Abstract
The benzoquinone ansamycins (BQAs) are a valuable class of antitumor agents that serve as inhibitors of heat shock protein (Hsp)-90. However, clinical use of BQAs has resulted in off-target toxicities, including concerns of hepatotoxicity. Mechanisms underlying the toxicity of quinones include their ability to redox cycle and/or arylate cellular nucleophiles. We have therefore designed 19-substituted BQAs to prevent glutathione conjugation and nonspecific interactions with protein thiols to minimize off-target effects and reduce hepatotoxicity. 19-Phenyl– and 19-methyl–substituted versions of geldanamycin and its derivatives, 17-allylamino-17-demethoxygeldanamycin and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), did not react with glutathione, whereas marked reactivity was observed using parent BQAs. Importantly, although 17-DMAG induced cell death in primary and cultured mouse hepatocytes, 19-phenyl and 19-methyl DMAG showed reduced toxicity, validating the overall approach. Furthermore, our data suggest that arylation reactions, rather than redox cycling, are a major mechanism contributing to BQA hepatotoxicity. 19-Phenyl BQAs inhibited purified Hsp90 in a NAD(P)H:quinone oxidoreductase 1 (NQO1)–dependent manner, demonstrating increased efficacy of the hydroquinone ansamycin relative to its parent quinone. Molecular modeling supported increased stability of the hydroquinone form of 19-phenyl-DMAG in the active site of human Hsp90. In human breast cancer cells, 19-phenyl BQAs induced growth inhibition also dependent upon metabolism via NQO1 with decreased expression of client proteins and compensatory induction of Hsp70. These data demonstrate that 19-substituted BQAs are unreactive with thiols, display reduced hepatotoxicity, and retain Hsp90 and growth-inhibitory activity in human breast cancer cells, although with diminished potency relative to parent BQAs.
Introduction
The 90-kDa heat shock protein (Hsp90) is an evolutionarily conserved molecular chaperone that functions to promote the conformational stabilization and activation of a wide subset of client proteins. Many of these proteins are essential in transducing proliferative and survival signals and adaptive responses to stress. In cancer cells, Hsp90 can serve as a molecular chaperone to prevent the misfolding or degradation of numerous overexpressed or mutated oncoproteins, including protein kinases, steroid receptors, and transcription factors. As a result, many cancers increasingly rely upon Hsp90 for growth, survival, and drug resistance (Whitesell and Lindquist, 2005). Inhibition of Hsp90 has attracted considerable interest in recent years as a potential therapeutic target for the development of a new generation of anticancer drugs that can block more than one cancer-causing pathway (Workman, 2004). Increased expression of Hsp90 is associated with disease progression in melanoma and diminished survival in breast, lung, and gastrointestinal stromal tumors (Normant et al., 2011). Thus, targeting Hsp90 may effectively treat numerous cancer types.
Hsp90 uses ATP hydrolysis to assist in the folding of client proteins to their mature, correctly folded forms (Pearl and Prodromou, 2006). Preventing Hsp90 from performing its chaperone function through the inhibition of ATP binding has been accomplished by a structurally diverse group of compounds. Of these compounds, the benzoquinone ansamycins (BQAs), including geldanamycin (GA), were the original class of compounds identified (Whitesell et al., 1994). However, in preclinical studies, GA demonstrated significant liver toxicity (Supko et al., 1995). Derivatives of GA, 17-allylamino-17-demethoxygeldanamycin (17-AAG), and 17-(dimethylaminoethylamino)-17-demethoxydeldanamycin (17-DMAG) have since emerged as candidate Hsp90 inhibitors. 17-AAG and 17-DMAG have progressed to phase I and phase II trials (Banerji et al., 2005; Modi et al., 2011; Pacey et al., 2011) and demonstrated activity in human epidermal growth factor receptor 2 (HER2)–positive, trastuzumab-refractory breast cancer (Modi et al., 2011). 17-AAG is poorly soluble and requires specialized vehicles for formulation and administration, so the considerably more water-soluble hydroquinone of 17-AAG (IPI-504) has been developed and is currently in clinical trials (Ge et al., 2006; Siegel et al., 2011). We have previously shown that hydroquinone ansamycins generated via NAD(P)H:quinone oxidoreductase 1 (NQO1) metabolism are more effective Hsp90 inhibitors than their respective parent quinones due to improved binding in the active site of Hsp90 (Guo et al., 2005).
Despite their clinical use, hepatotoxicity remains a problem with both 17-AAG and 17-DMAG. Hepatotoxicity of 17-AAG was found to be dose limiting in two separate phase I trials (Banerji et al., 2005; Solit et al., 2007), and, in the most recent phase II trial in advanced unresectable breast cancer, five patients developed grade 3/4 toxicities that were primarily hepatic and pulmonary. Based on these toxicity findings and lack of efficacy, 17-AAG was not recommended for further study for this indication (Gartner et al., 2012). 17-DMAG also demonstrated significant toxicities in phase I clinical trials, including hepatotoxicity as reflected by changes in liver function (Pacey et al., 2011). The toxicity of quinones, such as BQAs, arises from their ability to redox cycle and/or arylate cellular nucleophiles (Ross et al., 2000). These molecules are capable of both redox cycling to produce reactive oxygen species and reaction with thiols at the 19-substituent, leading to the formation of glutathione conjugates and adducts with cellular proteins (Guo et al., 2008). We have therefore designed 19-substituted BQAs (19BQAs) to prevent thiol reactivity as an approach to minimize off-target effects and reduce hepatotoxicity of this class of Hsp90 inhibitors. We have previously described the synthesis of 19BQAs, protein crystallography establishing that these new compounds bind to Hsp90 with a favored cis-amide conformation for inhibition, and preliminary evidence for Hsp90 inhibition in cellular systems (Kitson et al., 2013).
The aim of this study was to validate that novel 19BQAs did not react with thiols and to define their hepatotoxic potential relative to their parent unsubstituted quinones. 19-Substituted BQAs prevented nucleophilic attack of glutathione (GSH) and resulted in markedly reduced toxicity in freshly isolated hepatocytes, suggesting that arylation is a major mechanism of hepatotoxicity. Using three separate approaches, purified NQO1, human breast cancer cells isogenic for NQO1, and molecular modeling, we also demonstrated that 19BQAs inhibit Hsp90 in a NQO1-dependent manner, emphasizing the importance of the hydroquinone ansamycin. Finally, our work shows that the 19BQAs are effective Hsp90 inhibitors and induce growth-inhibitory effects in human breast cancer cells. When combined with diminished off-target toxicity, these novel 19BQA compounds may have a greater therapeutic window than their parent quinones.
Materials and Methods
Cell Culture and Reagents.
17-AAG, GA, and 17-DMAG were obtained from LC Laboratories (Woburn, MA); 19-phenyl 17-AAG, 19-phenyl GA, 19-phenyl 17-DMAG, 19-methyl GA, 19-methyl 17-AAG, and 19-methyl 17-DMAG were synthesized, as described (Kitson et al., 2013; Kitson and Moody, 2013), along with ES936 [5-methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione] (Guo et al., 2005). NADH, NADPH, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], bovine serum albumin, Percoll, and mouse anti–β-actin antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Yeast Hsp90 was obtained from Enzo Life Sciences (Farmingdale, NY). Recombinant human NQO1 (rhNQO1) was purified from Escherichia coli, as described previously (Beall et al., 1994). The activity of rhNQO1 was 4.5 μmol dichlorophenolindophenol/min/mg protein (Siegel et al., 2007). Malachite green phosphate assay kit was obtained from BioAssay Systems (Hayward, CA). Mouse anti-Hsp70, rabbit anti-Hsp90 antibody, and rabbit anti–Raf-1 antibodies were obtained from Enzo (Farmingdale, NY). Rabbit anti-Akt was from Cell Signaling Technology (Beverly, MA). Purified mouse and human liver microsomes were purchased from BD Biosciences (San Jose, CA).
Cell Lines.
The human breast cancer cell lines MDA468 and BT474 were obtained from American Type Culture Collection (Manassas, VA). Parental MDA468 cells are NQO1 null due to homozygous expression of the NQO1*2 polymorphism, whereas the MDA468/NQ16 cell line was stably transfected with wild-type NQO1 (Dehn et al., 2004). Cells were grown in a humidified incubator at 37°C with 5% CO2 in RPMI 1640 containing 10% (v/v) fetal bovine serum, (100 U/ml) penicillin, (100 µg/ml) streptomycin, and 4 mM glutamine. The murine TAMH hepatocyte cell line was provided by C. Franklin (Skaggs School of Pharmacy, University of Colorado) and was maintained as described (Wu et al., 1994).
Polarographic Studies.
Oxygen consumption was measured using a Clark electrode following the addition of BQAs to NADPH- or NADH-supplemented human or mouse liver microsomes. Reactions (3 ml) were performed at 37°C in 50 mM potassium phosphate buffer (pH 7.4) containing 1 mg/ml bovine serum albumin, human or mouse liver microsomes (0.4 mg), and 50 µM BQA. Reactions were started by the addition of 0.5 mM NADH or NADPH, and oxygen consumption was measured over 5 minutes. Baseline oxygen consumption rates were determined following addition of dimethylsulfoxide (DMSO) and substracted from measured rates with BQAs.
Reactions of BQAs with Glutathione.
The conjugation of BQAs by glutathione was measured by high-performance liquid chromatography (HPLC) following the incubation of BQAs with GSH in 50 mM potassium phosphate (pH 7.4) at 27°C. At the indicated times, reactions (1 ml) were stopped by the addition of acetic acid (15 µl) and directly analyzed by HPLC. Samples (50 µl) were separated at room temperature using a linear gradient (10–85% buffer B over 12 minutes) on a reverse-phase C18 (Luna II) column (5 µm, 4.6 × 250 mm; Phenomenex, Torrance, CA) using a flow rate of 1 ml/min and a detection wavelength of 270 nm. For HPLC analysis of GA and 17-AAG, the buffer system was buffer A, 0.1% (v/v) trifluoroacetic acid, and buffer B, acetonitrile, whereas for studies of 17-DMAG, 50 mM ammonium acetate was used in place of 0.1% trifluoroacetic acid as buffer A.
Primary Mouse Hepatocyte Isolation and Liver Toxicity Assays.
Male C57BL/6 mice (8–10 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) and kept in the Center of Laboratory Animal Care at the University of Colorado Denver for 1 week before sacrificing. All animal experiments were performed according to guidelines from the University of Colorado Denver Institutional Animal Care and Use Committee. Hepatocytes were isolated, as previously described (Cheng et al., 2010; Yin et al., 2010), by collagenase perfusion, gravity sedimentations, and a final Percoll density gradient centrifuge step. The yield of hepatocytes was, on average, 1 × 107 cells per liver with viability greater than 90%. Hepatocytes were plated in Williams’ medium E supplemented with 5% FBS, 20 mM HEPES buffer with 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Hepatocytes were seeded in 96-well plate at 1 × 104 cells/well and allowed to adhere for 2 hours before treatment with BQAs. Cells were exposed to 17-DMAG or 19-phenyl and 19-methyl derivatives (range from 0 to 100 μM) in Williams’ medium E for 24 hours. At the end of the incubation, a 10-µl aliquot of media was taken from each well to assess cytotoxicity via release of aspartate aminotransferase (AST). AST activity was measured using an assay kit (Teco Diagnostics, Anaheim, CA). Viability was also assessed using reduction of MTT to formazan (Guo et al., 2005, 2006).
TAMH cells were seeded in 96-well plate at 1 × 104 cells/well and allowed to adhere overnight. Cells were exposed to 17-DMAG or 19-phenyl and 19-methyl derivatives (range from 0 to 100 µM) in complete Dulbecco’s modified Eagle’s medium/F12 media for 72 hours. At the end of incubation, BQAs were removed and viability was assessed using MTT assay and compared with cells at time zero to determine median lethal concentration (LC50) concentrations.
Heat Shock Protein 90 ATPase Activity Assay.
Inhibition of Hsp90 ATPase activity was measured, as described previously (Guo et al., 2005). Briefly, purified yeast Hsp90 (2.5 μg) was incubated in 100 mM Tris-HCl (pH 7.4) containing 20 mM KCl, 6 mM MgCl2, 400 μM NADH, BQAs, or 19BQAs with or without 3.3 μg rhNQO1. Reactions (25 μl) were started by the addition of 1 mM ATP and allowed to proceed at 37°C for 3 hours. Reactions were then diluted with 225 μl 100 mmol/l Tri-HCl (pH 7.4) containing 20 mM KCl and 6 mM MgCl2 mixed thoroughly, and 80 μl was transferred to wells of a 96-well plate, followed by 20 μl malachite green reagent. After 10 minutes, trisodium citrate (83 mM) was added to stabilize the color, and plates were read at 650 nm.
Computational-Based Molecular Modeling.
All molecular modeling studies were conducted using Accelrys Discovery Studio 3.1 (Accelrys Software, San Diego, CA). The structural coordinates for human Hsp90 (Sreeramulu et al., 2009) were obtained from the Protein Data Bank (PDB ID 2K5B). The flexible docking algorithm (Koska et al., 2008), which allows for flexibility in both the ligand and the receptor, was used to predict the binding orientation of the quinone and hydroquinone forms of 19-phenyl-DMAG (19Ph-DMAG) within the ATP binding site of Hsp90. Hsp90-ligand complexes underwent energy minimization in situ using the conjugate gradient method (10,000 iterations with a root mean square cutoff of 0.01 kcal/mol). The following residue side chains were designated as flexible in the calculations: Glu47, Asn51, Asp54, Lys58, Asp93, Met98, Asn106, Leu107, Lys112, Phe138, and Thr184. Ligand-binding energies were calculated using the Poisson-Boltzmann implicit solvent model (Feig and Brooks, 2004).
Growth Inhibition Assay.
Growth inhibition in human breast cancer cell lines was measured using MTT assay, as described previously (Guo et al., 2005). Briefly, cells were seeded at 2 × 103 per well (96-well plate) in complete RPMI 1640 medium overnight. The next morning, the cells were treated with 19-phenyl BQAs, 19-methyl BQAs, or BQAs for 4 hours, after which cells were rinsed free of drug and incubated in fresh medium for an additional 72 hours.
Immunoblot Analysis.
MDA468 and MDA468/NQ16 cells were grown in 100-mm plates in complete RPMI 1640 medium to approximately 70% confluency. Cells were treated with DMSO, 19BQAs, or BQAs (1–5 μM) in 10 ml complete medium for 24 hours. Following drug treatment, cells were washed in PBS and then lysed by the addition of radioimmunoprecipitation assay lysis buffer (Boston Bioproducts, Ashland, MA) containing protease and phosphatase inhibitors and collected via scraping. Lysates were probe sonicated (10 seconds) on ice and then centrifuged at 13,000g for 5 minutes at 4°C to remove cellular debris. Protein concentration was determined on supernatant by the method of Lowry et al. (1951). Samples were heated to 90°C in 2× Laemmli buffer, and protein (20 μg) was separated by 12% SDS-PAGE (precast minigel; Bio-Rad, Hercules, CA) and then transferred to 0.4-μm polyvinylidene difluoride membranes. Membranes were blocked in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.2% (v/v) Tween 20, and 5% (w/v) nonfat milk for a minimum of 1 hour at room temperature. Anti-Hsp70, anti–Raf-1, and anti-Akt antibodies were incubated overnight at 4°C. All primary antibodies were diluted 1:1000, except β-actin (1:10,000). Horseradish peroxidase–labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were diluted 1:10,000 and added for 60 minutes. Proteins were visualized using enhanced chemiluminescence detection. Quantitation of immunoblots from three independent experiments was performed using Adobe Photoshop 7.0.
Statistical Analysis.
Statistical significance was determined using Prism 5.0 software (GraphPad Software, San Diego, CA). Two-way analysis of variance with concentration-group interaction was used for multiple comparisons with the application of SPSS v19.0 (SPSS, Chicago, IL). Statistical significance was defined as P < 0.05.
Results
19BQAs Do Not React with Glutathione.
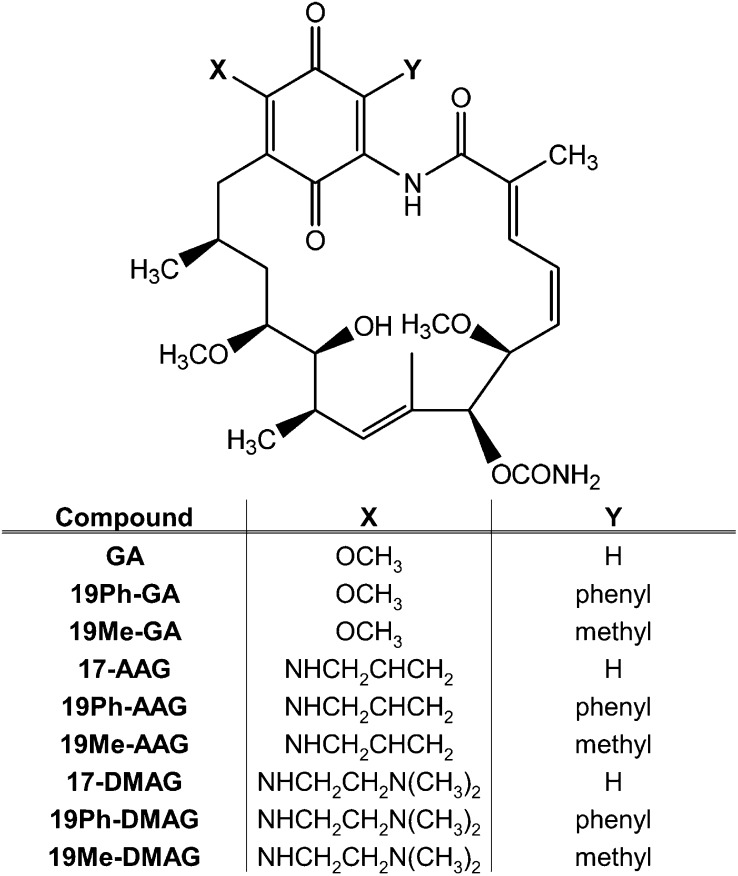
19-Substituted BQAs (Fig. 1) were synthesized, as described previously (Kitson et al., 2013), to remove the potential for arylation of cellular thiols and proteins. We initially used 19-phenyl– and 19-methyl–substituted BQAs in these studies to provide proof of principle for this class of agents. Previously, we had examined the reactivity of 19BQAs using a model thiol, N-acetylcysteine methyl ester with basic conditions, tetrahydrofuran, and elevated temperatures (Kitson et al., 2013). The current experiments were performed using the biologic thiol glutathione under physiologic conditions. The reactivity of GA, 17-AAG, and 17-DMAG and their 19-substituted derivatives with glutathione was measured by HPLC. The parent quinones (GA, 17-AAG, and 17-DMAG) readily formed glutathione adducts in the order of GA > 17-DMAG > 17-AAG, as we and others have previously reported (Cysyk et al., 2006; Guo et al., 2008). However, 19-phenyl– and 19-methyl–substituted compounds of all three BQA series did not react with GSH (Fig. 2), thus validating the rationale for their synthesis.
Fig. 1.
Chemical structures of BQAs and 19-substituted BQAs.
Fig. 2.
19-Substituted BQAs prevents GSH conjugation. HPLC analysis of the formation of BQA- and 19BQA-glutathione conjugates. Briefly, 50 μM BQAs, 19Ph-BQAs or 19Me-BQAs, and 5 mM glutathione were incubated in 50 mM potassium phosphate buffer (pH 7.4, 1 ml) at room temperature for 15 minutes (GA series), 3 hours (17-DMAG series), or 16 hours (17-AAG series). Following incubation, concentrations of unconjugated BQA and 19BQAs were measured by HPLC at 270 nm. Data are expressed as mean ± S.E.M. (n = 3) of remaining BQA or 19BQA concentration following incubation. Solid bars represent samples run in the absence of minus glutathione, whereas dashed bars represent sample run in the presence of glutathione. *P < 0.05 versus corresponding sample with no glutathione (two-tailed t test).
19BQAs Redox-Cycling Rates Compared with Parent-Unsubstituted Quinones.
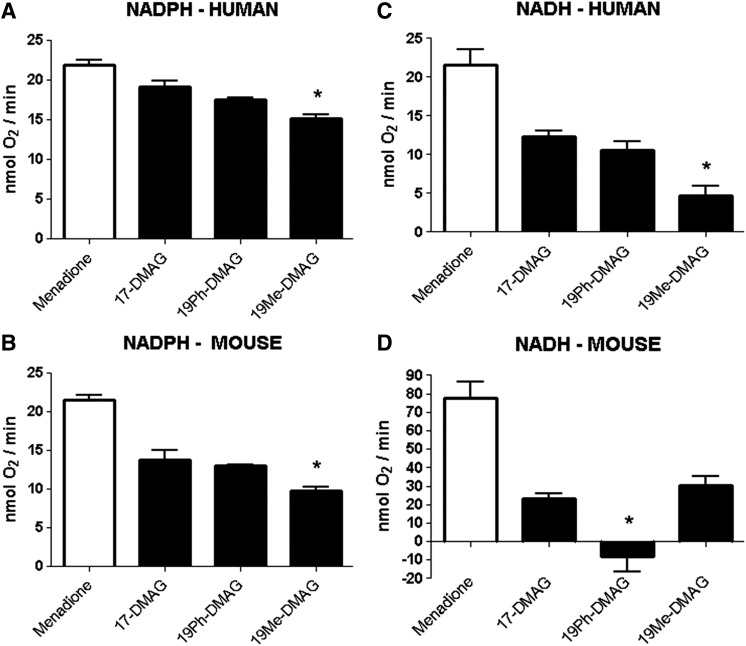
We have shown previously that the BQAs in general have relatively low rates of redox cycling (Guo et al., 2008), but in the current study we have compared the relative rates of redox cycling of 19Ph- and 19-methyl-DMAG (19Me-DMAG) with the parent quinone, 17-DMAG. NADH or NADPH was used as a cofactor to provide reducing equivalents for one-electron reductases present in liver microsomes. The rates of oxygen consumption were measured as indicators of redox-cycling rates of the studied compounds (Fig. 3, A–D). In general, 19-phenyl substitution of 17-DMAG showed no difference in redox-cycling rates relative to parent quinone, whereas 19Me-DMAG showed a significant decrease in rate. This was observed in NADH- and NADPH-supplemented human and NADPH-supplemented mouse liver microsomes. However, in NADH-dependent mouse liver microsomes, 19Me-DMAG consumed oxygen at a comparable rate to 17-DMAG, and 19Ph-DMAG showed less oxygen consumption than DMSO controls (Fig. 3D).
Fig. 3.
Redox cycling of BQAs and 19BQAs by mouse and human liver microsomes. The redox-cycling rates of BQAs and 19BQAs were determined in human (A and C) and mouse (B and D) liver microsomes supplied with NADH or NADPH by measuring the rates of oxygen consumption, as described in Materials and Methods. The naphthoquinone menadione was used as a positive control in these experiments. Data are expressed as mean ± S.E.M. (n = 3). *P < 0.05 versus parent BQA for each 19BQA (one-way analysis of variance with Tukey’s post-test).
19-Substituted BQAs Reduce Hepatotoxicity.
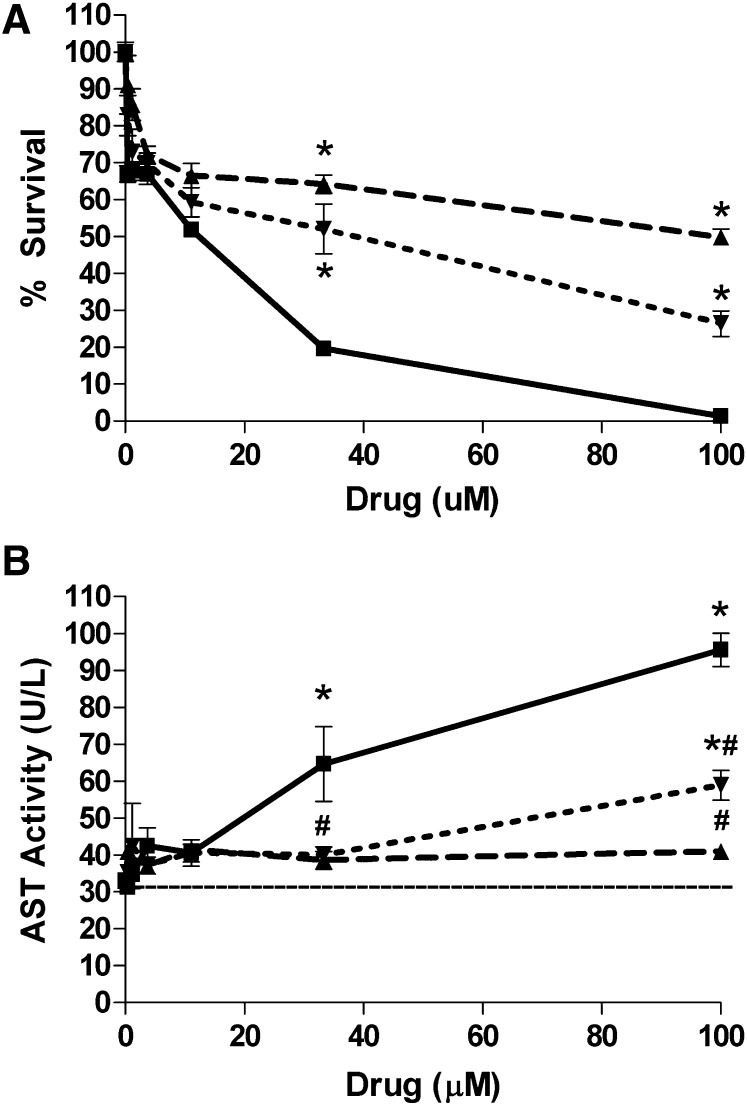
Further studies were conducted with 19-substituted derivatives of 17-DMAG to evaluate hepatotoxicity of these compounds compared with the parent quinone. 17-DMAG exhibited dose-dependent toxicity in freshly isolated mouse hepatocytes (Fig. 4). However, 19Ph-DMAG and 19Me-DMAG showed reduced hepatotoxicity compared with the unsubstituted parent quinone in terms of cell survival assessed using the MTT assay (Fig. 4A) and cytotoxicity via AST release (Fig. 4B). These results were confirmed in the mouse TAMH hepatocyte cell line: 17-DMAG was considerably more potent than 19Ph-DMAG and 19Me-DMAG in terms of growth inhibition and cell death (Table 1; Supplemental Fig. 1). Specifically, IC50 and LC50 concentrations in TAMH cells were decreased 230 and 65 times, respectively, with 19Ph-DMAG compared with the parent compound. 19Me-DMAG showed the least toxicity in TAMH cells with IC50 and LC50 concentrations 400 and 203 times lower, respectively, than 17-DMAG. The fact that 19 substitution of 17-DMAG precludes GSH conjugation, yet shows no consistent relationship between toxicity and rates of redox cycling and reduces damage to liver cells, suggests that arylation reactions are a major contributing mechanism to the hepatotoxicity of BQAs.
Fig. 4.
19-Substituted BQAs show reduced hepatotoxicity compared with parent quinones. Hepatocytes were isolated from naive male C57BL/6 mice and cultured in 96-well plates (1 × 104 cells/well). The cells were treated with increasing concentrations of 17-DMAG (▪), 19Ph-DMAG (▼), or 19Me-DMAG (▲) for 24 hours. DMSO-treated cells served as control. Cell viability (A) and cytotoxicity (B) were determined using MTT and AST release assays, respectively, as described in Materials and Methods. Data are expressed as mean ± S.E.M. (n = 4–8). Dashed line in B represents AST activity in DMSO-treated cells. *P < 0.05 versus untreated (DMSO) control; #P < 0.05 versus 17-DMAG at same concentration (one-way analysis of variance with Tukey’s post-test).
TABLE 1.
Hepatotoxicity of Hsp90 inhibitors in the TAMH cell line
Data expressed as mean ± S.D. of three independent experiments.
| Compound | IC50 | LC50 |
|---|---|---|
| µM | ||
| 17-DMAG | 0.02 ± 0.01 | 0.21 ± 0.01 |
| 19Ph-DMAG | 4.61 ± 0.39 | 13.83 ± 3.57 |
| 19Me-DMAG | 8.00 ± 0.37 | 42.67 ± 0.16 |
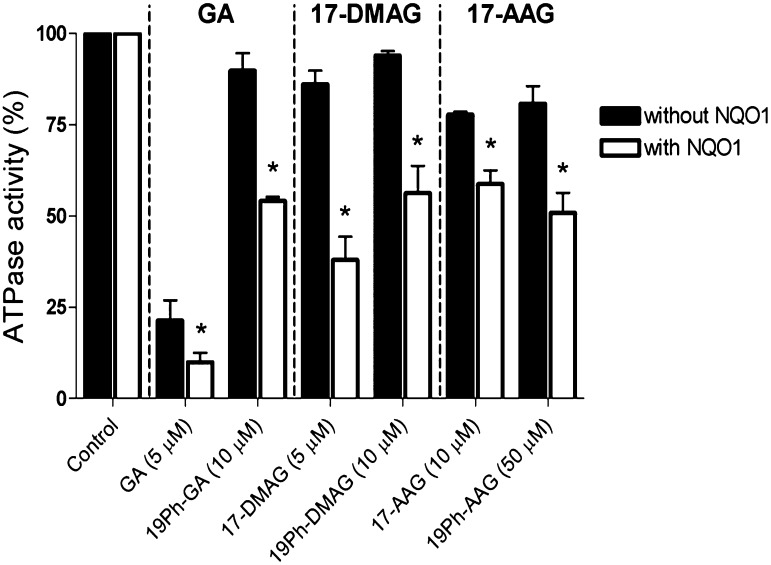
Inhibition of Hsp90 by 19BQAs Is NQO1 Dependent.
We assessed the ability of 19BQAs to inhibit purified yeast Hsp90 ATPase activity using the malachite green assay. Previous data demonstrated that the Hsp90 inhibition induced by GA, 17-AAG, and 17-DMAG was dependent upon metabolism by NQO1, consistent with the greater inhibitory activity of the hydroquinone relative to the parent quinone (Guo et al., 2005). These data were confirmed and extended to 19BQAs. Hsp90 ATPase activity was significantly decreased with both 19-phenyl BQAs and BQAs in the presence of NQO1 (Fig. 5). Similar data were obtained using 19-methyl BQAs (Supplemental Fig. 2).
Fig. 5.
Inhibition of yeast Hsp90 ATPase activity by BQAs and 19-phenyl BQAs. Yeast Hsp90 ATPase activity was measured in reactions with either vehicle (DMSO), BQAs, or 19BQAs at indicated concentrations in the presence or absence of rhNQO1. The reactions were analyzed after 3 hours, and phosphate concentrations were measured using the malachite green assay. Solid bars represent samples run in absence of rhNQO1, whereas open bars represent samples run in presence of rhNQO1. Data are expressed as mean ± S.E.M. (n = 3). *P < 0.05 versus corresponding sample without rhNQO1 (two-tailed t test).
In silico molecular docking of 19Ph-DMAG into the active site of human Hsp90 supported the in vitro results. Although the binding orientations of both the 19-phenyl-quinone (Data Supplement: 2K5B&19phquinone) and 19-phenyl–hydroquinone (Data Supplement: 2K5B&19phhydroquinone) were not radically different, the hydroquinone form was predicted to form additional hydrogen bond interactions with Hsp90 compared with the parent quinone (Supplemental Fig. 3; Supplemental Table 1). The hydroquinone form is predicted to form two additional hydrogen bond interactions with Asp54 and one with Asp93, while maintaining all of those hydrogen bonds involving the parent quinone. This most likely plays a role both in the enhanced favorability of the predicted binding energy (−18.9 kcal/mol for the quinone versus −30.2 kcal/mol for the hydroquinone) and in the increased binding affinity observed in vitro.
19-Phenyl BQAs Induce Growth Inhibition with Molecular Biomarkers of Hsp90 Inhibition in Human Breast Cancer Cell Lines.
To examine the role of NQO1 on Hsp90 inhibition ability and cytotoxicity of 19-substituted BQAs in human breast cancer, we used the BT474 and MDA468 cell lines. The MDA468 cell line was selected because it has no detectable NQO1 catalytic activity due to homozygous expression of the NQO1*2 polymorphism. We have previously stably transfected the MDA468 cell line with a plasmid containing the human NQO1*1 coding region to generate an isogenic cell line (MDA468/NQ16) expressing a high-level NQO1 protein and activity (Dehn et al., 2004) (Supplemental Fig. 4).
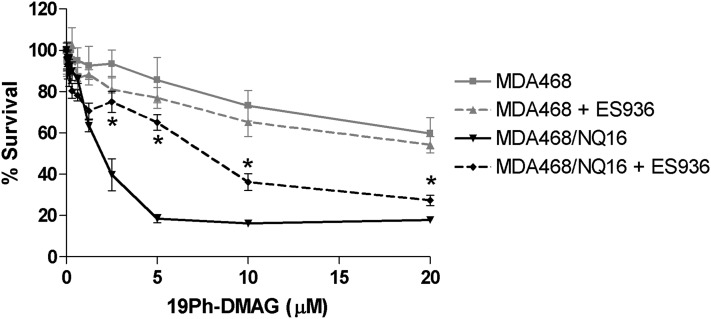
MDA468 or MDA468/NQ16 cells were treated with either 19-phenyl–substituted BQAs or parent BQAs for 4 hours. Cells were then washed free of drug, and growth inhibition was determined using the MTT assay after 72 hours. All 19-phenyl derivatives and parent quinones showed greater growth-inhibitory activity in NQO1-expressing MDA468/NQ16 cells, emphasizing the importance of the hydroquinone ansamycins to the Hsp90-inhibitory mechanism (Table 2). 19-Phenyl–substituted BQAs were also less potent than their respective parent quinones at inducing growth inhibition of MDA468/NQ16 cells. The IC50 values of all 19-phenyl derivatives and parent quinones in both MDA468 and MDA468/NQ16 cells are shown in Table 2 together with the fold increase in growth inhibition observed in cells containing high levels of NQO1. The role of NQO1 in growth inhibition induced by 17-DMAG and 19Ph-DMAG was further assessed in MDA468 and MDA468/NQ16 cells using the mechanism-based NQO1 inhibitor, ES936 (Guo et al., 2005). In these studies, cells were treated with either 17-DMAG or 19Ph-DMAG for 4 hours in the presence or absence of ES936 pretreatment. Two-way analysis of variance demonstrated that growth inhibition caused by 19Ph-DMAG was significantly decreased by pretreatment with ES936 in MDA468/NQ16 cells. In contrast, pretreatment with ES936 had no effect on 19Ph-DMAG–dependent growth inhibition in MDA468 cells (Fig. 6).
TABLE 2.
Growth inhibition induced by Hsp90 inhibitors in human breast cancer cell lines dependent upon NQO1 activity
Data expressed as mean ± S.D. of three independent experiments.
| Compound | IC50 |
IC50-Fold Difference | |
|---|---|---|---|
| MDA468 | MDA468/NQ16 | ||
| µM | |||
| GA | 0.06 ± 0.01 | 0.02 ± 0.01 | 3 |
| 19Ph-GA | 7.45 ± 0.51 | 1.19 ± 0.08 | 6 |
| 17-AAG | 10.05 ± 1.07 | 0.86 ± 0.16 | 11 |
| 19Ph-AAG | 88.37 ± 2.46 | 38.79 ± 10.90 | 2 |
| 17-DMAG | 0.61 ± 0.02 | 0.19 ± 0.02 | 3 |
| 19Ph-DMAG | 16.48 ± 2.14 | 3.28 ± 0.26 | 5 |
Fig. 6.
Effect of NQO1 inhibition on 19Ph-DMAG–dependent growth inhibition in human breast cancer cells. MDA468 cells lacking NQO1 activity or isogenic MDA468/NQ16 cells containing human NQO1 were treated with ES936 (100 nM) for 30 minutes to inhibit NQO1 activity prior to 19Ph-DMAG treatment. Growth inhibition with 4-hour 19Ph-DMAG treatment followed by 72-hour drug-free incubation was determined via MTT analysis. Data are expressed as mean ± S.E.M. (n = 4). *P < 0.05 versus same cell type with ES936 pretreatment (two-way analysis of variance with concentration-group interaction for multiple comparisons).
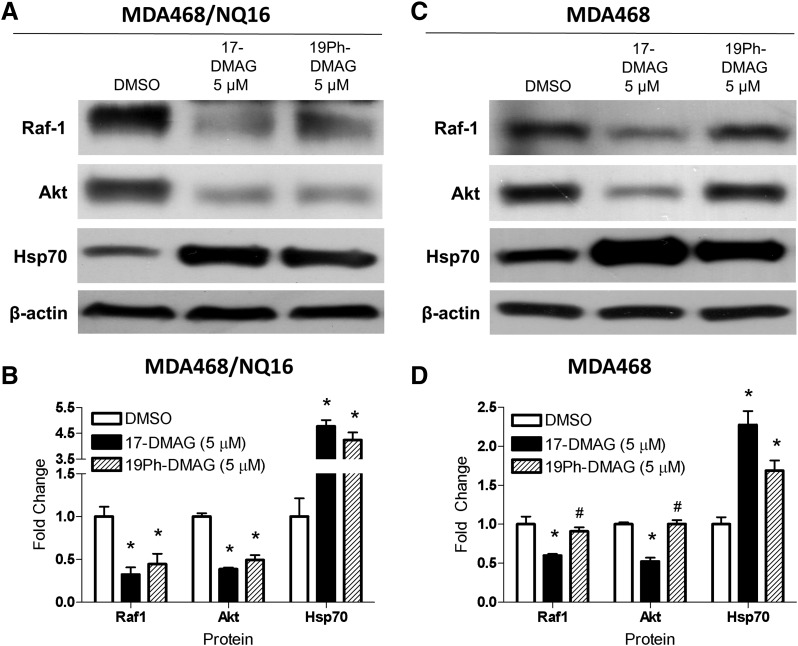
Expression levels of client proteins and Hsp70 were measured via immunoblot to confirm the molecular signature of cellular Hsp90 inhibition. Decreased levels of Hsp90 client proteins Raf-1 and Akt were observed with both 17-DMAG and 19Ph-DMAG in MDA468/NQ16 cells (Fig. 7, A and B). Raf-1 and Akt were also decreased with 17-DMAG in NQO1-deficient MDA468 cells but to a lesser degree compared with MDA468/NQ16 cells. 19Ph-DMAG had little effect on Hsp90 client protein in NQO1-deficient MDA468 cells (Fig. 7, C and D). A compensatory induction of Hsp70 was observed with both 17-DMAG and 19Ph-DMAG in MDA468/NQ16 and MDA468 cells. However, Hsp70 induction was more potent in MDA468/NQ16 cells, in which over 4-fold increase in expression was observed compared with only a 2.2- or 1.6-fold increase in MDA468 cells with 17-DMAG or 19Ph-DMAG, respectively. Although immunoblotting and MTT growth-inhibitory assays could not be performed under identical conditions due to cell number constraints, the NQO1-dependent anticancer activity of BQAs and 19BQAs was apparent in both situations. In general, 17-DMAG showed slightly greater effects on clients at equimolar concentrations than 19Ph-DMAG, consistent with the growth-inhibitory data. GA, 17-AAG, and their 19-phenyl derivatives had similar NQO1-dependent effects on client protein degradation and Hsp70 induction. Consistent with their increased growth-inhibitory capability, parent BQAs generally demonstrated greater potency (Supplemental Fig. 5).
Fig. 7.
Effect of 17-DMAG and 19Ph-DMAG on Hsp90 client proteins and heat shock protein induction in human breast cancer cells is NQO1 dependent. Immunoblot analysis of Hsp90 client protein degradation (Raf-1 and Akt) and Hsp70 compensatory response in MDA468/NQ16 (A) and MDA468 cells (C) treated with 17-DMAG or 19Ph-DMAG at 5 µM for 24 hours. DMSO-treated cells served as control. Blots are representative of three independent experiments. (B and D) Fold change of the indicated proteins is normalized to DMSO control and estimated by densitometry. Data are expressed as mean ± S.E.M. (n = 3). *P < 0.05 versus DMSO; #P < 0.05 versus 17-DMAG (one-way analysis of variance with Tukey’s post-test).
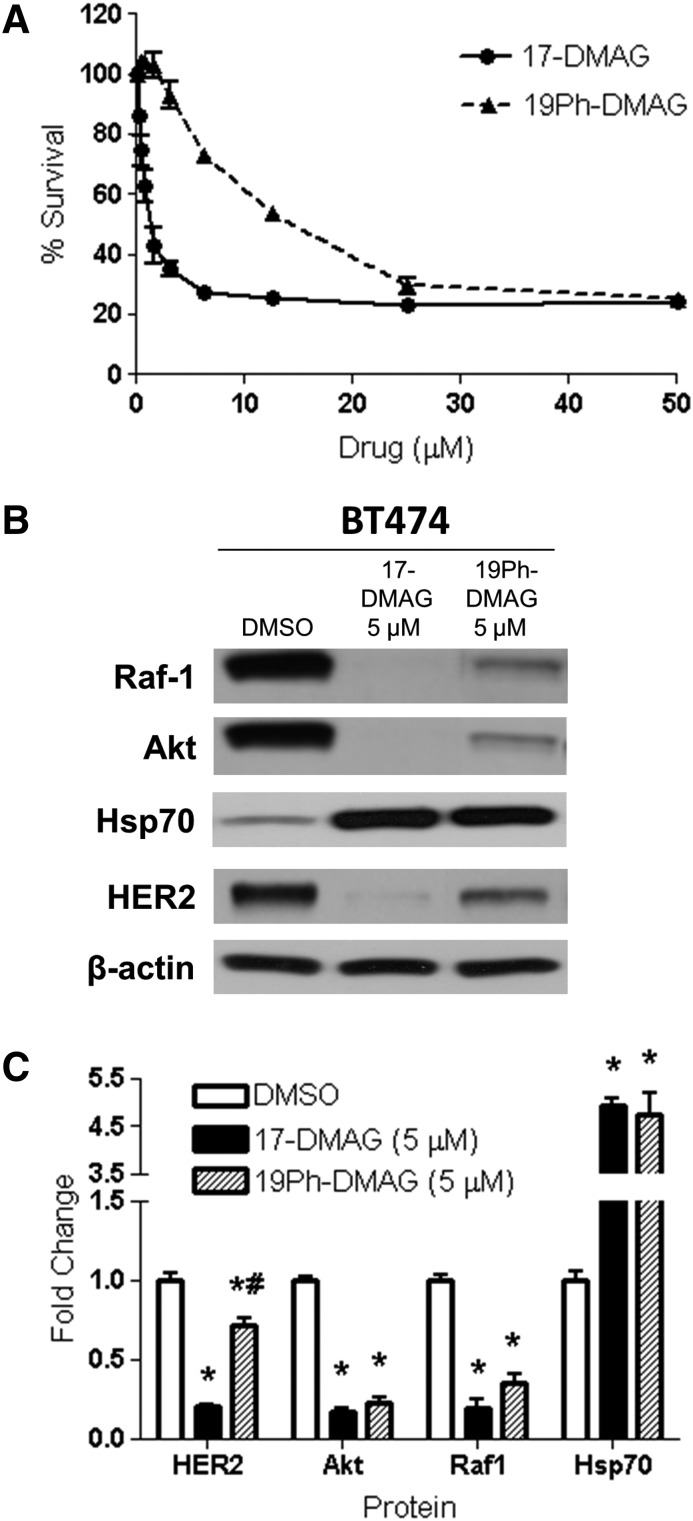
To extend our results to a HER2-positive human breast cancer cell line, we examined the effects of 19Ph-DMAG and 17-DMAG in BT474 cells. Both 19Ph-DMAG and 17-DMAG induced marked growth inhibition, with 17-DMAG demonstrating greater potency (Fig. 8A). These effects were reflected in decreased levels of Hsp90 client protein upregulation of Hsp70 expression with both compounds (Fig. 8, B and C). Changes in expression of Raf-1, Akt, and Hsp70 were similar with both 17-DMAG and 19Ph-DMAG, although 17-DMAG showed a significantly greater effect on HER2 degradation.
Fig. 8.
19Ph-DMAG inhibits growth in HER2-positive human breast cancer cells with molecular signatures of Hsp90 inhibition. (A) Growth inhibition following either 17-DMAG or 19Ph-DMAG treatment was measured by MTT analysis in BT474 cell lines. Data are expressed as mean ± S.E.M. (n = 3). (B) Immunoblot analysis of Hsp90 client proteins (HER2, Raf-1, and Akt) and Hsp70 compensatory response in BT474 cells treated with either 17-DMAG or 19Ph-DMAG at 5 µM for 24 hours. Blots are representative of three independent experiments. (C) Fold change of the indicated proteins is normalized to DMSO control and estimated by densitometry. Data are expressed as mean ± S.E.M. (n = 3) *P < 0.05 versus DMSO; #P < 0.05 versus 17-DMAG (one-way analysis of variance with Tukey’s post-test).
Discussion
Hsp90 is a protein chaperone that promotes the maturation and conformational stabilization of cellular proteins important in transducing proliferation and survival signaling. It has evolved as a promising anticancer target because, by blocking Hsp90, many client proteins that drive neoplasia can be simultaneously targeted, allowing a combinatorial anticancer blockade (Workman et al., 2007; Neckers and Workman, 2012). The BQA class of Hsp90 inhibitors has exhibited promising activity in phase I and phase II clinical trials, but their development has been hindered by toxicity concerns, particularly hepatotoxicity (Banerji et al., 2005; Solit et al., 2007; Pacey et al., 2011; Gartner et al., 2012). This has led to the development of Hsp90 inhibitors using nonquinone scaffolds, but these molecules may also be associated with their own characteristic toxicities (Rajan et al., 2011). In this work, we have explored an alternative route to optimizing therapy, employing Hsp90 inhibitors by examining mechanisms underlying the toxicity of the active class of BQA Hsp90 inhibitors and using chemical biology to minimize their toxicity and reduce off-target effects. The novel 19-substituted BQAs studied in this work showed reduced hepatotoxicity compared with their parent BQAs but retain Hsp90-inhibitory and anticancer activity in human breast cancer cells.
Quinones induce biologic toxicity as a function of their ability to arylate biologic nucleophiles and/or undergo redox-cycling reactions to generate reactive oxygen species. BQA Hsp90 inhibitors such as GA, 17-AAG, and 17-DMAG are known to interact with cellular thiols, including GSH, via addition at the electrophilic 19 position of the BQA molecule (Cysyk et al., 2006; Guo et al., 2008). To prevent the nucleophilic attack of thiols and other nucleophiles at the 19 position, we synthesized the 19-phenyl and 19-methyl derivatives of GA, 17-AAG, and 17-DMAG. The details of the synthesis of these novel 19-substituted compounds are provided elsewhere (Kitson et al., 2013). In this work, we used 19-phenyl and 19-methyl derivatives as proof of principle that the 19-substituted BQAs as a class have potential as Hsp90 inhibitors with diminished off-target toxicities and, consequently, a greater therapeutic index. In contrast to their parent BQAs, 19-substituted derivatives did not react with cellular thiols such as glutathione, which validated the rationale for their synthesis. Hepatotoxicity has been a significant problem with the BQA Hsp90 inhibitors from their initial development, as demonstrated by preclinical hepatotoxicity exhibited by GA up to the present day, with the most recent phase II trial of 17-AAG demonstrating significant hepatotoxicity (Gartner et al., 2012). To determine whether reduced reactivity with thiols translated to reduced hepatotoxicity, we used a model employing freshly isolated mouse hepatocytes. In this system, marked dose-dependent toxicity could be observed using 17-DMAG, but 19-phenyl or 19-methyl substitution showed reduced toxicity in mouse hepatocytes. This is an important demonstration of decreased off-target toxicity of the 19BQAs and is in agreement with previous data demonstrating lack of toxicity of these compounds in normal human umbilical vein endothelial cells (Kitson et al., 2013). Redox cycling can also contribute to quinone toxicity following one-electron reduction with subsequent production of reactive oxygen species. In liver, microsomal NADPH cytochrome P450 reductase and NADH cytochrome b5 reductase are the predominant one-electron reductases (Guo et al., 2008). Using mouse or human liver microsomes, we found no consistent relationship between toxicity and redox cycling of the 19-substituted DMAG series of compounds.
These data demonstrate that 19-substituted BQAs, which by design are incapable of arylation reactions, are markedly less hepatotoxic than their parent unsubstituted BQAs. Although this work still needs to be extended to in vivo studies, our mechanistic data suggest that arylation is a major mechanism responsible for hepatotoxicity. However, contributions of redox cycling to toxicity cannot be completely discounted because the studied compounds were still able to undergo one-electron reduction during liver microsome metabolism. This work indicates that the 19BQAs are less toxic than their parent quinones, and, if the Hsp90-inhibitory activity of this class of compounds can be maximized, they may have a greater therapeutic window than their unsubstituted parent compounds because of their inability to undergo arylation reactions.
We have previously defined the greater activity of the hydroquinone ansamycins toward inhibiting Hsp90 than their parent benzoquinone ansamycins. This is a result of a more favorable binding energy of the hydroquinone ansamycin in the Hsp90 active site, primarily due to increased hydrogen bonding (Guo et al., 2005). 19-Phenyl BQAs behaved in a similar fashion and retained their ability to inhibit purified Hsp90 in a NQO1-dependent manner, emphasizing the importance of the hydroquinone ansamycin in Hsp90 inhibition. The NQO1 dependence of 19-phenyl BQA–dependent Hsp90 inhibition translated to cellular studies, in which greater Hsp90 inhibition was observed in MDA468/NQ16 breast cancer cells with elevated NQO1 levels, relative to isogenic NQO1-deficient cells. Furthermore, the growth-inhibitory activity of 19Ph-DMAG could be abrogated using the NQO1 inhibitor ES936. 19-Phenyl BQAs caused degradation of Hsp90 client proteins, including Raf-1 and Akt and compensatory upregulation of Hsp70 to a greater extent in cells containing high NQO1 levels. However, growth-inhibitory potency of these first generation 19-phenyl–substituted compounds was not as great as their parent quinones. To verify these molecules also have potential in HER2-expressing breast cancer cells, we also examined reduction of Hsp90 clients, including HER2, induction of Hsp70, and growth-inhibitory effects in BT474 human breast cancer cells, and marked growth-inhibitory and Hsp90-inhibitory activity was observed with both 19Ph-DMAG and its parent quinone.
In summary, the limited series of 19BQAs that we have tested do not react with glutathione at the 19 position and exhibit reduced toxicity in primary mouse hepatocytes and a mouse hepatocyte cell line in comparison with their parent quinones. Furthermore, 19BQAs retain the ability to inhibit Hsp90 both in purified enzyme systems and in cellular systems. The development of 17-AAG and 17-DMAG as anticancer agents has recently been halted due to nonclinical reasons (Modi et al., 2011). This led to a pointed editorial asking why such active anticancer compounds, in trastuzamab-resistant breast cancer, for example, were no longer available (Arteaga, 2011). This leaves a void that novel, less toxic 19BQAs could conceivably fill. Clearly, optimization of the substituent at the 19 position needs to be performed for maximal inhibition of cellular Hsp90 and growth-inhibitory potency, but data obtained with prototype 19-phenyl BQA compounds to demonstrate proof of principle suggest that this class of molecule is worthy of further translational investigation.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of the Computational Chemistry and Biology Core Facility at the University of Colorado Anschutz Medical Campus.
Abbreviations
- 17-AAG
17-allylamino-17-demethoxygeldanamycin
- AST
aspartate aminotransferase
- BQA
benzoquinone ansamycin
- 19BQA
19-substituted BQA
- 17-DMAG
17-(dimethylaminoethylamino)-17-demethoxygeldanamycin
- 19Me-DMAG
19-methyl-DMAG
- 19Ph-DMAG
19-phenyl-DMAG
- DMSO
dimethylsulfoxide
- ES936
5-methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione
- GA
geldanamycin
- GSH
glutathione
- HER2
human epidermal growth factor receptor 2
- HPLC
high-performance liquid chromatography
- Hsp
heat shock protein
- LC50
median lethal concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NQO1
NAD(P)H:quinone oxidoreductase 1
- rhNQO1
recombinant human NQO1
Authorship Contributions
Participated in research design: Chang, Drechsel, Siegel, Moody, Ju, Ross.
Conducted experiments: Chang, Drechsel, Siegel, You, Backos.
Contributed new reagents or analytic tools: Kitson.
Performed data analysis: Chang, Drechsel, Siegel.
Wrote or contributed to the writing of the manuscript: Drechsel, Chang, Moody, Ross.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA51210] (to D.R.); and the Parkinson’s Disease Society UK (to C.J.M.).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Arteaga CL (2011) Why is this effective HSP90 inhibitor not being developed in HER2+ breast cancer? Clin Cancer Res 17:4919–4921. [DOI] [PMC free article] [PubMed]
- Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M et al. (2005) Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 23:4152–4161. [DOI] [PubMed]
- Beall HD, Mulcahy RT, Siegel D, Traver RD, Gibson NW, Ross D. (1994) Metabolism of bioreductive antitumor compounds by purified rat and human DT-diaphorases. Cancer Res 54:3196–3201 [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt MP, Ju C. (2010) Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem Pharmacol 80:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysyk RL, Parker RJ, Barchi JJ, Jr, Steeg PS, Hartman NR, Strong JM. (2006) Reaction of geldanamycin and C17-substituted analogues with glutathione: product identifications and pharmacological implications. Chem Res Toxicol 19:376–381 [DOI] [PubMed] [Google Scholar]
- Dehn DL, Winski SL, Ross D. (2004) Development of a new isogenic cell-xenograft system for evaluation of NAD(P)H:quinone oxidoreductase-directed antitumor quinones: evaluation of the activity of RH1. Clin Cancer Res 10:3147–3155 [DOI] [PubMed] [Google Scholar]
- Feig M, Brooks CL., 3rd (2004) Recent advances in the development and application of implicit solvent models in biomolecule simulations. Curr Opin Struct Biol 14:217–224 [DOI] [PubMed] [Google Scholar]
- Gartner EM, Silverman P, Simon M, Flaherty L, Abrams J, Ivy P, Lorusso PM. (2012) A phase II study of 17-allylamino-17-demethoxygeldanamycin in metastatic or locally advanced, unresectable breast cancer. Breast Cancer Res Treat 131:933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Normant E, Porter JR, Ali JA, Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J, et al. (2006) Design, synthesis, and biological evaluation of hydroquinone derivatives of 17-amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J Med Chem 49:4606–4615 [DOI] [PubMed] [Google Scholar]
- Guo W, Reigan P, Siegel D, Ross D. (2008) Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos 36:2050–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. (2005) Formation of 17-allylamino-demethoxygeldanamycin (17-AAG) hydroquinone by NAD(P)H:quinone oxidoreductase 1: role of 17-AAG hydroquinone in heat shock protein 90 inhibition. Cancer Res 65:10006–10015 [DOI] [PubMed] [Google Scholar]
- Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. (2006) The bioreduction of a series of benzoquinone ansamycins by NAD(P)H:quinone oxidoreductase 1 to more potent heat shock protein 90 inhibitors, the hydroquinone ansamycins. Mol Pharmacol 70:1194–1203 [DOI] [PubMed] [Google Scholar]
- Kitson RR, Chang CH, Xiong R, Williams HE, Davis AL, Lewis W, Dehn DL, Siegel D, Roe SM, Prodromou C, et al. (2013) Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat Chem 5:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson RR, Moody CJ. (2013) An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors–potential therapeutics in cancer and neurodegeneration. Chem Commun 49:8441–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koska J, Spassov VZ, Maynard AJ, Yan L, Austin N, Flook PK, Venkatachalam CM. (2008) Fully automated molecular mechanics based induced fit protein-ligand docking method. J Chem Inf Model 48:1965–1973 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S et al. (2011) HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 17:5132–5139. [DOI] [PubMed]
- Neckers L and Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76. [DOI] [PMC free article] [PubMed]
- Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, McDougall J, Wylie AA, Robison K, Caliri K, et al. (2011) The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene 30:2581–2586 [DOI] [PubMed] [Google Scholar]
- Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U, Roels B et al. (2011) A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res 17:1561–1570. [DOI] [PMC free article] [PubMed]
- Pearl LH, Prodromou C. (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem 75:271–294 [DOI] [PubMed] [Google Scholar]
- Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L et al. (2011) A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res 17:6831–6839. [DOI] [PMC free article] [PubMed]
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. (2000) NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129:77–97 [DOI] [PubMed] [Google Scholar]
- Siegel D, Jagannath S, Vesole DH, Borello I, Mazumder A, Mitsiades C, Goddard J, Dunbar J, Normant E, Adams J, et al. (2011) A phase 1 study of IPI-504 (retaspimycin hydrochloride) in patients with relapsed or relapsed and refractory multiple myeloma. Leuk Lymphoma 52:2308–2315 [DOI] [PubMed] [Google Scholar]
- Siegel D, Kepa JK, and Ross D (2007) Biochemical and genetic analysis of NAD(P)H:quinone oxidoreductase 1 (NQO1). Curr Protoc Toxicol Chapter 4:Unit 4.22. [DOI] [PubMed] [Google Scholar]
- Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, Kelly WK, DeLaCruz A, Curley T, Heller G et al. (2007) Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res 13:1775–1782. [DOI] [PMC free article] [PubMed]
- Sreeramulu S, Gande SL, Göbel M, Schwalbe H. (2009) Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37, a kinome chaperone-cochaperone, by triterpene celastrol. Angew Chem Int Ed Engl 48:5853–5855 [DOI] [PubMed] [Google Scholar]
- Supko JG, Hickman RL, Grever MR, Malspeis L. (1995) Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol 36:305–315 [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5:761–772 [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 91:8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P. (2004) Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett 206:149–157 [DOI] [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N. (2007) Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci 1113:202–216 [DOI] [PubMed] [Google Scholar]
- Wu JC, Merlino G, Cveklova K, Mosinger B, Jr, Fausto N. (1994) Autonomous growth in serum-free medium and production of hepatocellular carcinomas by differentiated hepatocyte lines that overexpress transforming growth factor alpha 1. Cancer Res 54:5964–5973 [PubMed] [Google Scholar]
- Yin H, Cheng L, Holt M, Hail N, Jr, Maclaren R, Ju C. (2010) Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology 51:1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.