Abstract
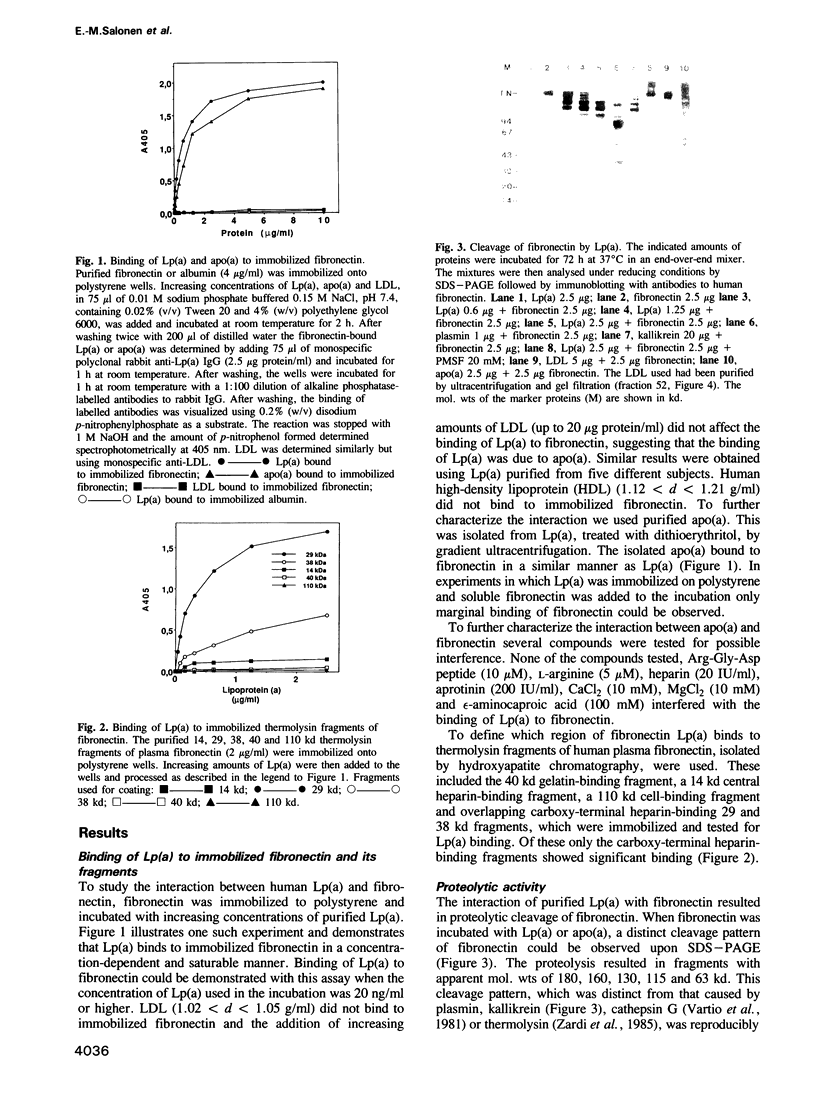
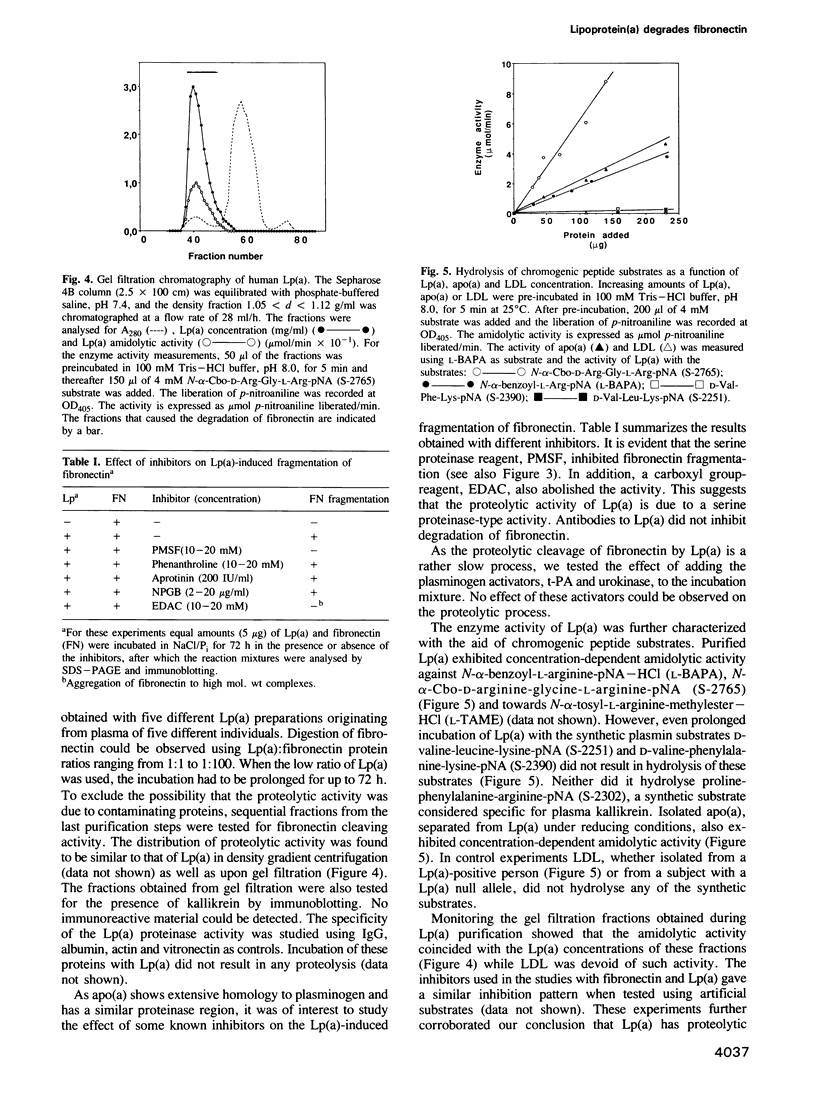
The plasma concentration of human lipoprotein(a) [Lp(a)] is correlated with the risk of heart disease. A distinct feature of the Lp(a) particle is the apolipoprotein (a) [apo(a)], which is associated with apoB-100, the main protein component of low-density lipoprotein. We now report that apo(a), which has extensive homology to plasminogen, binds to immobilized fibronectin. The binding of Lp(a) was localized to the C-terminal heparin-binding domain of fibronectin. Incubation of Lp(a) with fibronectin resulted in fragmentation of fibronectin. The cleavage pattern, as visualized by gel electrophoresis and immunoblotting, was reproducibly obtained with Lp(a) purified from five different individuals and was distinct from that obtained upon proteolysis of fibronectin by plasmin or kallikrein. The use of synthetic peptide substrates demonstrated that the amino acid specificity for Lp(a) was arginine rather than lysine. The proteolytic activity of Lp(a) was localized to apo(a) and experiments with inhibitors indicated that the proteolytic activity was of serine proteinase-type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Armstrong V. W., Cremer P., Eberle E., Manke A., Schulze F., Wieland H., Kreuzer H., Seidel D. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis. 1986 Dec;62(3):249–257. doi: 10.1016/0021-9150(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Borsi L., Castellani P., Balza E., Siri A., Pellecchia C., De Scalzi F., Zardi L. Large-scale procedure for the purification of fibronectin domains. Anal Biochem. 1986 Jun;155(2):335–345. doi: 10.1016/0003-2697(86)90443-4. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Fless G. M., Kohr W. J., McLean J. W., Xu Q. T., Miller C. G., Lawn R. M., Scanu A. M. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc Natl Acad Sci U S A. 1987 May;84(10):3224–3228. doi: 10.1073/pnas.84.10.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehnholm C., Garoff H., Renkonen O., Simons K. Protein and carbohydrate composition of Lp(a)lipoprotein from human plasma. Biochemistry. 1972 Aug 15;11(17):3229–3232. doi: 10.1021/bi00767a015. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Garoff H., Simons K., Aro H. Purification and quantitation of the human plasma lipoprotein carrying the Lp(a) antigen. Biochim Biophys Acta. 1971 May 25;236(2):431–439. doi: 10.1016/0005-2795(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Salisbury B. G. Fibronectin stimulates macrophage uptake of low density lipoprotein-heparin-collagen complexes. Arteriosclerosis. 1988 May-Jun;8(3):263–273. doi: 10.1161/01.atv.8.3.263. [DOI] [PubMed] [Google Scholar]
- Fless G. M., ZumMallen M. E., Scanu A. M. Isolation of apolipoprotein(a) from lipoprotein(a). J Lipid Res. 1985 Oct;26(10):1224–1229. [PubMed] [Google Scholar]
- Frank S. L., Klisak I., Sparkes R. S., Mohandas T., Tomlinson J. E., McLean J. W., Lawn R. M., Lusis A. J. The apolipoprotein(a) gene resides on human chromosome 6q26-27, in close proximity to the homologous gene for plasminogen. Hum Genet. 1988 Aug;79(4):352–356. doi: 10.1007/BF00282175. [DOI] [PubMed] [Google Scholar]
- Gaubatz J. W., Heideman C., Gotto A. M., Jr, Morrisett J. D., Dahlen G. H. Human plasma lipoprotein [a]. Structural properties. J Biol Chem. 1983 Apr 10;258(7):4582–4589. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar K. A., Gavish D., Breslow J. L., Nachman R. L. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989 May 25;339(6222):303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- Harvie N. R., Schultz J. S. Studies of Lp-lipoprotein as a quantitative genetic trait. Proc Natl Acad Sci U S A. 1970 May;66(1):99–103. doi: 10.1073/pnas.66.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., Qunici G. B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 1981 Jan-Feb;38(1-2):51–61. doi: 10.1016/0021-9150(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Kratzin H., Armstrong V. W., Niehaus M., Hilschmann N., Seidel D. Structural relationship of an apolipoprotein (a) phenotype (570 kDa) to plasminogen: homologous kringle domains are linked by carbohydrate-rich regions. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1533–1544. doi: 10.1515/bchm3.1987.368.2.1533. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Gersdorf E., Menzel H. J., Duba C., Cleve H., Humphries S., Utermann G. The gene for the Lp(a)-specific glycoprotein is closely linked to the gene for plasminogen on chromosome 6. Hum Genet. 1989 Jan;81(2):149–152. doi: 10.1007/BF00293891. [DOI] [PubMed] [Google Scholar]
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987 Nov 12;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Miles L. A., Fless G. M., Levin E. G., Scanu A. M., Plow E. F. A potential basis for the thrombotic risks associated with lipoprotein(a). Nature. 1989 May 25;339(6222):301–303. doi: 10.1038/339301a0. [DOI] [PubMed] [Google Scholar]
- Pande H., Calaycay J., Lee T. D., Legesse K., Shively J. E., Siri A., Borsi L., Zardi L. Demonstration of structural differences between the two subunits of human-plasma fibronectin in the carboxy-terminal heparin-binding domain. Eur J Biochem. 1987 Jan 15;162(2):403–411. doi: 10.1111/j.1432-1033.1987.tb10616.x. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Rhoads G. G., Dahlen G., Berg K., Morton N. E., Dannenberg A. L. Lp(a) lipoprotein as a risk factor for myocardial infarction. JAMA. 1986 Nov 14;256(18):2540–2544. [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Saksela O., Vartio T., Vaheri A., Nielsen L. S., Zeuthen J. Plasminogen and tissue-type plasminogen activator bind to immobilized fibronectin. J Biol Chem. 1985 Oct 5;260(22):12302–12307. [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. Immobilization of viral and mycoplasma antigens and of immunoglobulins on polystyrene surface for immunoassays. J Immunol Methods. 1979;30(3):209–218. doi: 10.1016/0022-1759(79)90095-4. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. Rapid solid-phase enzyme immunoassay for antibodies to viruses and other microbes: effects of polyethylene glycol. J Immunol Methods. 1981;41(1):95–103. doi: 10.1016/0022-1759(81)90277-5. [DOI] [PubMed] [Google Scholar]
- Seman L. J., Breckenridge W. C. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a). Biochem Cell Biol. 1986 Oct;64(10):999–1009. doi: 10.1139/o86-133. [DOI] [PubMed] [Google Scholar]
- Simons K., Ehnholm C., Renkonen O., Bloth B. Characterization of the Lp(a) lipoprotein in human plasma. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):459–466. doi: 10.1111/j.1699-0463.1970.tb04328.x. [DOI] [PubMed] [Google Scholar]
- Skorstengaard K., Thøgersen H. C., Vibe-Pedersen K., Petersen T. E., Magnusson S. Purification of twelve cyanogen bromide fragments from bovine plasma fibronectin and the amino acid sequence of eight of them. Overlap evidence aligning two plasmic fragments, internal homology in gelatin-binding region and phosphorylation site near C terminus. Eur J Biochem. 1982 Nov 15;128(2-3):605–623. doi: 10.1111/j.1432-1033.1982.tb07007.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., von Smitten K., Vaheri A. Fibronectin and atherosclerosis. Acta Med Scand Suppl. 1980;642:165–170. doi: 10.1111/j.0954-6820.1980.tb10949.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. E., McLean J. W., Lawn R. M. Rhesus monkey apolipoprotein(a). Sequence, evolution, and sites of synthesis. J Biol Chem. 1989 Apr 5;264(10):5957–5965. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J Clin Invest. 1987 Aug;80(2):458–465. doi: 10.1172/JCI113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Weber W. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 1983 Apr 18;154(2):357–361. doi: 10.1016/0014-5793(83)80182-3. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Salonen E. M., Vartio T. Fibronectin in formation and degradation of the pericellular matrix. Ciba Found Symp. 1985;114:111–126. doi: 10.1002/9780470720950.ch8. [DOI] [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. W., Hitchens J., Magnani H. N., Khan M. A study of methods of identification and estimation of Lp(a) lipoprotein and of its significance in health, hyperlipidaemia and atherosclerosis. Atherosclerosis. 1974 Sep-Oct;20(2):323–346. doi: 10.1016/0021-9150(74)90016-1. [DOI] [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Balza E., Borsi L., Castellani P., Rocco M., Siri A. Elution of fibronectin proteolytic fragments from a hydroxyapatite chromatography column. A simple procedure for the purification of fibronectin domains. Eur J Biochem. 1985 Feb 1;146(3):571–579. doi: 10.1111/j.1432-1033.1985.tb08690.x. [DOI] [PubMed] [Google Scholar]



