Abstract
Objectives
A number of studies have demonstrated a trophic effect of gastrin on pancreatic cancer cells in vitro. Pernicious anemia is a clinical condition characterized by chronic hypergastrinemia. The aim of this study is to determine if pernicious anemia is a risk factor for pancreatic cancer.
Methods
This study is a retrospective cohort study using the Health Improvement Network (THIN) database, which contains comprehensive health information on 7.5 million patients in the United Kingdom from 1993–2009. All patients with pernicious anemia in the study cohort were identified and composed the exposed group. Each exposed patient was matched on practice site, sex, and age with up to four unexposed patients without pernicious anemia. The outcome was incident pancreatic cancer. The hazard ratio and 95% confidence intervals (CI) were estimated using multivariable Cox regression analysis.
Results
We identified 15,324 patients with pernicious anemia and 55,094 unexposed patients. Mean follow up time was similar between groups (exposed 4.31 [standard deviation (SD) 3.38] years, unexposed 4.63 [SD 3.44] years). The multivariable adjusted hazard ratio for pancreatic cancer associated with pernicious anemia was 1.16 (95% CI 0.77–1.76, p=0.47).
Conclusions
There is no significant association between pernicious anemia and the risk of pancreatic cancer.
Keywords: Gastrin, pancreatic cancer, hypergastrinemia, pernicious anemia
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer related deaths worldwide.(1) Elucidating risk factors for the development of PDAC is important as it could lead to new approaches to early diagnosis and potential therapeutic targets.
Pernicious anemia (PA) is an autoimmune condition with autoantibody-mediated destruction of gastric parietal cells manifest primarily as achlorhydria and subsequent up-regulation of serum gastrin production.(2) Gastrin is a trophic hormone secreted by gastric antral cells. It may have a role in the development and growth of PDAC. In vitro studies of human PDAC cell lines have demonstrated constitutive expression of gastrin.(3) Gastrin has been shown to stimulate pancreatic cancer cell growth via binding to gastrin receptors with propagation of pancreatic cancer growth in an autocrine fashion.(4, 5) Furthermore, over-expression of gastrin can lead to increased tumor growth in xenografted PDAC cell lines while treatment with gastrin receptor antagonists or decreased expression of gastrin receptors may inhibit tumor growth and increase apoptosis.(6–9) However, the role of gastrin in pancreatic cancer development remains controversial as other investigators did not observe abundant expression of gastrin receptors in PDAC. (10–12)
Two previous studies have reported a possible association between PA and PDAC. (13, 14) However, both studies were limited by a small sample size and by the use of the expected PDAC incidence in the general population as the reference.
In this study, we assess the association between PA and PDAC in a large population-based cohort with a concurrent unexposed group without PA. We hypothesized that PA, via the induction of hypergastrinemia, might be a risk factor for the development of PDAC. The results of this study could have implications for other common clinical circumstances that are associated with hypergastrinemia such as gastric acid suppressive therapy with proton pump inhibitors (PPIs).
Methods
We performed a retrospective cohort study using The Health Improvement Network (THIN), a large population-based electronic medical records database from the United Kingdom (UK). This study was approved by the Institutional Review Board at the University of Pennsylvania and by the Scientific Review Committee of THIN.
Data Source
The THIN database contains comprehensive electronic medical records data on approximately 7.5 million patients seen by general practitioners in 415 practices in the UK between 1993 and 2009. The demographic and geographic distributions of the THIN population are similar to those of the general population within the UK. The THIN database includes data on patient demographics, medical diagnoses, and prescription drug information. Each medical diagnosis entry is associated with a date of diagnosis. All diagnostic codes are stored in the database using Read codes, and all medications are stored using British National Formulary (BNF) codes. Several additional dates are noted in THIN: registration date is the date when patients were first registered with a practice; vision date is the date that the practice began using the In Practice Visions software, the electronic medical record software that collects information for the THIN database; the acceptable mortality rating (AMR) date defines the earliest date of optimal mortality reporting for that practice.(15) All practices are instructed to follow the standardized protocol of entering information and transmitting information to the central database. Data quality is monitored through routine analysis of the entered data(16) and has been found to be suitable for epidemiological research.(17) Under the UK National Health Services, 98% of the UK population receive their healthcare through general practitioners; therefore the data in THIN reflect the general healthcare delivery pattern for the entire UK population.
Study cohort
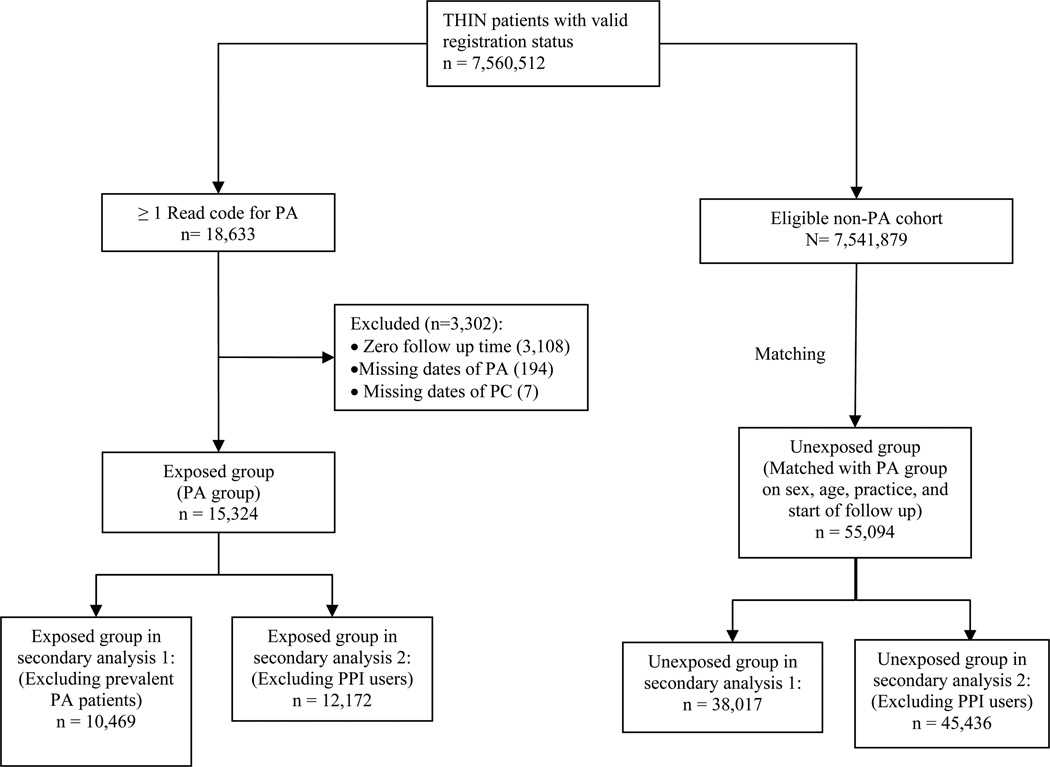
All patients receiving care from a THIN practitioner from 1993 to 2009 were potentially eligible for inclusion in the study. Patients were excluded if their registration status was anything other than ‘acceptable’ (i.e., patients with incomplete documentation or out of sequence date of birth, date of entry into the database, date of death, or date of exit from the database; not male or female; not permanently registered). All patients with the diagnosis of PDAC prior to the diagnosis of PA were excluded from the study.
Exposed group
The exposed group consisted of all patients from the eligible study cohort with one or more Read codes for PA. Follow-up time for the exposed group began at the date of first diagnosis entry of PA, the date of registration, the vision date or the AMR date, whichever occurred later, and ended at first diagnosis of PDAC or the end of follow-up in the THIN database, whichever is earlier. End of follow up in the THIN database was represented by either exit from the database, death, or discontinuation of practice enrollment in the database.
Unexposed group
The unexposed group consisted of a sample of patients from the remainder of the study cohort without a diagnosis of PA. For each exposed patient, we identified all subjects in the cohort without a diagnosis of PA who were of the same sex, from the same practice site, and of similar age (+/− 2.5 years) as the matched exposed patient. These patients were considered as eligible matched unexposed patients. The expected gain in statistical precision by including more than 4 unexposed patients per exposed patient is generally limited; thus for efficiency of analysis, we randomly selected up to 4 (i.e., all eligible unexposed patients were included if there were fewer than 4 available) of these eligible matched unexposed patients for each exposed patient.
The unexposed patients were assigned the same follow-up start date as their matched exposed patient. Follow up time for the unexposed group ended at time of first diagnosis of PDAC or at end of follow up in the THIN database, whichever is earlier. Unexposed patients were assigned a similar start date as their counterpart exposed patients in order to ensure that patients were followed for similar calendar periods.
Outcome of interest
The outcome of interest was diagnosis of PDAC based on Read codes. A previous study in THIN demonstrated that the incidence of PDAC in THIN was similar to the incidence in the entire population of the UK.(18)
Statistical analysis
The baseline characteristics at the start of follow-up were compared between the patients with and without PA using chi square tests for categorical variables and t-tests for continuous variables. The crude incidence of PDAC in each comparison group was estimated by dividing the number of incident cases in the group by the total person-years of follow-up in the same group.
Cox regression model was used to assess the association between PA and PDAC. Hazard ratio (HR) was calculated with and without adjusting for covariate information. In addition to sex and age, we examined the following covariates: body mass index (BMI) (ie., <20, 20–25, >25, missing), the last recorded smoking status before the beginning of follow-up period (ie., current, past, never, unknown), diabetes mellitus, chronic pancreatitis, and chronic hepatitis B infection. These covariates were selected because they may be associated with the risk of PDAC and in some instances PA.(19, 20) Diabetes mellitus, chronic pancreatitis, and chronic hepatitis B infection were analyzed as covariates if they were present prior to the start of follow up. Potential confounders were included in the Cox model if the prevalence of the confounder was >0.25% in either study group at the start of follow-up and if its inclusion in the respective Cox model changed the sex- and age-adjusted HR by >10%. This approach has been shown to be superior to other methods of selecting confounders in observational studies.(21) The assumption of proportional hazards over time was tested using the Schoenfeld test.
Secondary analyses
Two secondary analyses were performed by varying the definition of the exposed or unexposed group. In the first secondary analysis, only incident cases of PA were used in the exposed group; exposed cases were restricted to those patients who had a PA diagnosis at least one year after the date of registration. This secondary analysis was conducted because a prior study showed that large database studies can overestimate incidence rates of disease in the first year after registration.(22)
Second, to avoid potential selection bias, a subgroup analysis was conducted after eliminating any unexposed patient with use of greater than one year of cumulative PPI therapy. PPI therapy may lead to hypergastrinemia and is not indicated in patients with PA.
Our study included 15,324 exposed subjects, matched with approximately four times as many unexposed patients, with a mean follow up of 4.63 years. The observed incidence of PDAC in the unexposed group in this study was 37.28/100,000 person years. Thus, our study was able to detect a HR of 1.7 with 80% power and alpha of 0.05 in our primary analysis.
Results
Table 1 shows the comparison of the exposed and unexposed groups. Mean age and sex of patients were similar between the exposed and unexposed groups. Mean follow up time was similar between groups (exposed, 4.31 [SD 3.38] years, unexposed 4.63 [SD 3.44] years).
Table 1.
Patient characteristics
| Characteristics |
Pernicious Anemia, n=15,324 |
Non-pernicious anemia, n=55,094 |
p value |
|---|---|---|---|
| Mean Age (SD) | 68.79 yrs (+/−17.36) | 66.66 yrs (+/−16.91) | p<0.001 |
| Male (%) | 4,874 (31) | 16,787(31) | p=.002 |
| Current smokers (%) | 3,561 (19) | 12,672 (17) | |
| Former smokers (%) | 2,880 (23) | 9,479 (23) | |
| Non-smokers (%) | 8,173 (53) | 30,074 (55) | |
| Missing smoking data (%) | 710 (5) | 2,869 (5) | |
| Mean BMI (kg/m2) | 26.17 (+/− 5.40) | 26.10 (+/− 4.72) | p=0.223 |
| BMI <25 (%) | 5,054 (33) | 15,770 (28) | |
| BMI 25–30 (%) | 3,734 (24) | 13,054(24) | |
| BMI >30 (%) | 2,198 (14) | 6,212 (11) | |
| Missing BMI data (%) | 4,338 (28) | 20,058 (36) | |
| DM (%) | 2,031 (13) | 2,900 (5.26) | p<0.001 |
| Chronic hepatitis B (%) | 21 (0.14) | 63 (0.11) | p=0.472 |
| Chronic pancreatitis (%) | 27 (0.18) | 26 (0.05) | p<0.001 |
| Pancreatic cancer (%) | 30 (0.20) | 95 (0.17) | p=0.544 |
Thirty incident PDAC cases were identified in the exposed group and 95 cases in the unexposed group, yielding an incidence of 45.38/100,000 person years in the exposed group and 37.28/100,000 person years in the unexposed group. The incidence of PDAC observed in this study is comparable to the incidence of PDAC previously reported in the UK for similar age groups.(23) In the 30 patients who had PA and developed PDAC, the mean duration of PA prior the diagnosis of PDAC is 6.38 years with a SD of 5.10 years.
The unadjusted HR for the risk of PDAC associated with PA was 1.23 (95% CI 0.81–1.85, p=0.33).
A priori, we decided to include age and sex in the final model. However, none of the other potential confounders met our pre-specified confounder selection criteria (i.e., altering the unadjusted HR by >10%). Thus, the final HR for PDAC associated with PA, after adjustment for age and sex only, was 1.16 (95% CI 0.77–1.76, p=0.47). The test for proportionality was nonsignificant (p=0.17).
The results from the two secondary analyses showed similar results as the primary analysis (Table 2). The analysis restricting the exposed group to incident PA, defined as PA diagnosed greater than 1 year after enrollment, yielded a sex- and age- adjusted HR for PDAC associated with PA of 1.11 (95% CI 0.69–1.79, p=0.67). Finally, after excluding all patients who received greater than 1 year of cumulative PPI therapy, the sex-and age-adjusted HR was 1.29 (95% CI 0.83–2.01, p=0.25).
Table 2.
The HRs for PC associated with various definitions of exposure as well as after exclusion of PPI users among the unexposed group.
| PA group | Non-PA group |
Unadjusted HR(95% CI) |
Adjusted* HR (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Total (n) |
PC (n) |
Total (n) |
PC (n) |
||||
| Primary analysis | 15,324 | 30 | 55,094 | 95 | 1.23 (0.81–1.85) | 1.16 (0.77–1.76) | |
| Secondary analyses | Excluding prevalent PA patients | 10,469 | 22 | 38,017 | 72 | 1.16 (0.72–1.87) | 1.11 (0.69–1.79) |
| Excluding PPI users | 12,172 | 27 | 45,436 | 78 | 1.40 (0.90–2.17) | 1.29 (0.83–2.01) | |
Adjusted for sex and age only because none of the other potential confounders met our a priori confounder selection criteria (i.e., altering the crude HR by >10%).
Discussion
We observed no significant association between PA and PDAC in this large population-based cohort study. From a mechanistic standpoint, this finding suggests that although hypergastrinemia may affect the growth of pancreatic cancer cells in vitro, it may not be involved in the initiation of PDAC.
In contrast to our findings, two previous studies suggested a moderately positive association between PA and PDAC. (13, 14) However, both studies included a small convenience sample of PA patients, raising the concern for potential selection bias. More importantly, both studies estimated standardized incidence ratios based on the expected population PDAC incidence. As such, neither study could adequately account for potential confounding. Other studies have assessed the cancer risk in patients with PA and not found an increased incidence of PDAC (24–26). With its large population-based study design and the inclusion of a concurrent unexposed reference group, our study provides a more definitive characterization of the association between PA and PDAC than these previous studies.
Our results also have several clinical implications. There are other clinical conditions resulting in serum hypergastrinemia such as chronic PPI therapy or atrophic gastritis associated with H. pylori infection. The extent of hypergastrinemia in these conditions is less pronounced compared to PA (27, 28). Therefore, the fact that we did not observe a significant association between PA and PDAC would provide some reassurance that PPI therapy, even at high doses, is unlikely to increase the risk of PDAC. Indeed a recent study observed no significant association between PPI use and PDAC.(29) A previous study assessing the relationship between H. pylori infection and PDAC demonstrated a statistically significant association.(30) Our data would suggest that the mechanism underlying this association is likely independent of hypergastrinemia.
There are several potential limitations to this study. BMI information was not available in a substantial fraction of study patients. However, there is no established association between BMI and PA, which is consistent with our finding that BMI was not a confounder. Furthermore, in an exploratory analysis restricted to patients with available BMI data, BMI also was not a significant confounder and there was no association between PA and PDAC (data not shown). Additionally, we did not have information on alcohol intake. However, there is no strong evidence that alcohol use would be associated with PA. Alcohol use also has not been consistently associated with PDAC.(31, 32) Alcohol use may be associated with PDAC through the induction of chronic pancreatitis, but chronic pancreatitis was not a confounder in this study. The diagnoses of PA and PDAC were based on Read codes and there is a possibility of misclassification. However, the results did not change when we used a more specific definition of PA exposure (i.e., newly diagnosed PA), arguing against the presence of substantial misclassification of PA status. The observed incidence of PDAC in this database was consistent with previously published PDAC incidence in the UK,(23) supporting the validity of the PDAC diagnosis in our study. Our study included a mean follow up time per patient of 4.31 and 4.6 years, respectively. This may pose one potential limitation; there is the possibility that an association between PA and PDAC exists but is not detected until longer duration of PA is present. In a study by Tally et al. assessing the risk of colon cancer in a cohort of 150 patients with PA, the RR of colon cancer in any time period (mean follow up of 11 years) was non-significant; however, the RR of colon cancer within the first 5 years after diagnosis was found to be increased at 4.1 and this was statistically significant (33). If pancreas cancer behaved in a similar fashion to colon cancer, we would have expected to detect such an association. Finally, this is a retrospective study using a population based medical database; there is the possibility of unidentified confounders which may have caused residual confounding.
In summary, PA, a condition characterized by chronic profound hypergastrinemia, does not appear to increase the risk of developing PDAC. As such, lower levels of hypergastrinemia induced by PPI therapy are unlikely to increase the risk of PDAC.
Additional research needs to be conducted to evaluate the role of hypergastrinemia in patients with established pancreatic cancer.
Figure 1.
Patient selection flow chart
Acknowledgements
This study is funded by the NIH T32-DK007740 to P.S.; NIH Clinical and Translational Science Award UL1 RR024134 to P.S. and K.H.; NIH K08-DK088945 to A.D.R.
Footnotes
None of the authors has any conflict of interest to declare.
Author Contribution Statements:
-
-Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
-
-No conflicts of interest exist for this author.
-
-Study concept and design; critical revision of the manuscript for important intellectual content
-
-No conflicts of interest exist for this author.
-
-Acquisition of data; critical revision of the manuscript for important intellectual content; administrative, technical, or material support
-
-No conflicts of interest exist for this author.
-
-Study design; statistical analysis
-
-No conflicts of interest exist for this author.
-
-Study concept and design; critical revision of the manuscript for important intellectual content; study supervision
-
-No conflicts of interest exist for this author.
Contributor Information
Pari Shah, Department of Medicine, Gastroenterology and Nutrition Service, Memorial Sloan Kettering Cancer Center, New York, NY 10065.
Andrew D. Rhim, Department of Medicine, Division of Gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Kevin Haynes, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Wei-Ting Hwang, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Yu-Xiao Yang, Department of Medicine, Division of Gastroenterology and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
References
- 1.Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol. 2010;6:246–254. [PMC free article] [PubMed] [Google Scholar]
- 2.Lewin KJ, Dowling F, Wright JP, et al. Gastric morphology and serum gastrin levels in pernicious anaemia. Gut. 1976;17:551–560. doi: 10.1136/gut.17.7.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JP, Hamory MW, Verderame MF, et al. Quantitative analysis of gastrin mRNA and peptide in normal and cancerous human pancreas. Int J Mol Med. 1998;2:309–315. doi: 10.3892/ijmm.2.3.309. [DOI] [PubMed] [Google Scholar]
- 4.Smith JP, Shih A, Wu Y, et al. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. The Am J Physiol. 1996;270:R1078–R1084. doi: 10.1152/ajpregu.1996.270.5.R1078. [DOI] [PubMed] [Google Scholar]
- 5.Smith JP, Stanley WB, Verderame MF, et al. The functional significance of the cholecystokinin-C (CCK-C) receptor in human pancreatic cancer. Pancreas. 2004;29:271–277. doi: 10.1097/00006676-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Chua YJ, Cunningham D. Adjuvant treatment for resectable pancreatic cancer. J Clin Oncol. 2005;23:4532–4537. doi: 10.1200/JCO.2005.17.954. [DOI] [PubMed] [Google Scholar]
- 7.Matters GL, Harms JF, McGovern CO, et al. Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas. 2009;38:e151–e161. doi: 10.1097/MPA.0b013e3181a66fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilliam AD, Broome P, Topuzov EG, et al. An International Multicenter Randomized Controlled Trial of G17DT in Patients With Pancreatic Cancer. Pancreas. 2012;41:374–379. doi: 10.1097/MPA.0b013e31822ade7e. [DOI] [PubMed] [Google Scholar]
- 9.Fino KK, Matters GL, McGovern CO, et al. Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1244–G1252. doi: 10.1152/ajpgi.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reubi JC, Waser B, Gugger M, et al. Distribution of CCK1 and CCK2 Receptors in Normal and Diseased Human Pancreatic Tissue. Gastroenterology. 2003;125:98–106. doi: 10.1016/s0016-5085(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 11.Jang JY, Sun-Whe K, Ja-Luk K, et al. Presence of CCK A, B receptors and effect of gastric and cholecystokinin on growth of pancreatobiliary cancer cell lines. World J Gastroenterol. 2005;11(6):803–809. doi: 10.3748/wjg.v11.i6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monstein HJ, Ohlsson B, Axelson J. Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines. Scand J Gastroenterol. 2001;36(7):738–743. doi: 10.1080/003655201300192003. [DOI] [PubMed] [Google Scholar]
- 13.Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious Anemia and Subsequent Cancer: A Population Based Cohort Study. Cancer. 1993;71(3):745–750. doi: 10.1002/1097-0142(19930201)71:3<745::aid-cncr2820710316>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Borch K, Kullman E, Hallagen S, et al. Increased Incidence of Pancreatic Neoplasia in Pernicious Anemia. World J Surg. 1988;12:866–870. doi: 10.1007/BF01655502. [DOI] [PubMed] [Google Scholar]
- 15.Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Safe. 2009;18:76–83. doi: 10.1002/pds.1688. [DOI] [PubMed] [Google Scholar]
- 16.Bourke A, Dattani H, Robinson M. Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care. 2004;12:171–177. doi: 10.14236/jhi.v12i3.124. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Safe. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 18.Haynes K, Forde KA, Schinnar R, et al. Cancer incidence in The Health Improvement Network. Pharmacoepidemiol and Drug Safe. 2009;18:730–736. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
- 19.Ben Q, Li Z, Liu C, et al. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from china. Pancreas. 2012;41:435–440. doi: 10.1097/MPA.0b013e31822ca176. [DOI] [PubMed] [Google Scholar]
- 20.Pandol S, Gukovskaya A, Edderkoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Safe. 2005;14:443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 23. [last accessed 6/2012];Office for National Statistics. ( http://ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-262496;)
- 24.Mellemkjaer L, Gridley G, Møller H, et al. Pernicious anaemia and cancer risk in Denmark. Br J Cancer. 1996;73(8):998–1000. doi: 10.1038/bjc.1996.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinton LA, Gridley G, Hrubec Z, et al. Cancer risk following pernicious anaemia. Br J Cancer. 1989;59(5):810–813. doi: 10.1038/bjc.1989.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlson BM, Ekbom A, Wacholder S, et al. Cancer of the upper gastrointestinal tract among patients with pernicious anemia: a case-cohort study. Scand J Gastroenterol. 2000 Aug;35(8):847–851. doi: 10.1080/003655200750023228. [DOI] [PubMed] [Google Scholar]
- 27.Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci. 2007;52(10):2482–2489. doi: 10.1007/s10620-006-9419-3. [DOI] [PubMed] [Google Scholar]
- 28.Lanzon Miller S, Pounder RE, Hamilton MR, et al. Twenty-four-hour intragastric acidity and plasma gastrin concentration in healthy subjects and patients with duodenal or gastric ulcer, or pernicious anaemia. Aliment Pharmacol Ther. 1987;1(3):225–237. doi: 10.1111/j.1365-2036.1987.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 29.Bradley MC, Murray LJ, Cantwell MM, et al. Proton pump inhibitors and histamine-2-receptor antagonists and pancreatic cancer risk: a nested case-control study. Br J Cancer. 2012;106:233–239. doi: 10.1038/bjc.2011.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93:937–941. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 31.Tramacere I, Scotti L, Jenab M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer. 2010;126:1474–1486. doi: 10.1002/ijc.24936. [DOI] [PubMed] [Google Scholar]
- 32.Velema JP, Walker AM, Gold EB. Alcohol and pancreatic cancer. Insufficient epidemiologic evidence for a causal relationship. Epidemiol Rev. 1986;8:28–41. doi: 10.1093/oxfordjournals.epirev.a036294. [DOI] [PubMed] [Google Scholar]
- 33.Talley NJ, Chute CG, Larson DE, et al. Risk for colorectal adenocarcinoma in pernicious anemia. A population based cohort study. Ann Intern Med. 1989;111(9):738–742. doi: 10.7326/0003-4819-111-9-738. [DOI] [PubMed] [Google Scholar]