Abstract
Escherichia coli heat-shock proteins GroES and GroEL are essential cytoplasmic proteins, which have been termed 'chaperonins' because of their ability to assist protein assembly of bacteriophage capsids and multimeric enzymes of foreign origin. In this report we show that temperature-sensitive mutations in groES and groEL genes cause defective export of the plasmid-encoded beta-lactamase (Bla) in vivo. Since efficient translocation of proteins across biological membranes is thought to be supported by cytoplasmic factors that protect presecretory molecules from being misfolded, these results suggest that both GroES and GroEL proteins possess a chaperone function by which they facilitate export of Bla. The translocation of other secretory proteins, however, appears to depend minimally on GroE, suggesting that GroE interacts only with a specific class of secreted proteins.
Full text
PDF
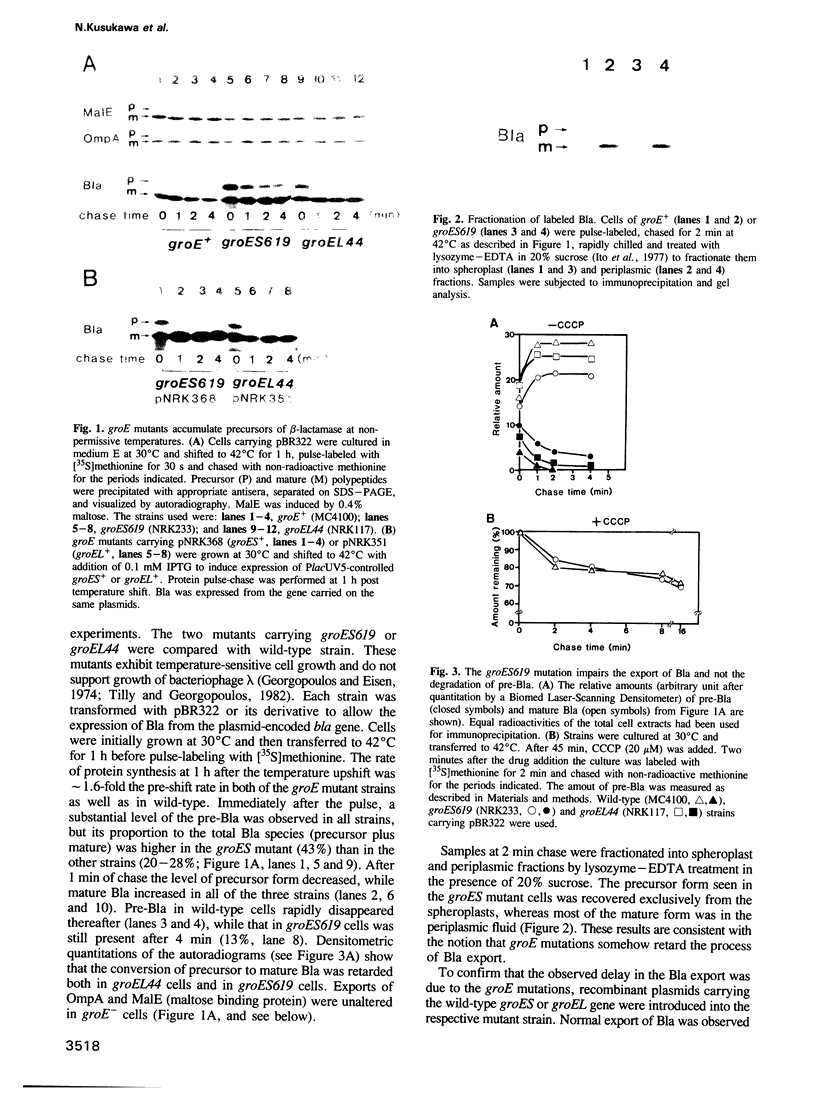



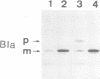
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Crooke E., Brundage L., Rice M., Wickner W. ProOmpA spontaneously folds in a membrane assembly competent state which trigger factor stabilizes. EMBO J. 1988 Jun;7(6):1831–1835. doi: 10.1002/j.1460-2075.1988.tb03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Schekman R. The role of stress proteins in membrane biogenesis. Trends Biochem Sci. 1988 Oct;13(10):384–388. doi: 10.1016/0968-0004(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P. M., Li P., Kumamoto C. A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989 Feb;171(2):813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusukawa N., Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988 Jul;2(7):874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Liberek K., Georgopoulos C., Zylicz M. Role of the Escherichia coli DnaK and DnaJ heat shock proteins in the initiation of bacteriophage lambda DNA replication. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6632–6636. doi: 10.1073/pnas.85.18.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minsky A., Summers R. G., Knowles J. R. Secretion of beta-lactamase into the periplasm of Escherichia coli: evidence for a distinct release step associated with a conformational change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4180–4184. doi: 10.1073/pnas.83.12.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]