Abstract
Efficiency of intracoronary (IC) adenoviral vector transfection is impaired by the vascular endothelium. Ischemia and substances that increase vascular permeability (sodium nitroprusside, nitroglycerin) may augment adenoviral vector transfection efficiency (TE). We tested whether TE of adenoviral vector following IC infusion is improved by nitrates or by ischemia. Fluoroscopically guided angioplasty balloon catheters occluded the coronary artery in Yorkshire pigs and delivered adenoviral type 5 vector encoding the luciferase gene (Ad5Luc, 1011 viral particles). TE (luciferase activity) was minimal and was not augmented by IC co-administration of 50 μg/min sodium nitroprusside to nonischemic myocardium. Two (but not one) 3-min episodes of occlusion tended to increase luciferase activity (p=0.06), and luciferase activity was further increased by IC co-administration of nitroglycerin (p<0.001). After 75 min of coronary artery occlusion, luciferase activity was greater than with shorter periods of ischemia, and was significantly greater in the ischemia-reperfused zone compared to the border zone 3 and 14 days after infusion; there was no transfection in nonischemic myocardium. IC delivery of Ad5Luc into post-ischemic myocardium caused no local inflammation or hemodynamic instability. We conclude that the uptake of IC Ad5 to ischemic reperfused myocardium validates use of IC Ad5 delivery protocols in future human gene therapy trials in patients following myocardial ischemia.
Shi and colleagues evaluate whether the transfection efficiency of adenovirus type 5 vector following intracoronary infusion in Yorkshire pigs can be improved either by increasing vascular permeability with nitrates or by ischemia. Transgene activity increased with two 3-minute periods of ischemia as well as with co-administration of nitroglycerin. Longer ischemic periods that result in myocardial infarction led to significantly greater transfection activity in the ischemia-reperfused area, at both 3 and 14 days after infusion.
Introduction
Intracoronary infusion of adenoviral gene therapy vectors provides homogenous gene transfer to the whole myocardium, but shows low transfection efficiency (TE; Katz et al., 2011). The barrier function of the vascular endothelium is one of the main contributors to low TE (Sasano et al., 2007). Several agents that increase vascular permeability have been shown to increase efficiency of gene transfer, such as sodium nitroprusside (SNP; Roth et al., 2004), nitroglycerin (NTG), vascular endothelial growth factor (VEGF; Nagata et al., 2001), substance P (Iwatate et al., 2003), and histamine (Greelish et al., 1999). In addition, ischemia reperfusion (I/R) also increases vascular permeability, thereby potentially increasing TE (Tilton et al., 1985, 1989). Low-level or transient ischemia increases the permeability/surface area ratio of the coronary vascular bed (Harris et al., 1984). A brief (1 min) episode of myocardial ischemia has been reported to increase adenoviral vector diffusion into the interstitial space and to the cardiomyocytes in a Langendorff perfusion model (Logeart et al., 2000). However, it is unknown whether I/R affects adenoviral type 5 (Ad5) gene transfer in vivo. The present studies reported here were designed to enhance TE to expand and improve the results seen in the Angiogenic Gene Therapy (AGENT) trials in some subgroups of patients (Henry et al., 2007). Using a closed-chest pig model of intracoronary (IC) infusion and angioplasty balloon-induced coronary artery occlusion, this study tested the hypothesis that nitrates (SNP or NTG) or incremental periods of I/R increase TE following IC administration of adenovirus type 5 encoding the luciferase reporter gene (Ad5Luc). Clinically applicable 3-min occlusion periods, which do not cause myocardial damage but may increase vascular permeability, and 75-min left anterior descending coronary artery (LAD) occlusion/reperfusion, which leads to myocardial infarction, were used as the ischemic intervals.
Materials and Methods
Viral vector preparation
The test article Ad5Luc was provided by Tissue Repair Company (Cardium Therapeutics, Inc., San Diego, CA) and contained a replication incompetent recombinant human Ad5. The vector encoded a reporter gene expressing luciferase (Luc) using a cytomegalovirus promoter. The Ad5Luc vials contained 1 ml of Ad5Luc at a concentration of 1011 viral particles (vp)/ml in GTS buffer (2.5% glycerol, 25 mM NaCl, 20 mM Tris buffer, pH 8.0), and were kept frozen at −70°C. The infectious titer of Ad5Luc preparation was 6.8×1010 plaque-forming units/ml (as determined by the standard plaque-forming assay). The frozen test articles were thawed at room temperature no longer than 30 min prior to dilution and administration. We used aliquots from the same lot for all the studies described. The appropriate amount of Ad5Luc was diluted with 0.9% sterile normal saline USP to a final volume of 5 ml, immediately prior to administration. The Ad5Luc vector was infused into the LAD and/or into the left circumflex coronary artery (LCx) in several different protocols via the distal lumen of a coronary catheter (UltraFuse-X, Boston Scientific, Natick, MA) at a rate of 2 ml/min using a dual syringe pump (Harvard Apparatus, Holliston, MA). When vector infusion was complete, the catheter system was manually flushed with 2 ml of saline over 1 min to flush the remaining virus from the dead space of the catheter infusion system.
Surgical preparation
The experimental procedures were performed in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH publication No. 85-23, revised 1996) and were approved by The Institutional Animal Care and Use Committee of Emory University. Yorkshire cross female domestic pigs (25–35 kg) were selected based on the well-established model of myocardial infarction (Halkos et al., 2008) and previous experience with adenovector DNA delivery to the myocardium (French et al., 1994).
Animals were premedicated with an intramuscular injection of a cocktail containing ketamine (22 mg/kg), acepromazine (1.1 mg/kg), and atropine (0.05 mg/kg). Cefazolin (25 mg/kg) and aspirin (325 mg) were administered intravenously. Anesthesia was induced with an intravenous injection of thiopental (10 mg/kg) and maintained by continuous inhalation of isoflurane (1%–2%). Ventilatory rates were adjusted to maintain pCO2 between 30 and 40 mm Hg; pO2 greater than 100 mm Hg; arterial blood pH was maintained between 7.35–7.45. If unable to counteract acidemia by changing respiratory settings, acidemia was treated with sodium bicarbonate (2–10 ml; 8.4% solution). Arterial blood gases were taken periodically to ensure physiological status. Surface EKG lead II was used to continuously measure electrocardiographic data. Hemodynamic data were collected by Emka Technologies software (Falls Church, VA).
Cardiac catheterization
Under sterile conditions, both femoral arteries were isolated via groin cut-downs for the insertion of 8F sheaths. Animals were heparinized (200 U/kg) for anticoagulation. Angiography was used to evaluate coronary anatomy and measure the diameter of the LAD and LCx at the intended site of balloon placement. Amiodarone (8 mg/kg, Bioniche Pharma USA, Lake Forest, IL) was administered intravenously over 30–40 minutes before coronary artery occlusion. An appropriately sized angioplasty balloon catheter (3.0–4.0 mm, Ultrafuse-X, Boston Scientific, Natick, MA) was selected based on the arterial diameter measurements determined by contrast angiography with a camera angle of 30° left anterior oblique, and was advanced into the proximal LAD or LCx at the level of the first or second branch. In coronary artery occlusion studies (Protocols 2 and 3), the balloon was inflated to 6–8 atm based on the catheter's nominal inflation chart. Complete coronary artery occlusion and position of the balloon were confirmed by angiography. Lidocaine (80–160 mg total, Hospira, Lake Forest, IL) was administered during coronary artery occlusion for prophylaxis of ventricular arrhythmias. Episodes of ventricular fibrillation were immediately treated with electrical cardioversion delivered at 200 J (Hewlett-Packard, Andover, MA).
Experimental protocols
The studies were conducted in a randomized, blinded, and placebo-controlled manner. Protocol 1 was designed to evaluate the uptake of Ad5Luc in nonischemic myocardium with or without SNP. Eight animals were equally divided into two groups (saline treated and SNP treated). An IC infusion of saline (control) or SNP (50 μg/min) into the LAD by a second lumen of the coronary catheter was started 3 min before vector infusion. Ad5Luc (total dose 10 11 vp) was infused through the central perfusion lumen of the coronary catheter. SNP administration was continued for the duration of Ad5Luc infusion. Luciferase activity (LA) in myocardial tissues was evaluated on day 3 after Ad5Luc infusion.
In protocol 2, four groups (four animals in each group) were assigned to examine if short-term ischemia would increase TE after 3 days of reperfusion when Ad5luc infusion (total dose 10 11 vp) was given as follows: Group 2A, 1 min after a single cycle of 3-min coronary arterial occlusion (immediate infusion group); Group 2B, 8 min after a single 3-min coronary artery occlusion (delayed infusion group); Group 2C, two cycles of 3-min coronary artery occlusion separated by 5 min of reperfusion (Ad5Luc was infused starting 1 min after the beginning of the second 3-minute occlusion cycle); Group 2D, same as Group 3 but with a co-infusion of NTG (50 μg/min) starting 2 min after completion of the first occlusion and continuing until completion of Ad5Luc infusion.
In protocol 3, a total of 24 animals were used to test whether Ad5Luc transfection was increased by an extended period (75 min) of coronary artery occlusion followed by 3 days (saline control and Ad5Luc groups, n=4 each) or 14 days (saline control and Ad5Luc groups, n=8 each) of reperfusion. The occluded–reperfused LAD received 60% of the total Ad5Luc dose (6×1010 vp), while the nonischemic LCx received 40% of the Ad5Luc dose (4×1010 vp), each infused at a rate of 2 ml/min starting 15 min after onset of reperfusion.
Regional myocardial blood flow
In protocol 3, regional myocardial blood flow was measured using 15-μm diameter microspheres (BioPAL, Worcester, MA) at the following four time points: 1) baseline, 2) end of ischemia (to quantify collateral blood flow in area at risk), 3) 25 min of reperfusion (after infusion of adenovirus or saline control), and 4) 72 hr of reperfusion. Myocardial samples were obtained from the central ischemic zone (area at risk [AAR]), the border zone, and a remote nonischemic zone, and divided into epicardial and endocardial halves. The samples were rinsed according to manufacturer's instructions (BioPAL, Worcester, MA), weighed, and dessicated in an oven at 90°C for 24 hr, and the dry weights were recorded. The samples were then sent to the supplier (BioPAL) for neutron activation and detection of radioactivity. Blood flow was calculated as (Qr×Rt)/Rr and expressed as milliliters per minute per gram of wet weight, where Qr=reference flow rate (ml/min); Rt=tissue sample radioactivity (decays/min); and Rr=reference sample radioactivity (decays/min).
Tissue luciferase assay
In all three protocols, samples of myocardium were snap frozen and stored in liquid nitrogen until analyzed. The tissue was powdered under liquid nitrogen and vortexed in 1 ml of potassium phosphate lysis buffer for 10 min. Samples were then spun at 12,000×g for 20 min at 4°C, and the supernatant was removed for assay. The Luciferase Assay System (Promega, Madison, WI) or the QuantiLum Recombinant Luciferase (Promega) kits were used to measure luciferase levels in the cardiac supernatant. Twenty microliters of supernatant were placed in a glass test tube, injected with 100 μl of substrate, and chemiluminescence was read on a Bethold AutoLumat Plus LB 953 luminometer (Bethold Technologies, Bad Wildbad, Germany). Luciferase activity (LA) values were expressed as picograms of activity per gram of tissue wet weight.
Histological analysis
In protocol 3, myocardial tissue samples from the right atrium (RA), right ventricle (RV), nonischemic left ventricular myocardium (NIZ), the border zone adjacent to the area at risk (BZ), and central area at risk (AAR) were fixed in 10% buffered formalin and embedded in paraffin, and the paraffin blocks of each were cut and stained with hematoxylin and eosin. The histological slides were scored for inflammatory characteristics (macrophage infiltration), hemorrhagic changes within the myocardium, and tissue mineralization (characteristic of necrosis). The extent of each lesion was given a score of normal (0), minimal (1), mild (2), moderate (3), or severe (4).
To visualize the expression of luciferase, immunohistochemical staining was performed on tissue samples from the left ventricular perfusion areas of interest in protocol 1. Transmural biopsy samples from myocardium perfused by the LAD or the LCx were harvested, fixed in formalin, and embedded in paraffin. Tissue sections were mounted on slides, deparaffinized, and blocked for nonspecific binding with 10% goat serum. The slides were incubated in primary (rabbit anti-luciferase antibody, CR2029RAP Cortex 1:200 to 1:500 dilution, Cortex Biochem, San Leandro, CA) and secondary (goat anti-rabbit IgG, Vector BA1000, 1:500 dilution, Vector Laboratories, Burlingame, CA) antibodies. Slides were substrated with Vector ABC–peroxidase Elite (Vector Laboratories, Burlingame, CA) for 1 hr, counterstained in 1:10 dilution of hematoxylin, and dehydrated. All slides were stained at the same time and under the same conditions to ensure that there were no day to day variations in staining conditions, and photomicrographs were taken without knowledge of the group assignment.
Area at risk and infarct size
Infarct size was assessed after excision of the heart using the double-staining technique. Briefly, after excision the aorta was cannulated and perfused with Unisperse Blue dye (Ciba Geigy, Hawthorne, NY) to outline the AAR, while the LAD was simultaneously perfused for 15 min at 37°C with 1% triphenyltetrazolium chloride (TTC, Sigma, St. Louis, MO) via an 18-gauge catheter inserted just distal to the site of occlusion (identified from cineangiogram) to quantify the necrotic myocardium; pressure during infusion of blue dye and TTC was set at 80 mm Hg. The left ventricle (LV) was then cut into transverse slices and weighed. The slices were placed in formalin overnight to enhance the contrast. After 24 hr, the slices were digitally photographed on both sides. The tissue was separated into nonischemic (blue), ischemic necrotic (TTC pale), and ischemic nonnecrotic (TTC red) areas and weighed. The AAR was expressed as a percentage of the LV mass (AAR/LV), and the infarct size was expressed as a percent of the area of necrosis (AN) mass relative to the AAR (AN/AAR).
Polymerase chain reaction
The whole genome DNA was extracted from myocardial tissues (25 mg) using DNeasy Blood & Tissue Kit (Qiagen, Chatsworth, CA). For PCR analysis of Ad5Luc, primers were designed (forward: 5′-GCCCGGCGCCATTCTATCC-3′; reverse 5′-GGGCGCAACTGCAACTCCG-3′) targeting the luciferase region of the viral vector. Polymerase chain reaction (PCR) was performed in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) in a final volume of 25 μl including 250 ng of the DNA template, 12.5 μl of 2X GoTaq hot start green master mix (Promega, Madison, WI), and 0.2 μM primers. In a negative control, the DNA template was replaced by nuclease-free water. The cycling conditions were 95°C for 2 min followed by 38 cycles of 45 sec at 94°C, 45 sec at 65°C, 45 sec at 72°C, and a final elongation for 5 min at 72°C. Ten microliters of PCR products, as well as 5 μl of PCR marker (Promega) containing 30–40 ng of each DNA fragment, were separated by electrophoresis on a 2% agarose gel and visualized with ethidium bromide under UV fluorescence. The predicted size of the PCR amplicon was 284 bp.
Statistical analysis
Data were expressed as the mean±standard error of the mean (SEM). All data were tested for normality of distribution. If data were normally distributed, a one-way analysis of variance (ANOVA) was used for single measurement data (infarct size, luciferase activity, etc), followed by Student-Newman-Keuls post hoc multiple comparisons test if significant differences were identified among groups. In protocol 2, variability was constrained by log transformation of the data, and means of the log transformed data were compared to remote nonischemic myocardium by t-test; Bonferroni's correction was used for multiple comparisons. Repeated measurement data from hemodynamics and regional blood flow were analyzed by repeated measures analysis of variance followed by post hoc analysis with Student-Newman-Keuls for multiple comparisons if significant time or group differences were identified. Luciferase activity and regional myocardial blood flow (microspheres) were measured in the target (ischemic reperfused) perfusion bed, the zone adjacent to (border zone), and contralateral to (remote zone) the target perfusion bed. Significance was assigned if the adjusted p value <0.05.
Results
The effect of SNP on TE
Protocol 1 tested the hypothesis that IC administration of SNP increased TE in nonischemic myocardium in vivo. In the saline-treated groups in which no SNP was infused, myocardial LA values were comparably very low in the left ventricular remote, border and Ad5Luc infusion zones (Table 1). IC SNP infusion (50 μg/min, started 3 min before infusion of Ad5Luc) did not increase LA (n=4, Table 1), indicating that SNP alone is not sufficient to increase Ad5 uptake and TE in nonischemic myocardium. Immunohistochemistry confirmed similar uptake of Ad5 (using the luciferase marker gene) between SNP and saline control, and showed furthermore that luciferase activity primarily appeared in the perivascular tissue (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hgtb).
Table 1.
Effect of Intracoronary Administration of Sodium Nitroprusside on Luciferase Expression in Left Ventricular Nonischemic Myocardial Samples Taken From the Remote Zone, Border Zone, and Ad5Luc Perfusion Zone
| Remote zone | Border zone | Ad5Luc perfusion zone | |
|---|---|---|---|
| Saline | 2.447±2.447 | 2.470±1.735 | 0±0 |
| SNP | 0.019±0.019 | 0±0 | 0.158±0.158 |
Luciferase expression is expressed as picogram of activity per gram of tissue wet weight (means±SEM, n=4).
The effect of short episodes of ischemia on TE
Protocol 2 tested whether short periods (3 min) of coronary artery occlusion, not causing infarction, could increase Ad5 TE in vivo. A single 3-min occlusion of either the LAD or LCx was followed by Ad5Luc infusion 1 min (“immediate”) after start of reperfusion (under the high blood flow conditions of reactive hyperemia). LA was not detectable in the AAR myocardium. Delay of Ad5Luc infusion by 8 min, to avoid the peak blood flow during reactive hyperemia, failed to increase TE (5.5±5.5 pg/g, n=4) over that observed at immediate infusion.
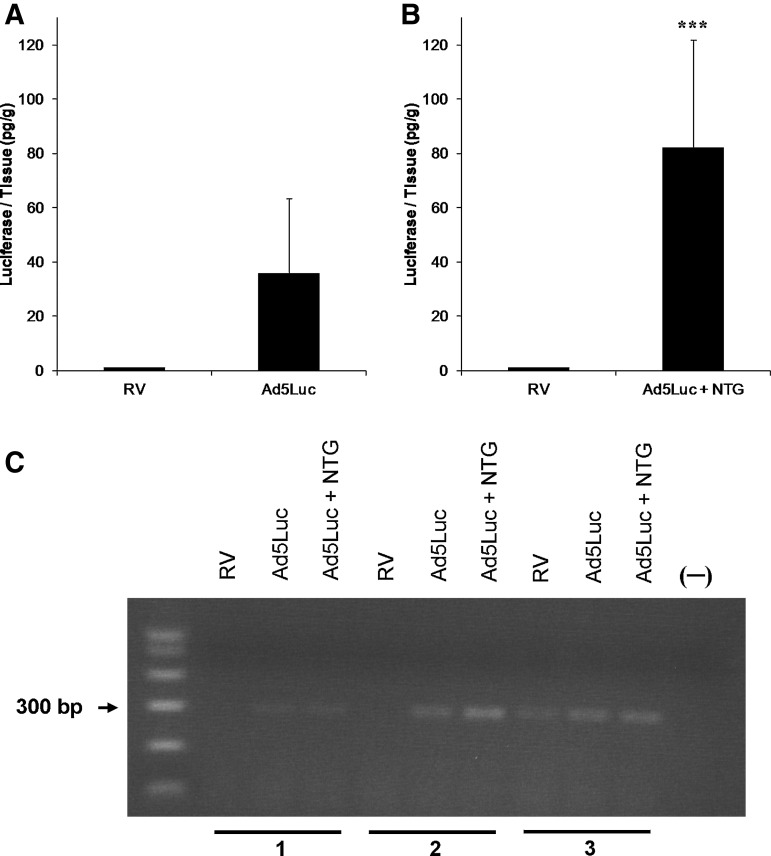
We then tested if two episodes of balloon occlusion increased TE. Luciferase assay showed that LA tended to be greater when Ad5Luc was infused during the second balloon occlusion (35.9±27.5 pg/g, n=4, p=0.06) compared to RV samples (Fig. 1A). LA was significantly (p<0.001) greater compared to remote myocardium when IC NTG (50 μg/min) was applied 2 min after finishing the first occlusion and continuing until completion of Ad5Luc delivery (82.3±39.6 pg/g, n=4) (Fig. 1B). There was no significant difference in LA in the infusion zones between the vehicle Ad5Luc and the NTG Ad5Luc groups (p=0.15). Consistent with the LA data, the PCR products of RV, which were used as a negative control, displayed no signal (animals 1 and 2) or very weak signal (animal 3) (Fig. 1C). Both Ad5Luc and Ad5Luc+NTG treatments showed positive adenoviral transduction, and NTG treatment appeared to further increase PCR product bands compared to Ad5Luc alone.
FIG. 1.
Luciferase expression in ventricular myocardium. (A) Two cycles of coronary artery occlusions separated by a 5-min reperfusion interval with adenoviral type 5 vector (Ad5Luc) infused during the second occlusion resulted in measurable luciferase activity (LA) in most animals (n=4), but it was not significantly different from the right ventricle (RV) samples (p=0.06 between Ad5Luc and RV by t test after logarithmic transformation). (B) The increase in LA was more pronounced when nitroglycerin (NTG) was co-administered (n=4, ***p<0.001 between Ad5Luc+NTG group and RV group). Values are presented as mean±SEM. (C) Polymerase chain reaction (PCR) detection of Ad5Luc DNA in myocardial tissues. Tissues were obtained from three animals. The samples were obtained 3 days after intracoronary delivery of Ad5Luc. The animals were subjected to two short episodes of balloon occlusion. Nuclease-free water served as the DNA template in PCR amplification for negative control (−). The whole-genome DNA was extracted from animals 1, 2, and 3. The RV PCR products displayed no (animal 1 and 2) or very weak signal (animal 3). Both Ad5Luc and Ad5Luc+NTG treatments showed positive adenoviral transduction, and NTG treatment appeared to further enhance Ad5Luc DNA signal.
The effect of prolonged ischemia on TE
Protocol 3 tested TE of Ad5Luc in a closed-chest myocardial infarction model Halkos et al., 2008 measured 3 or 14 days following 75-min ischemia and IC infusion of Ad5Luc into both the LAD (occluded) and LCx (nonoccluded) coronary arteries. The severity of occlusion was confirmed by coronary arterial angiography (Fig. 2) and coronary blood flow in the AAR by microspheres (Fig. 3). Subepicardial (0.90±0.31 ml/min/g and 0.93±0.20 ml/min/g, respectively) and subendocardial (1.06±0.28 ml/min/g and 0.96±0.15 ml/min/g, respectively) blood flow were similar in the saline control and Ad5Luc groups at baseline. The blood flow to both regions decreased comparably in both groups during ischemia; tissue blood flow (percentage of baseline) averaged 13.5% in subepicardium, and 12.7% in subendocardium in the two groups at the end of ischemia, with no differences between the two groups. At 25 min of reperfusion, myocardial blood flow increased to levels comparable to the respective baseline values with no significant group differences (p=0.11). Blood flow returned to baseline at day 3, again without group differences.
FIG. 2.
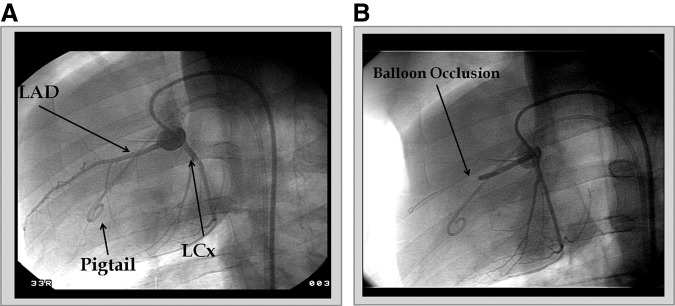
Coronary artery angiography taken at 30° left anterior oblique angle. (A) Angiogram taken during baseline showing the position of the pigtail catheter, the left anterior descending coronary artery (LAD), and left circumflex coronary artery (LCx). (B) Angiogram taken using the same angle during balloon occlusion of the LAD.
FIG. 3.
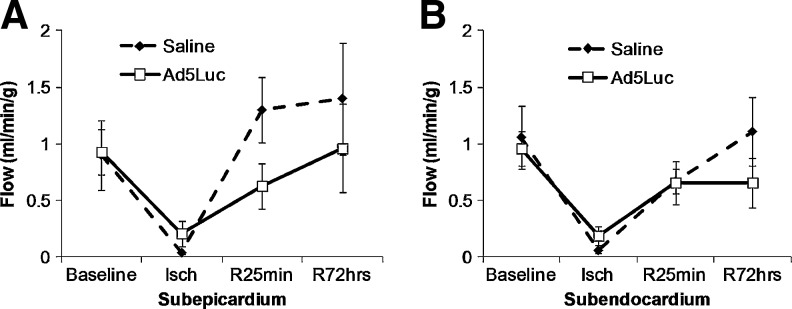
Regional myocardial blood flow in the area at risk (AAR, n=4) during baseline, end of ischemia (Isch), and 25 min (R25min) and 72 hr (R72hours) of reperfusion. (A) Saline-treated group and Ad5Luc group demonstrated similar myocardial blood flow at baseline in the subepicardium. The blood flow decreased significantly in the LAD zone during ischemia. At 25 min of reperfusion, myocardial blood flow increased to levels comparable to the respective baseline values. (B) Blood flow in the subendocardium was similar between the two groups at all timepoints measured. Values are presented as mean±SEM.
Hemodynamic data for protocol 3 at baseline, end of 75 min of ischemia, and 72 hr of reperfusion are shown in Table 2. Blood pressure and heart rate were comparable between groups at baseline. Ischemia was associated with ST-segment elevation after coronary artery occlusion and a decrease in systemic mean arterial pressure, but with no group differences. Heart rate and blood pressure were comparable to baseline values after 3 days of reperfusion, again without group differences.
Table 2.
Hemodynamic Data During Baseline, End of 75-Min Ischemia, and After 72 Hr of Reperfusion
| Parameters | |||||
|---|---|---|---|---|---|
| Time | Groups | HR | MAP | LVESP | LVEDP |
| Baseline | Saline | 116.8±15.8 | 78.1±2.7 | 80.3±4.2 | 11.7±3.5 |
| Ad5Luc | 100.0±9.4 | 82.6±3.4 | 85.1±3.6 | 10±1.8 | |
| Ischemia | Saline | 88.3±11.0 | 63.5±3.8 | 64.2±2.6 | 16±2.7 |
| Ad5Luc | 85.6±11.5 | 67.1±2.8 | 68.1±7.2 | 21.3±4.7 | |
| Day 3 | Saline | 100.2±21.1 | 67.7±6.5 | 69.7±5.5 | 17.6±1.1 |
| Ad5Luc | 92.7±1.3 | 65.2±10.2 | 64.6±4.6 | 14.5±4.5 | |
HR, heart rate (beats/min); MAP, mean aortic pressure (mm Hg); LVESP, left ventricular end-systolic pressure (mm Hg); LVEDP, left ventricular end-diastolic pressure (mm Hg). Values are expressed as mean±SEM.
The AAR (percentage of the LV mass) created by balloon occlusion followed by 72 h of reperfusion averaged 37.5±4.6% in the saline group (n=4) and 48.1±3.0% (n=3) in Ad5Luc group (Table 3, p=0.137). The AAR was on average smaller in the 14-day reperfusion groups. Notwithstanding, infarct size, expressed as a percentage of the AAR, was comparable among groups in the 3-day and 14-day reperfusion protocols.
Table 3.
Infarct Size Measurement in Left Ventricle Expressed as a Percentage of Mass of Left Ventricle or Area at Risk at Day 3 (N=4) or Day 14 (N=8) After Start of Reperfusion
| Reperfusion time | Groups | AAR/LV (%) | AN/LV (%) | AN/AAR (%) |
|---|---|---|---|---|
| Day 3 | Saline | 37.5±4.6 | 25.7±4.9 | 67.2±6.2 |
| Ad5 Luc | 48.1±3.0 | 25.4±4.9 | 52.1±7.2 | |
| Day 14 | Saline | 20.9±3.6 | 12.4±2.4 | 53.0±6.1 |
| Ad5 Luc | 21.9±3.0 | 13.2±2.1 | 58.1±2.9 |
AN, area of necrosis. Values are expressed as mean±SEM.
The average LA and PCR data for protocol 3 are shown in Fig. 4. Using luciferase activity assay, there was no LA in the saline group, consistent with absence of background tissue luciferase expression/activity (Fig. 4A). In the Ad5Luc group, there was robust LA in the AAR (90.41±26.53 pg/g, n=4), which was significantly greater than that in the border zone adjacent to the ischemic region (9.58±6.08 pg/g, n=4, p<0.001) or in the remote zone of the LV (0.24±0.59 pg/g, n=4, p<0.001; Fig. 4A). In the remote nonischemic zone perfused by the LCx the 95% confidence interval included zero, suggesting no vector uptake, despite infusion of Ad5Luc to this (LCx) bed, which is consistent with a lack of LA in protocol 1. Brief ischemia in adjacent myocardium may precondition remote areas of myocardium (Przyklenk et al., 1993). However, our data show no increase of LA in the remote nonischemic LCx zone, so this mechanism of remote conditioning for TE can be excluded. At day 14, the highest LA signal was still observed in the target ischemic (LAD) myocardium (18.38±10.10 pg/g, n=8). However, LA was significantly lower in the ischemic reperfused and border zones compared to the same regions on day 3 (p<0.001). At day 3, transmural tissues sampled from AAR showed strong adenoviral transduction (PCR products) in all three representative animals (animals 4, 5, and 6, Fig. 4B). The PCR products from the border zone displayed a weak signal while the nonischemic zone exhibited either no signal (animals 4 and 6) or a very weak signal (animal 5).
FIG. 4.
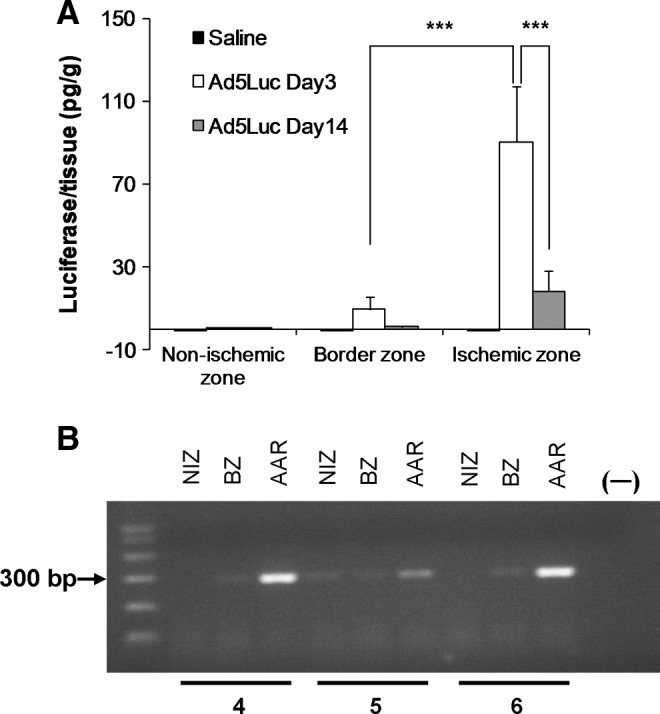
Adenovector transfection evaluated by tissue luciferase activity (A) and PCR (B) in nonischemic, border zone, and ischemic zone of the left ventricular myocardium after 75 min of LAD occlusion. 0 bar values represent no LA expression. (A) No LA was detected in any region of left ventricle in the saline-treated animals on day 3 or day 14 (n=4). In the Ad5Luc group, the ischemic zone exhibited robust LA, which was significantly greater than that in the border zone adjacent to the AAR (***p<0.001, n=4). At day 14, the ischemic zone still demonstrated the strongest LA compared to nonischemic zone or border zone (n=8). However, the LA at day 14 was significantly reduced in infarct and border zones compared to that on day 3 (***p<0.001, n=8). Values are presented as mean±SEM. (B) PCR detection of Ad5Luc DNA in myocardial tissues. The tissues were obtained from three animals (animals 4, 5, and 6). The samples were obtained 3 days after intracoronary delivery of Ad5Luc. Tissues that were obtained from the AAR showed strong adenoviral transduction in all three animals. The PCR product from the border zone (BZ) showed a weak signal. Nonischemic zone (NIZ) displayed no signal (animals 4 and 6) or very weak expression (animal 5).
Ad5Luc infusion can potentially induce myocardial inflammation, especially under the present experimental conditions in which AdLuc was infused directly to the acutely ischemic myocardium. Therefore, we evaluated whether infiltration of macrophages as a marker of myocardial inflammation occurred after Ad5Luc administration compared to a saline infusion, in addition to the markers of cell injury secondary to infarction (mineralization and hemorrhage). A summary of the scores is presented in Fig. 5. The average score in every category was 2 or less, suggesting the histological changes in both groups were mild or minimal. There was less inflammatory macrophage infiltration in the RV in the AdLuc group compared to the saline control group, but the difference was insignificant (p=0.12. Fig. 5A). There was a significantly lower hemorrhagic score in the area at risk and border zone of the Ad5luc group compared to the saline control group (p<0.001. Fig. 5B). There was no group difference in cell degeneration (calcification, mineralization) in the control and the Ad5Luc-treated pigs in any of the areas sampled (Fig. 5C).
FIG. 5.
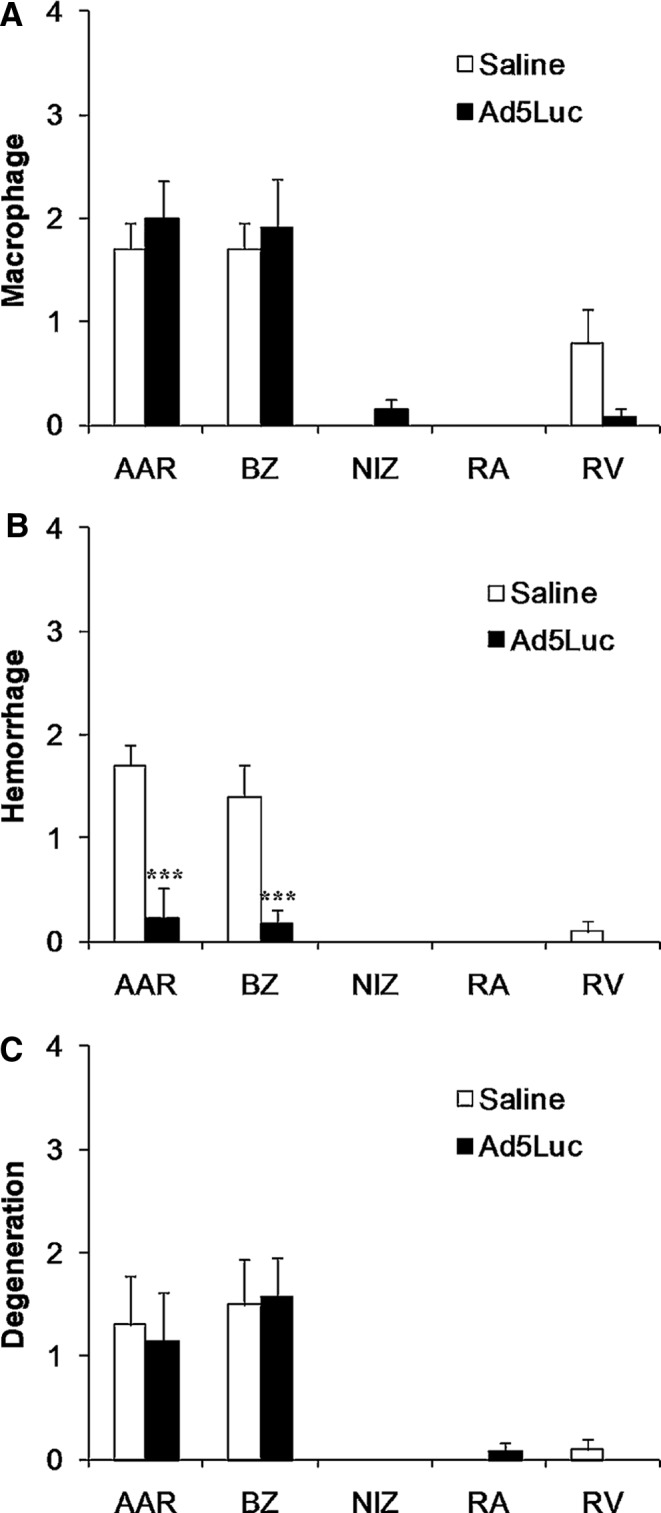
Histological scores for the presence of (A) macrophages, (B) hemorrhage, and (C) cell degeneration after 75 min of LAD occlusion and 14 days of reperfusion with and without Ad5Luc infusion (n=8). The highest score was <2 in all these studies. The ischemic AAR zone and the border zone (BZ) showed a higher score than the NIZ, right atrium (RA), and RV for the presence of macrophages, hemorrhage, and cell degeneration. The difference between the control and Ad5Luc group was not statistically significant for the presence of macrophages and cell degeneration, but was significantly lower in the Ad5Luc group for hemorrhage (***p<0.001). Values are presented as mean±SEM.
Discussion
The present study demonstrated that there was very low TE in nonischemic myocardium, with or without co-infusion of the nitric oxide donor SNP. Ad5Luc was taken up and expressed to varying degrees in myocardial regions exposed to two brief consecutive short-term cycles of transient I/R. Infusion during the second 3-min coronary occlusion likely increased vector dwell time. The TE was significantly higher relative to the RV myocardium control segment when Ad5Luc was co-infused with NTG. These data were consistent with the findings using PCR to visualize the presence of virus. Prolonged (75 min) ischemia caused myocardial infarction and significant Ad5 TE in the infarct zone, without hemodynamic abnormalities or myocardial inflammation in this I/R model. There was significantly less hemorrhage in the Ad5Luc group.
Adenoviral vector was chosen rather than adeno-associated viral (AAV) vectors. The duration of adenovector gene transfection is transient, probably limited by methylation (Brooks et al., 2004) and immune response, and is therefore a good vector choice in situations in which short transfection is desirable, such as healing of a myocardial infarction or stimulating angiogenesis. AAV gene transfection results in prolonged expression and is a good vector in situations in which long-term expression is required such as chronic congestive heart failure. There have been three published clinical trials exposing 450 patients to IC adenovector (Ad5-FGF4) with no safety concerns (Grines et al., 2002, 2003; Henry et al., 2007). However the efficacy results in these studies were inconsistent, with a trend to greater efficacy in patients with more severe disease. The present studies expanded and improved on the TE results reported in the AGENT trials in some subgroups of patients.
Previous studies showed that endothelial permeability barrier function reduced the TE of viral vectors administered into the coronary vasculature (Greelish et al., 1999; Iwatate et al., 2003; Nagata et al., 2001; Roth et al., 2004; Sasano et al., 2007). In protocol 1, we tested the hypothesis that IC administration of SNP, a nitric oxide donor that augments endothelial permeability, increased TE in nonischemic myocardium in vivo. Our results suggest that SNP alone is not sufficient to increase Ad5 uptake and TE in nonischemic myocardium over the minimal uptake during saline infusion. Roth et al. (2004) reported that IC infusion of SNP (50 μg/ml) increased Ad5ACVI vector uptake (measured as adenylyl cyclase activity) fourfold. Simoes et al. (2001) found that SNP increased vascular permeability in the hamster cheek pouch preparation under conditions of I/R, but this was not observed under nonischemic conditions by Feletou et al. (1996) using the same preparation. Our results are in agreement with those of Simoes et al. (2001) and Feletou et al. (1996), but not with the data by Roth et al. (2004) although a similar dose of SNP was used.
Ischemia is well documented to increase the permeability surface area ratio in myocardium (Harris et al., 1984), and a brief episode of myocardial ischemia was reported to increase viral TE in a Langendorff perfusion model (Logeart et al., 2000). Accordingly, we tested whether short periods of coronary artery occlusion could raise Ad5 TE in vivo (protocol 2). Our results show that there was a trend toward increased luciferase activity when Ad5Luc was infused during the second balloon occlusion. The co-infusion of NTG led to a statistically significant increase in Ad5 TE relative to the RV.
There are several factors that affect adenoviral vector uptake, such as vascular permeability, intravascular exposure (dwell) time of viral vectors, and vector binding by the coxsackie-adenovirus receptor (CAR). The present study suggests that the coexistence of several of these factors is necessary to enhance TE after IC administration of Ad5 vectors. Although both short-term ischemia (Harris et al., 1984; Logeart et al., 2000) and nitric oxide (Roth et al., 2004) have been reported to increase vascular permeability in the heart, applying them alone in the present study was not sufficient to increase Ad5 TE. However, when two consecutive 3-min occlusion–reperfusion cycles (vascular permeability increase) were combined with Ad5Luc administration during balloon occlusion (dwell time prolongation), moderate Ad5Luc gene transfer was observed. Adding a third factor (IC co-administration of NTG) significantly increased TE to a mean value similar to that observed after prolonged ischemia causing myocardial infarction. Induced CAR expression in both cardiomyocytes and coronary capillary endothelium has been observed in the infarct zone (Fechner et al., 2003). Indeed, the majority of earlier studies in different preclinical models evaluating gene transfer efficiency of IC delivery of Ad5 vectors demonstrated the need for applying a combination of factors (e.g., substances to increase vascular permeability, high perfusion pressure, coronary artery occlusion combined with coronary sinus occlusion) to significantly increase TE in isolated perfused rabbit hearts (Donahue et al., 1998; Logeart et al., 2000), in intact rabbits (Logeart et al., 2001), and in pigs (Emani et al., 2003; Szelid et al., 2002). Sasano et al. (2007) developed an IC adenovector delivery protocol in piglets that consisted of oral administration of the phosphodiesterase type 5 inhibitor sildenafil (25 mg), balloon occlusion to prevent virus backflow, and IC infusion of a perfusate containing VEGF (0.5 μg/ml), NTG (250 μg/ml), adenosine (5 mg/ml), and Ca2+ (1.0 mM). The study showed that reducing concentrations of VEGF, NTG, or adenosine impaired TE, while decreasing Ca2+ concentration improved TE. In addition, great cardiac vein occlusion plus dual infusion through the LAD and great cardiac vein increased TE by more than twofold. These data confirm that the combination of multiple factors are needed to enhance myocardial TE after IC delivery of Ad5 vectors.
The immune status of the animals used in the studies could also affect gene transfection. However, the pigs used in the study were “naïve” animals, most likely devoid of anti-Ad5 antibodies before treatment. Moreover, it was reported earlier that intentionally inducing anti-Ad5 antibodies by pre-immunization with Ad5LacZ made no significant impact on the efficacy of IC administration of Ad5FGF-4 in pigs (Roth et al., 2006).
The much longer occlusion time of 75 min generated pronounced and reproducible gene transfer efficiency in the ischemic-reperfused myocardial zone. This observation may be explained by vascular hyperpermeability during prolonged I/R. Release of pro-inflammatory factors (histamine, thrombin, etc.) as well as activated neutrophils may also contribute to increased disruption of endothelial cell–cell junctions, may contract the cytoskeleton, and consequentially may increase the intercellular space and vascular permeability (Kumar et al., 2009). In addition to vascular permeability, another important factor determining Ad5 TE is the expression of CAR in cardiac myocytes (Pinkert et al., 2009). Fechner et al. (2003) demonstrated that after ligation of the LAD coronary artery in rats, CAR expression is induced locally in cardiomyocytes from the infarct zone and on the capillary endothelium.
We conclude that TE is very low in nonischemic myocardium. The robust TE shown in the protocol with 75-min ischemia without additional myocardial inflammation above that observed by I/R suggests that Ad5 could be applied in patients suffering from acute myocardial infarction. The efficient transfection of Ad5Luc delivered by IC route during transient balloon-induced I/R of the myocardium without any hemodynamic alterations can be considered in clinical trial protocol designs using IC administration of Ad5 to patients with chronic myocardial ischemic conditions, such as angina or heart failure during elective cardiac catheterization.
Supplementary Material
Acknowledgments
These studies were supported by a small business innovative research (SBIR) grant from the National Institutes of Health (National Heart, Lung and Blood Institute), and Cardium Therapeutics, Inc. The authors are grateful for the continued support of the Carlyle Fraser Heart Center of Emory University Hospital Midtown.
Author Disclosure Statement
Robert L. Engler is a consultant and stockholder for Cardium Therapeutics. Garbor M. Rubanyl is an employee and stockholder of Cardium Therapeutics. No other competing financial interests exist.
References
- Brooks A.R. Harkins R.N. Wang P., et al. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- Donahue J.K. Kikkawa K. Thomas A.D., et al. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5:630–634. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- Emani S.M. Shah A.S. Bowman M.K., et al. Catheter-based intracoronary myocardial adenoviral gene delivery: importance of intraluminal seal and infusion flow rate. Mol. Ther. 2003;8:306–313. doi: 10.1016/s1525-0016(03)00149-7. [DOI] [PubMed] [Google Scholar]
- Fechner H. Noutsias M. Tschoepe C., et al. Induction of coxsackievirus-adenovirus-receptor expression during myocardial tissue formation and remodeling: identification of a cell-to-cell contact-dependent regulatory mechanism. Circulation. 2003;107:876–882. doi: 10.1161/01.cir.0000050150.27478.c5. [DOI] [PubMed] [Google Scholar]
- Feletou M. Bonnardel E. Canet E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: role of nitric oxide. Br. J. Pharmacol. 1996;118:1371–1376. doi: 10.1111/j.1476-5381.1996.tb15547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B.A. Mazur W. Geske R.S., et al. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- Greelish J.P. Su L.T. Lankford E.B., et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat. Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- Grines C.L. Watkins M.W. Helmer G., et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- Grines C.L. Watkins M.W. Mahmarian J.J., et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J. Am. Coll. Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- Halkos M.E. Zhao Z.Q. Kerendi F., et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res. Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- Harris T.R. Overholser K.A. Collins J.C. Tracer exchange in the normal and ischemic coronary circulation. Fed. Proc. 1984;43:164–170. [PubMed] [Google Scholar]
- Henry T.D. Grines C.L. Watkins M.W., et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J. Am. Coll. Cardiol. 2007;50:1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Iwatate M. Gu Y. Dieterle T., et al. In vivo high-efficiency transcoronary gene delivery and Cre-LoxP gene switching in the adult mouse heart. Gene Ther. 2003;10:1814–1820. doi: 10.1038/sj.gt.3302077. [DOI] [PubMed] [Google Scholar]
- Katz M.G. Swain J.D. Tomasulo C.E., et al. Current strategies for myocardial gene delivery. J. Mol. Cell Cardiol. 2011;50:766–776. doi: 10.1016/j.yjmcc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. Shen Q. Pivetti C.D., et al. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeart D. Hatem S.N. Rucker-Martin C., et al. Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery. Hum. Gene Ther. 2000;11:1015–1022. doi: 10.1089/10430340050015329. [DOI] [PubMed] [Google Scholar]
- Logeart D. Hatem S.N. Heimburger M., et al. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum. Gene Ther. 2001;12:1601–1610. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- Nagata K. Marban E. Lawrence J.H., et al. Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J. Mol. Cell Cardiol. 2001;33:575–580. doi: 10.1006/jmcc.2000.1322. [DOI] [PubMed] [Google Scholar]
- Pinkert S. Westermann D. Wang X., et al. Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation. 2009;120:2358–2366. doi: 10.1161/CIRCULATIONAHA.108.845339. [DOI] [PubMed] [Google Scholar]
- Przyklenk K. Bauer B. Ovize M., et al. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Roth D.M. Lai N.C. Gao M.H., et al. Nitroprusside increases gene transfer associated with intracoronary delivery of adenovirus. Hum Gene Ther. 2004;15:989–994. doi: 10.1089/hum.2004.15.989. [DOI] [PubMed] [Google Scholar]
- Roth D.A. McKirnan M.D. Canestrelli I., et al. Intracoronary delivery of an adenovirus encoding fibroblast growth factor-4 in myocardial ischemia: effect of serum antibodies and previous exposure to adenovirus. Hum. Gene Ther. 2006;17:230–238. doi: 10.1089/hum.2006.17.230. [DOI] [PubMed] [Google Scholar]
- Sasano T. Kikuchi K. McDonald A.D., et al. Targeted high-efficiency, homogeneous myocardial gene transfer. J. Mol. Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes C. Svensjo E. Bouskela E. Effects of L-NA and sodium nitroprusside on ischemia/reperfusion-induced leukocyte adhesion and macromolecular leakage in hamster cheek pouch venules. Microvasc. Res. 2001;62:128–135. doi: 10.1006/mvre.2001.2324. [DOI] [PubMed] [Google Scholar]
- Szelid Z. Sinnaeve P. Vermeersch P., et al. Preexisting antiadenoviral immunity and regional myocardial gene transfer: modulation by nitric oxide. Hum. Gene Ther. 2002;13:2185–2195. doi: 10.1089/104303402320987879. [DOI] [PubMed] [Google Scholar]
- Tilton R.G. Williamson E.K. Cole P.A., et al. Coronary vascular hemodynamic and permeability changes during reperfusion after no-flow ischemia in isolated, diltiazem-treated rabbit hearts. J. Cardiovasc. Pharmacol. 1985;7:424–436. doi: 10.1097/00005344-198505000-00003. [DOI] [PubMed] [Google Scholar]
- Tilton R.G. Daugherty A. Sutera S.P., et al. Myocyte contracture, vascular resistance, and vascular permeability after global ischemia in isolated hearts from alloxan-induced diabetic rabbits. Diabetes. 1989;38:1484–1491. doi: 10.2337/diab.38.11.1484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.