. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 15.Blakely C, Jahan T. Emerging antiangiogenic therapies for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2011;11:1607–18. doi: 10.1586/era.11.146. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 17.Bonnesen B, Pappot H, Holmstav J, et al. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: relation to prognosis. Lung Cancer. 2009;66:314–8. doi: 10.1016/j.lungcan.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Donnem T, Al-Saad S, Al-Shibli K, et al. Co-expression of PDGF-B and VEGFR-3 strongly correlates with lymph node metastasis and poor survival in non-small-cell lung cancer. Ann Oncol. 2010;21:223–31. doi: 10.1093/annonc/mdp296. [DOI] [PubMed] [Google Scholar]
- 19.Ohta Y, Endo Y, Tanaka M, et al. Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res. 1996;2:1411–6. [PubMed] [Google Scholar]
- 20.Fontanini G, Vignati S, Boldrini L, et al. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861–5. [PubMed] [Google Scholar]
- 21.Imoto H, Osaki T, Taga S, et al. Vascular endothelial growth factor expression in non-small-cell lung cancer: prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115:1007–14. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 22.Fredriksson L, Li H, Fieber C, et al. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shikada Y, Yonemitsu Y, Koga T, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65:7241–8. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 24.McDermott U, Ames RY, Iafrate AJ, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–46. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Hiroi S, Torikata C. Expression in lung carcinomas of platelet-derived growth factor and its receptors. Lab Invest. 1997;77:431–6. [PubMed] [Google Scholar]
- 26.Donnem T, Al-Saad S, Al-Shibli K, et al. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008;3:963–70. doi: 10.1097/JTO.0b013e3181834f52. [DOI] [PubMed] [Google Scholar]
- 27.Andersen S, Donnem T, Al-Saad S, et al. Angiogenic markers show high prognostic impact on survival in marginally operable non-small cell lung cancer patients treated with adjuvant radiotherapy. J Thorac Oncol. 2009;4:463–71. doi: 10.1097/JTO.0b013e3181991d18. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–43. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 29.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–45. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 32.Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 33.Holopainen T, Huang H, Chen C, et al. Angiopoietin-1 overexpression modulates vascular endothelium to facilitate tumor cell dissemination and metastasis establishment. Cancer Res. 2009;69:4656–64. doi: 10.1158/0008-5472.CAN-08-4654. [DOI] [PubMed] [Google Scholar]
- 34.Takanami I. Overexpression of Ang-2 mRNA in non-small cell lung cancer: association with angiogenesis and poor prognosis. Oncol Rep. 2004;12:849–53. [PubMed] [Google Scholar]
- 35.Tanaka F, Ishikawa S, Yanagihara K, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62:7124–9. [PubMed] [Google Scholar]
- 36.Andersen S, Donnem T, Al-Shibli K, et al. Prognostic impacts of angiopoietins in NSCLC tumor cells and stroma: VEGF-A impact is strongly associated with Ang-2. PLoS One. 2011;6:e19773. doi: 10.1371/journal.pone.0019773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213. doi: 10.1042/BJ20101603. [DOI] [PubMed] [Google Scholar]
- 39.Marek L, Ware KE, Fritzsche A, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudek AZ, Mahaseth H. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Cancer Invest. 2005;23:193–200. doi: 10.1081/cnv-200055949. [DOI] [PubMed] [Google Scholar]
- 41.Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One. 2011;6:e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki H, Shitara M, Yokota K, et al. Increased FGFR1 copy number in lung squamous cell carcinomas. Mol Med Report. 2012;5:725–8. doi: 10.3892/mmr.2011.715. [DOI] [PubMed] [Google Scholar]
- 44.Slodkowska J, Sikora J, Roszkowski-Sliz K, et al. Expression of vascular endothelial growth factor and basic fibroblast growth factor receptors in lung cancer. Anal Quant Cytol Histol. 2000;22:398–402. [PubMed] [Google Scholar]
- 45.Brattstrom D, Bergqvist M, Hesselius P, et al. Elevated preoperative serum levels of angiogenic cytokines correlate to larger primary tumours and poorer survival in non-small cell lung cancer patients. Lung Cancer. 2002;37:57–63. doi: 10.1016/s0169-5002(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 46.Iwasaki A, Kuwahara M, Yoshinaga Y, et al. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eur J Cardiothorac Surg. 2004;25:443–8. doi: 10.1016/j.ejcts.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn H, Kopff C, Konrad J, et al. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer. 2004;44:167–74. doi: 10.1016/j.lungcan.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Fischer H, Taylor N, Allerstorfer S, et al. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408–19. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 50.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Avastin. AVASTIN. Vol. 2012 South San Francisco, CA: Genentech, Inc; 2012. ® (bevacizumab) Solution for intravenous infusion [package insert] [Google Scholar]
- 52.Schmidinger M, Bellmunt J. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev. 2010;36:416–24. doi: 10.1016/j.ctrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reck M, Barlesi F, Crino L, et al. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Ann Oncol. 2012;23:1111–20. doi: 10.1093/annonc/mdr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hainsworth JD, Fang L, Huang JE, et al. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol. 2011;6:109–14. doi: 10.1097/JTO.0b013e3181f94ad4. [DOI] [PubMed] [Google Scholar]
- 56.Wozniak AJ, Moon J, Thomas CR, et al. SWOG S0533: A pilot trial of cisplatin (C)/etoposide (E)/radiotherapy (RT) followed by consolidation docetaxel (D) and bevacizumab (B) (NSC-704865) in three cohorts of patients (pts) with inoperable locally advanced stage III non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl):Abstract 7018. [Google Scholar]
- 57.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol. 2012;30:3953–9. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 58.Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol. 2012;30:2829–36. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 59.Amgen. Independent data monitoring committee recommends resuming enrollment of non-squamous NSCLC patients in the motesanib MONET1 trial. http://www.amgen.com/media/media_pr_detail.jsp?year=2009&releaseID=1255738".
- 60.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–6. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–42. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 62.Gatzemeier U, Eisen T, Santoro A, et al. Sorafenib (S) + gemcitabine/cisplatin (GC) vs GC alone in the first-line treatment of advanced non-small cell lung cancer (NSCLC): Phase III NSCLC research experience utilizing sorafenib (NEXUS) trial. Ann Oncol. 2010;21(Suppl 8):viii7. [Google Scholar]
- 63.Heist RS, Wang XF, Hodgson L, et al. CALGB 30704: A randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl):Abstract 7513. doi: 10.1097/JTO.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horn L, Sandler AB. Emerging data with antiangiogenic therapies in early and advanced non-small-cell lung cancer. Clin Lung Cancer. 2009;10 (Suppl 1):S7–16. doi: 10.3816/CLC.2009.s.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol. 2012;30:2070–8. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 66.Lara PN, Jr, Douillard JY, Nakagawa K, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2965–71. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 67.Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27:3836–41. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 68.Giles FJ, Bellamy WT, Estrov Z, et al. The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) Leuk Res. 2006;30:801–11. doi: 10.1016/j.leukres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 69.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–82. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 70.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 71.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 72.Sloan B, Scheinfeld NS. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs. 2008;9:1324–35. [PubMed] [Google Scholar]
- 73.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doebele RC, Conkling P, Traynor AM, et al. A phase I, open-label dose-escalation study of continuous treatment with BIBF 1120 in combination with paclitaxel and carboplatin as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2012;23:2094–102. doi: 10.1093/annonc/mdr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reck M, Kaiser R, Mellemgaard A, et al. Nintendanib (BIBF 1120) + Docetaxel in NSCLC Patients Progressing after One Prior Chemotherapy Regimen: LUME-Lung 1, a Randomized, Double-blind, Phase III trial. Presented at: the 49th Annual Meeting of the American Society for Clinical Oncology; May 31 – June 4, 2013; Chicago, IL. 2013. [Google Scholar]
- 76.Hammerman PS, Sos ML, Ramos AH, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawano O, Sasaki H, Endo K, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54:209–15. doi: 10.1016/j.lungcan.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–21. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Arcangelo M, D’Incecco A, Cappuzzo F. Rare mutations in non-small-cell lung cancer. Future Oncol. 2013;9:699–711. doi: 10.2217/fon.13.16. [DOI] [PubMed] [Google Scholar]
- 82.Minuti G, D’Incecco A, Cappuzzo F. Targeted therapy for NSCLC with driver mutations. Expert Opin Biol Ther. 2013;13:1401–12. doi: 10.1517/14712598.2013.827657. [DOI] [PubMed] [Google Scholar]
- 83.Arbiser JL. Why targeted therapy hasn’t worked in advanced cancer. J Clin Invest. 2007;117:2762–5. doi: 10.1172/JCI33190. [DOI] [PMC free article] [PubMed] [Google Scholar]