Abstract
Viral RNA dependent polymerases (vRdPs) are present in all RNA viruses; unfortunately, their sequence similarity is too low for phylogenetic studies. Nevertheless, vRdP protein structures are remarkably conserved. In this study, we used the structural similarity of vRdPs to reconstruct their evolutionary history. The major strength of this work is in unifying sequence and structural data into a single quantitative phylogenetic analysis, using powerful a Bayesian approach.
The resulting phylogram of vRdPs demonstrates that RNA-dependent DNA polymerases (RdDPs) of viruses within Retroviridae family cluster in a clearly separated group of vRdPs, while RNA-dependent RNA polymerases (RdRPs) of dsRNA and +ssRNA viruses are mixed together. This evidence supports the hypothesis that RdRPs replicating +ssRNA viruses evolved multiple times from RdRPs replicating +dsRNA viruses, and vice versa. Moreover, our phylogram may be presented as a scheme for RNA virus evolution. The results are in concordance with the actual concept of RNA virus evolution. Finally, the methods used in our work provide a new direction for studying ancient virus evolution.
Introduction
RNA viruses evolve rapidly. Since viral RNA-dependent polymerases (vRdP) miss the proofreading activity they produce a high percentage of mutated variants [1]. These variants face a strong evolutionary pressure by the host immune system and a highly competitive environment between relative viruses [2]. These factors lead to a rapid diversification in the primary structure of all viral genes and proteins, and a swift establishment of new virus strains [3]–[5].
Despite these fast changes in the sequences of viral proteins, functions that are crucial for efficient virus reproduction must be preserved [6]. Therefore, proteins involved in important steps of the virus life cycle accumulate mutations slower and preserve a higher degree of conservation [6]. The most conserved proteins among RNA viruses are polymerases, helicases, proteases and methyltransferases [7].
Contrary to the primary structure, the tertiary structure of most proteins sharing a common evolutionary origin remains conserved [8], [9]. The most conserved part of the protein is usually the core structure essential for protein function. The core is often surrounded by less conserved structures modifying the protein function. Changes in these additional structures often lead to minor changes in protein character (e. g., different substrate specificity), but the major protein function remains unchanged.
Morphological description of protein structure can help in reconstructing protein evolutionary history. In this approach, protein structural features are encoded in a character matrix where the rows describe the individual proteins and the columns describe the individual features. This is similar to the approach used for reconstructing the evolutionary relations among fossil species [10]. Morphological data can also be coupled with sequence data to enforce the incoming information [11], [12]. This approach may also be applied to proteins. For example, mixed morphological and sequence data were used to reconstruct the evolution of aminoacyl tRNA synthetases class I [13] and the protein kinase-like superfamily [14].
Among all viral proteins, vRdPs display the highest degree of conservation. Genes coding for vRdPs were found in all non-satellite RNA viruses and RNA viruses reproducing via a DNA intermediate [15]. All vRdPs contain seven typical sequence motifs (G, F, A, B, C, D and E) [16], [17] that incorporate conserved amino acid residues crucial for polymerase function [18], [19].
Moreover, vRdPs share remarkable structural homology. The protein structural fold resembles a right hand with subdomains termed fingers, palm and thumb [20]–[23]. The palm subdomain is structurally well conserved among all vRdPs. Finger and thumb subdomains are more variable, but they can be fully aligned only among RNA-dependent RNA polymerases (RdRPs) of +ssRNA viruses [21]. For most vRdPs, the finger, palm and thumb subdomains accommodate seven conserved structural motifs (homomorphs), each bearing one of the conserved sequence motif described before [24].
All vRdPs evolved from one common ancestral protein [16], [20]. In the past, sequence similarity among vRdPs was used in attempts to reconstruct RNA virus evolutionary history [7], [16], [25]–[31]. Unfortunately, this sequence similarity was shown to be too low to produce an accurate sequence alignment for further phylogenetic analysis [32].
In our current work, we used the structural similarity of vRdPs to reconstruct their evolutionary history. We used the similarities of vRdPs protein structures to produce a highly accurate structure based sequence alignment for our subsequent studies. Moreover, we picked 21 biochemical and structural features of each polymerase and encoded them into the matrix that was used in a phylogenetic analysis to particularize results obtained from structure based sequence alignment analysis. In our phylogenetic analysis, we used Bayesian clustering algorithms, which are ideal for reconstruction of complicated phylogenetic relationships. The resulting phylogenetic tree describing the evolution of vRdPs has high statistical support for most branches. As vRdPs are the only universal gene in all RNA viruses, our phylogenetic tree can be understood as a scheme of RNA virus evolution.
Materials and Methods
Selection of vRdPs for further phylogenetic studies
To find structurally homologous vRdPs, we employed the DALI server [33] using the structure of Dengue virus type 3 (DENV3) RdRP as a query (PDB number 2J7W-A). The program was run under the default conditions. DALI server automatically screens the PDB database to select structurally homologous proteins and lists them according to a decreasing Z-score, a quantitative expression of protein structure similarity [33]. Only protein structures having similarity Z score higher than 2 were taken in account since hits with lower Z-score are most likely incidental hits. The vRdPs were selected among the listed protein structures. They were assigned to the individual virus species classified into genera and families according to the actual ICTV virus taxonomy [34]. Representative structures were selected using the following criteria: (1) Maximally two polymerases from two different viruses were selected from one genus (the exception was four viruses from genus Enterovirus). (2) Structures with bound substrate, substrate analogue and/or template nucleic acid were favored. (3) High resolution structures were preferred. (4) Structures without any mutation were favored. As polymerases are very active enzymes changing their topology in response to many external stimuli (bound template/nucleotide/product, actual step of polymerization cycle, etc.), the criteria for structure selection was set up to select polymerase structures under identical conditions.
The same process described above was done using three structures with the lowest structure homology to 2J7W-A as queries using the DALI sever: 3V81-C (human immunodeficiency virus 1 - HIV1), 2R7W-A (simian rotavirus - SRV) and 2PUS-A (infectious bursal disease virus - IBDV). Sets of structures selected in these three runs were compared with the first set to insure no adequate structures were missed.
Construction of structure superposition and structure based sequence alignment
Structures of selected vRdPs were superimposed using the DALI server multiple structural alignment tool [33]. DALI created structure based sequence alignment was validated and improved using the default settings in T-Coffee Expresso [35]. The resulting alignment was verified by comparison with previously published vRdP alignments [17], [24], [31], [36], [37].
The structure based sequence alignment was analyzed using the JOY server under the default conditions [38]. JOY is a program used for annotation of protein sequence alignments with 3D structural features. It is necessary in understanding the conservation of specific amino acid residues in a specific environment. JOY contains various algorithms such as DSSP [39] used for secondary structure classification. Sequence consensus and sequence conservation were calculated in Chimera implemented algorithms [40], [41].
Analysis of the vRdPs structural similarities between vRdPs
Analysis of conserved amino acid residues and sequence motifs in the structural based sequence alignment as well as presence/absence of conserved structural features was done manually according to criteria previously used in describing vRdPs [20], [24], [42]. Comparative results were encoded into a 21-column character matrix where each column represents a single selected character typical of some but not all vRdPs. The matrix row represents each evaluated polymerase. Structural characters were coded to MrBayes as standard data (0–9). These characters were set as unordered allowing them to move from one state to another (character designated “0” can change to “2” without passing “1”).
Construction of phylogenetic tree
Best fitting model of amino acid substitutions was tested in PROTTEST 2.4 [43] under the Akaike information criterion [44] and the Bayesian information criterion [45]. As results of the two tests were not consistent, we decided to use the most complex model, the general time reversible (GTR) model with a proportion of invariable sites and a gamma-shaped distribution of rates across sites [46], [47]. Bayesian phylogenetic analysis was performed using MrBayes v3.1.2 [48]. Bayesian analysis consisted of two runs with four chains (one cold and three heated), and was run for 10 million generations sampled every 100 generations. The first 25% of samples were discarded as a burning period. Although the average standard deviation of split frequencies was much lower than 0.01, convergence of runs and chains was verified using the AWTY [49]. Analysis was run for sequence data alone and for mixed data (sequence alignment and structural character matrix) with equal settings for analysis.
Results
Formation of representative set of vRdPs
The DALI server queried using the Dengue virus RdRP (2J7W-A) found 745 hits with structure similarity Z-score 2 or higher. Using the criteria described in the Material and methods section, we selected 21 vRdPs protein structures among these hits. In our subsequent query, no additional protein structures were selected from 844, 743 and 575 hits identified using 3V81-C (HIV1), 2R7W-A (SRV), and 2PUS-A (IBDV).
To ensure we did not miss any relevant structure, we browsed the PDB [50] using names of all RNA virus genera listed in the ICTV database. No additional structures were found. A preliminary notice was found about the successful crystallization of Thosea asigna virus RdRP (genus Permutotetravirus, family Permutotetraviridae), but the structure has not yet been published [51].
The final list included 22 vRdPs from 22 virus species in 17 virus genera and 8 virus families (see Table 1 for details). All viral families were classified in the Baltimore classes III (double stranded RNA viruses), IV (positive sense single stranded RNA viruses), and VI (Positive-sense single-stranded RNA viruses that replicate through a DNA intermediate). No polymerases of any virus classified in Baltimore class V (negative sense single stranded RNA viruses) were identified, since there was no known protein structure of any RNA dependent RNA polymerase for these viruses.
Table 1. The list of selected vRdPs.
| Baltimore class | family | genus | virus | abbre-viation | viral RNA dependent polymerase | ||||
| PDB | str. | res. [Å] | cocrystallized molecules | citation | |||||
| +ssRNA viruses | Caliciviriade | Lagovirus | Rabbit hemorrhagic disease virus | RHEV | 1KHV | B | 2,5 | Lu2+ | [90] |
| Norovirus | Murine norovirus | MuNORV1 | 3UQS | A | 2 | SO4 2− | [91] | ||
| Norovirus | NORV | 3BSO | A | 1,74 | Mg2+, CTP, RNA | [92] | |||
| Sapovirus | Sapporo virus | SappV | 2CKW | A | 2,3 | [93] | |||
| Flaviviridae | Flavivirus | Dengue virus 3 | DENV3 | 2J7W | A | 2,6 | Zn2+, GTP | [94] | |
| Japanese encephalitis virus | JEV | 4K6M | A | 2,6 | SAH, SO4 2−, Zn2+ | [95] | |||
| Hepacivirus | Hepatitis C virus 1 | HCV1 | 1NB6 | A | 2,6 | Mn2+, UTP | [96] | ||
| Pestivirus | Bovine viral diarrhea virus | BVDV1 | 1S49 | A | 3 | GTP | [97] | ||
| Leviviridae | Allolevivirus | Enterobacterio phage Qβ | Qβ | 3AVX | A | 2,41 | Ca2+, 3′dGTP, RNA | [98] | |
| Picornaviridae | Aphthovirus | Foot and mouth disease virus | FMDV | 2E9Z | A | 3 | Mg2+, UTP, PPi, RNA | [99] | |
| Enterovirus | Humane rhinovirus 16 A | HuRV16A | 1XR7 | A | 2,3 | [100] | |||
| Coxsackie virus B3 | CoxVB3 | 3CDW | A | 2,5 | PPi | [101] | |||
| Humane rhinovirus 1B | HuRV1B | 1XR6 | A | 2,5 | K+ | [100] | |||
| Poliovirus 1 | PolV | 3OLB | A | 2,41 | Zn2+, ddCTP, RNA | [42] | |||
| ds RNA viruses | Birnaviridae | Aquabirnavirus | Infectious pancreatic necrosis virus | IPNV | 2YI9 | A | 2,2 | Mg2+ | [102] |
| Avibirnavirus | Infectious bursal disease virus | IBDV | 2PUS | A | 2,4 | [103] | |||
| Cystoviridae | Cystovirus | Pseudomonas phage phi6 | Φ6 | 1HI0 | P | 3 | Mn2+, Mg2+, GTP, DNA | [62] | |
| Reoviridae | Orthoreovirus | Mammalian orthoreovirus 3 | MORV3 | 1N35 | A | 2,5 | Mn2+, 3′dCTP, RNA | [104] | |
| Rotavirus | Simian rotavirus Sa11 | SRV | 2R7W | A | 2,6 | GTP, RNA | [105] | ||
| Reverse tran- scribing viruses | Retroviridae | Gammaretrovirus | Moloney murine leukemia virus | MoMLV | 1RW3 | A | 3 | [106] | |
| Lentivirus | Human immunodeficiency virus 2 | HIV2 | 1MU2 | A | 2,35 | SO4 2− | [107] | ||
| Human immunodeficiency virus 1 | HIV1 | 3V81 | C | 2,85 | nepavirine, DNA | [108] | |||
The vRdPs selected as described in Material and methods were assigned to individual viral species, genera, families and Baltimore groups. For each individual vRdP its PDB code (PDB), used protein strand (column str.), resolution (column res.) and cofactor, substrate, template, product molecules (column co-crystallized molecules) are listed.
Structure superposition of vRdPs
The vRdPs from our collection represents a wide range of proteins that are different in protein size and other parameters (see Table 1). Many of them bear additional domains with non-polymerase activities that are conserved only among closely related proteins. These domains were not taken into account for subsequent analysis.
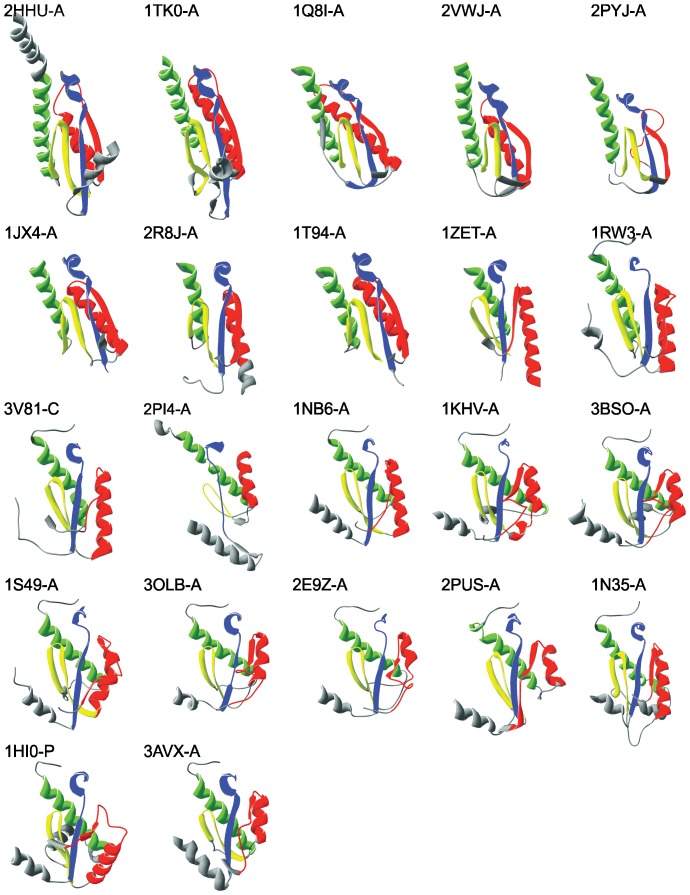
Primary and tertiary structures of domains bearing polymerase activity are similar in all selected proteins. Subdomains finger (F), palm (P), and thumb (T) are collinearly arranged in all vRdPs succeeding always as F1-P1-F2-P2-T from N- to C-terminus (see Figure S1 for details) [20]–[23]. Polymerase domains of selected vRdPs were superpositioned and structures typical for each of the selected viral families are highlighted in Figure 1 (for schematic structure of all vRdPs see Figure S2). Structural superposition shows a conserved architecture of vRdP subdomains and the seven conserved structural homomorphs previously described [24] are clearly visible.
Figure 1. Protein structures of selected vRdPs representatives.
Nine representatives of the selected vRdPs were chosen. Their structures are shown as a ribbon diagram. All molecules are oriented in the same orientation with finger subdomain on the left, the palm on the bottom and the thumb on the right. The catalytic site is positioned in the centre of each molecule and in some protein structures it is enclosed by the finger tips located at the top of each protein structure. Conserved protein structures typical of vRdPs (homomorphs) are highlighted by colours: violet (hmG), dark blue (hmF), dark green (hmA), light green (hmB), yellow (hmC), orange (hmD) red (hmE), and pink (hmH). Molecular rendering in this figure were created with Swiss PDB Viewer.
An additional eighth structural helix-turn-helix motif was observed in the thumb subdomain, we call homomorph H (hmH). Despite the poorly conserved sequence of homomorph H, the structural motif is well conserved in all vRdPs (see Figure 1). To characterize its conservativeness, we calculated its RMSD among all vRdPs and compared it with the RMSD of homomorph D (hmD) that is similar in size. Results showed that hmH is as conserved as the well-established hmD (see Table S1 for further details).
Structural similarities among vRdPs
The structure similarity Z-score was calculated for all polymerase couples (see Table 2) showing extremely high protein structure similarities among vRdPs from viruses classified into one viral genus (see genus Enterovirus as the best example). The similarities among the vRdPs of viruses classified in the same family are slightly lower, but still very high (see family Picornaviridae as the best example). RdRPs of all +ssRNA viruses (except enterobacteriophage Qβ - Qβ) form a cluster of relatively highly similar structures, while structures of pseudomonas phage Φ6 (Φ6), Qβ and Birnaviridae RdRPs are moderately similar, and structures of reoviral RdRPs and retroviral RdDPs are similar only distantly to RdRPs of +ssRNA virus (see Table 2 for details).
Table 2. Comparison of structure similarity Z-score of all vRdPs.
| DENV | JEV | BVDV1 | HCV1 | PolV1 | HuRV16 | HuRV1B | CoxVB3 | FMDV | NORV | MuNORV1 | RHEV | SappV | Φ6 | Qβ | IBDV | IPNV | SRV | MORV3 | HIV1 | HIV2 | ||
| 2J7W-A | 4K6M-A | 1S49-A | 1NB6-A | 3OLB-A | 1XR7-A | 1XR6-A | 3CDW-A | 2E9Z-A | 3BSO-A | 3UQS-A | 1KHV-B | 2CKW-A | 1HI0-P | 3AVX-A | 2PUS-A | 2YI9-A | 2R7W-A | 1N35-A | 3V81-C | 1MU2-A | ||
| JEV | 4K6M-A | 42,9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BVDV1 | 1S49-A | 22,8 | 21,7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HCV1 | 1NB6-A | 20,5 | 17,4 | 27,4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PolV1 | 3OLB-A | 18,1 | 16,8 | 25,3 | 21,5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HuRV16 | 1XR7-A | 18,2 | 16,6 | 25,1 | 20,9 | 52,4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HuRV1B | 1XR6-A | 18,0 | 16,5 | 24,8 | 20,7 | 52,2 | 56,7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CoxVB3 | 3CDW-A | 18,0 | 16,3 | 25,2 | 21,0 | 53,1 | 52,4 | 53,1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| FMDV | 2E9Z-A | 19,2 | 17,2 | 26,5 | 21,6 | 41,5 | 41,3 | 41,0 | 41,6 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NORV | 3BSO-A | 20,5 | 17,5 | 27,1 | 23,8 | 32,0 | 32,3 | 38,1 | 31,8 | 32,4 | - | - | - | - | - | - | - | - | - | - | - | - |
| MuNORV1 | 3UQS-A | 20,9 | 17,7 | 28,0 | 25,2 | 31,1 | 31,5 | 31,2 | 31,4 | 32,2 | 51,0 | - | - | - | - | - | - | - | - | - | - | - |
| RHEV | 1KHV-B | 18,7 | 17,9 | 27,4 | 24,3 | 32,4 | 33,0 | 32,9 | 33,0 | 32,4 | 39,3 | 42,7 | - | - | - | - | - | - | - | - | - | - |
| SappV | 2CKW-A | 17,5 | 15,0 | 24,7 | 20,6 | 30,4 | 30,8 | 30,8 | 30,9 | 30,8 | 39,1 | 39,4 | 43,9 | - | - | - | - | - | - | - | - | - |
| Φ6 | 1HI0-P | 14,8 | 10,6 | 4,1 | 16,4 | 17,2 | 17,0 | 16,9 | 17,7 | 15,7 | 18,5 | 19,1 | 17,7 | 14,1 | - | - | - | - | - | - | - | - |
| Qβ | 3AVX-A | 11,1 | 7,7 | 14,8 | 14,1 | 14,0 | 13,5 | 13,6 | 14,5 | 13,8 | 13,2 | 14,4 | 14,9 | 12,6 | 12,3 | - | - | - | - | - | - | - |
| IBDV | 2PUS-A | 8,4 | 6,6 | 10,7 | 9,5 | 12,1 | 12,1 | 11,9 | 12,6 | 12,9 | 13,4 | 13,3 | 12,6 | 12,9 | 9,5 | 6,0 | - | - | - | - | - | - |
| IPNV | 2YI9-A | 9,8 | 6,7 | 13,9 | 12,9 | 12,4 | 12,3 | 12,1 | 13,0 | 13,5 | 15,5 | 14,2 | 14,0 | 13,2 | 10,7 | 7,7 | 42,5 | - | - | - | - | - |
| SRV | 2R7W-A | 8,9 | 9,0 | 10,2 | 10,5 | 9,7 | 9,4 | 8,3 | 8,4 | 9,3 | 9,4 | 9,1 | 10,4 | 8,5 | 9,9 | 7,8 | 4,6 | 4,6 | - | - | - | - |
| MORV3 | 1N35-A | 6,5 | 4,0 | 10,3 | 7,6 | 7,8 | 7,3 | 7,1 | 7,8 | 8,1 | 7,9 | 7,9 | 8,1 | 8,0 | 8,4 | 8,0 | 6,5 | 6,6 | 15,4 | - | - | - |
| HIV1 | 3V81-C | 4,7 | 1,6 | 6,3 | 6,5 | 5,4 | 5,5 | 4,9 | 4,8 | 5,3 | 5,5 | 5,7 | 5,7 | 4,9 | 3,8 | 5,8 | 2,8 | 2,3 | 4,0 | 5,9 | - | - |
| HIV2 | 1MU2-A | 5,4 | 4,0 | 7,9 | 7,4 | 6,2 | 6,6 | 6,8 | 6,9 | 6,1 | 7,6 | 7,9 | 6,5 | 7,4 | 5,5 | 7,7 | 3,6 | 4,3 | 4,6 | 5,1 | 28,5 | - |
| MoMLV | 1RW3-A | 4,7 | 3,4 | 7,9 | 6,2 | 7,2 | 7,4 | 7,0 | 6,8 | 6,0 | 7,6 | 6,8 | 7,5 | 7,4 | 4,9 | 6,2 | 2,6 | 3,0 | 4,0 | 3,9 | 18,2 | 20,7 |
Individual vRdP structures are introduced by a PBD code-strain and they are assigned to a virus species. Note that structure similarity Z-score is high among vRdPs originating from viruses classified in the same genus (see genus Enterovirus (written in bold) as the best example). Structural similarity is somewhat lower but still high among vRdPs from viruses classified in the same family (see family Picornaviridae (written in italic) as the best example). Structural similarity of vRdPs from viruses classified in different families is significantly lower and is decreasing with excepted phylogenetic relationship. Compare all other families to family Picornaviridae.
We also quantified 21 attributes previously used for vRdPs description and encoded them into a 21-column character matrix (see Table 3). Features were selected and quantified manually according to criteria previously used for describing vRdPs [20], [24], [42] and are included in the Text S1.
Table 3. Matrix describing individual features used in phylogenetic analysis of vRdPs.
| Virus | Family | Genus | PDB ID | Chain | Features | ||||||||||||||||||||
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | |||||
| DENV3 | Flaviviridae | Flavivirus | 2J7W | A | 0 | 0 | 0 | 0 | 0 | 0 | N | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| JEV | Flaviviridae | Flavivirus | 4K6M | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| BVDV1 | Flaviviridae | Pestivirus | 1S49 | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HCV1 | Flaviviridae | Hepacivirus | 1NB6 | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| PolV1 | Picornaviridae | Enterovirus | 3OLB | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| HuRV16 | Picornaviridae | Enterovirus | 1XR7 | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| HuRV1B | Picornaviridae | Enterovirus | 1XR6 | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| CoxVB3 | Picornaviridae | Enterovirus | 3CDW | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| FMDV | Picornaviridae | Aphthovirus | 2E9Z | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| NORV | Caliciviriade | Norovirus | 3BSO | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| MuNORV1 | Caliciviriade | Norovirus | 3UQS | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| RHEV | Caliciviriade | Lagovirus | 1KHV | B | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| SappV | Caliciviriade | Sapovirus | 2CKW | A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Φ6 | Cystoviridae | Cystovirus | 1HI0 | P | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 2 |
| Qβ | Leviviridae | Allolevivirus | 3AVX | A | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| IBDV | Birnaviridae | Avibirnavirus | 2PUS | A | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 |
| IPNV | Birnaviridae | Aquabirnavirus | 2YI9 | A | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 |
| SRV | Reoviridae | Rotavirus | 2R7W | A | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 3 |
| MORV3 | Reoviridae | Orthoreovirus | 1N35 | A | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 3 |
| HIV1 | Retroviridae | Lentivirus | 3V81 | C | 1 | 1 | 2 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| HIV2 | Retroviridae | Lentivirus | 1MU2 | A | 1 | 1 | 2 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| MoMLV | Retroviridae | Gammaretrovirus | 1RW3 | A | 1 | 1 | 2 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
Individual vRdP structures are introduced by PBD code-strain and they are assigned to a virus species. Rows in the matrix represent vRdPs, while the compared features are listed as 21 columns. Compared features are: (A) polymerase product - 0 RNA, 1 DNA; (B) polymerase template - 0 RNA, 1 both DNA and RNA; (C) NA synthesis initiation - 0 de novo, 1 protein primer, 2 RNA primer; (D) overall polymerase domain architecture as described in [23] - 0 active site is encircled by finger tips, 1 active site is open (fingers subdomain do not touch thumb subdomain); (E) polymerase core organization - 0 ABC, 1 CAB; (F) motif F length - 0 normal (motif is F2 is present), 1 short (motif F2 is absent), 2 long (insertion is present in motif F); (G) motif F structure - 0 ββα(310)β, 1 βββ, 2 ββ; (H) F - A (C) motif connection - 0 short (≤35 amino acid residues), 1 long structured (>35 amino acid residues); (I) motif A structure - 0 -310, 1 βα, 2 β310; (J) A–B motif connection - 0 ααββ, 1 αββαββ, 2 ββ; (K) length of helix in motif B - 0 normal (≤21 amino acid residues), 1 long (>22 amino acid residues); (L) kink in motif B - 0 absent, 1 present; (M) B - C (D) motifs connection - 0 very short (≤5 amino acid residues), 1 loop (6–14 amino acid residues), 2 long helical (≥15 amino acid residues, at least 8 amino acid residues long helix); (N) motif C length - 0 short (10 amino acid residues), 1 long (>10 amino acid residues); (O) C (B)–D motifs connection - 0 short loop (≤5 amino acid residues), 1 long loop (>5 amino acid residues); (P) motif D structure - 310α-, 1 α-, 2αβ; (Q) position of helix in motif D - 0 normal position, 1 shifted position; (R) D–E motif connection - 0 short (<20 amino acid residues), 1 long structured (<20 amino acid residues); (S) motif E structure - 0 wide, 1 narrow; (T) thumb domain size - 0 large (>180 amino acid residues), 1 small (<180 amino acid residues); (U) priming motif - 0 none, 1 priming loop in thumb subdomain, 2 priming loop in palm subdomain, 3 polymerase C terminal part. Symbols α, β, 310, and L mean α helix, β strand, 310 helix, and loop, respectively.
Automatically created structure based alignment of selected vRdPs including annotated structural features is depicted in Figures 2, 3, and 4.
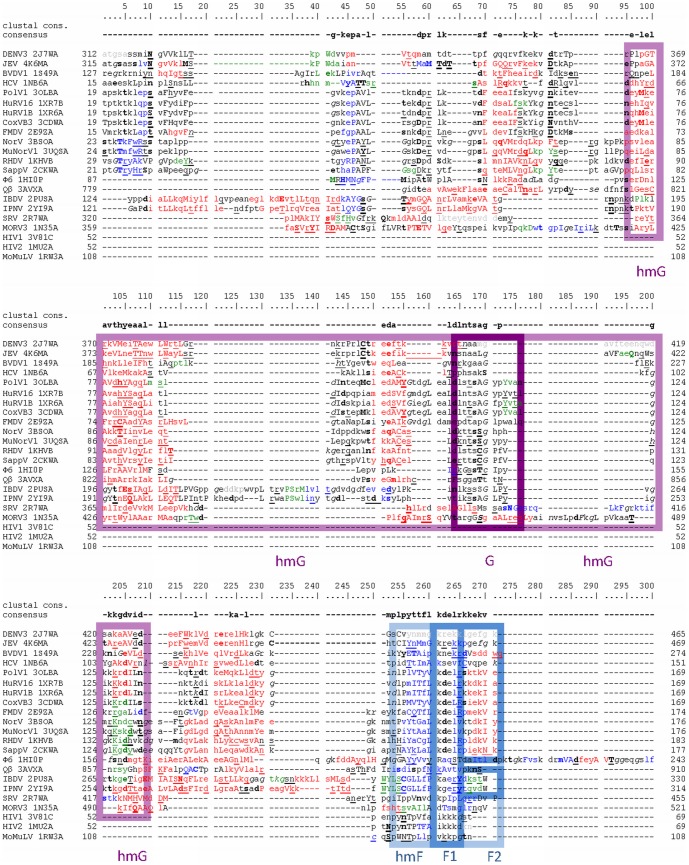
Figure 2. Structure based sequence alignment of vRdPs finger subdomain.
vRdPs are listed at the beginning of each row by the name of the virus encoding the appropriate vRdP followed by vRdP PBD code. The number at the beginning and at the end of each row indicates the position of the first and last amino acid residue on the appropriate row in the full-length protein bearing polymerase activity (including all additional protein domains). The numbering above the alignment describes position of individual amino acid residues in the alignment. Amino acid residues forming α helices, 310 helices, and β strands are written by red, green, and blue, respectively. Solvent accessible amino acid residues are written in lower case letters; solvent inaccessible by upper case letters. Amino acid residues with positive phi torsion angle, amino acid residues hydrogen bound to main-chain amide, or amino acid residues hydrogen bound to main-chain carbonyl are underlined, written in bold, or in italic, respectively. Most frequent amino acid residues at each alignment position are listed in a row called consensus. Highly conserved positions (more than 80%) are indicated by uppercase violet letters. The 100% conserved amino acid residues are shown by uppercase red letters. Most upper row shows Clustal calculated consensus. Amino acid residues in conserved sequence motifs G and F typical for all vRdPs are highlighted by violet and dark blue colour frames. Amino acid residues it the conserved structural homomorhps hmG and hmF are highlighted the same but lighter colours.
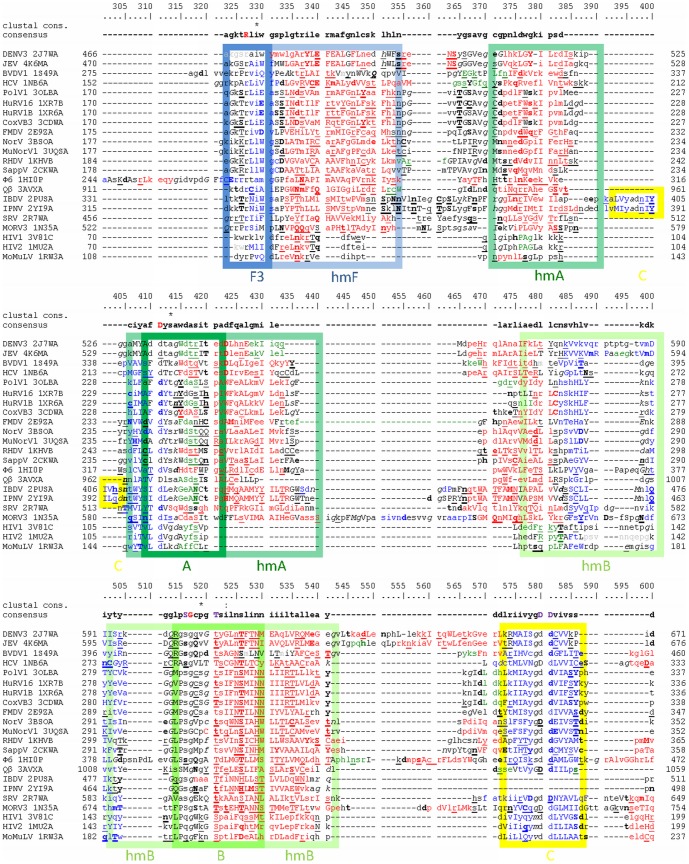
Figure 3. Structure based sequence alignment of vRdPs palm subdomain.
Alignment of vRdPs is as in Figure 2. Amino acid residues in conserved sequence motifs F, A, B, and C are highlighted by dark blue, dark green, light green, and yellow frames. Amino acid residues it the conserved structural homomorhps are highlighted the same but lighter colours. The only three 100% conserved amino acid residues in the entire alignment (an arginine residue at position 327 in motif F, an aspartate residue at position 411 in motif, and a glycine residue at position 517 in motif B). The fourth 100% conserved amino acid residue is an aspartate residue in motif C. Despite this aspartate residue is superpostionable in protein structures, it is placed on different position in structure based sequence alignment of protein primary structures thanks to cyclic permutation in IBDV and IPNV RdRPs (see position 397 for birnaviral RdRPs and position 580 for remaining vRdPs).
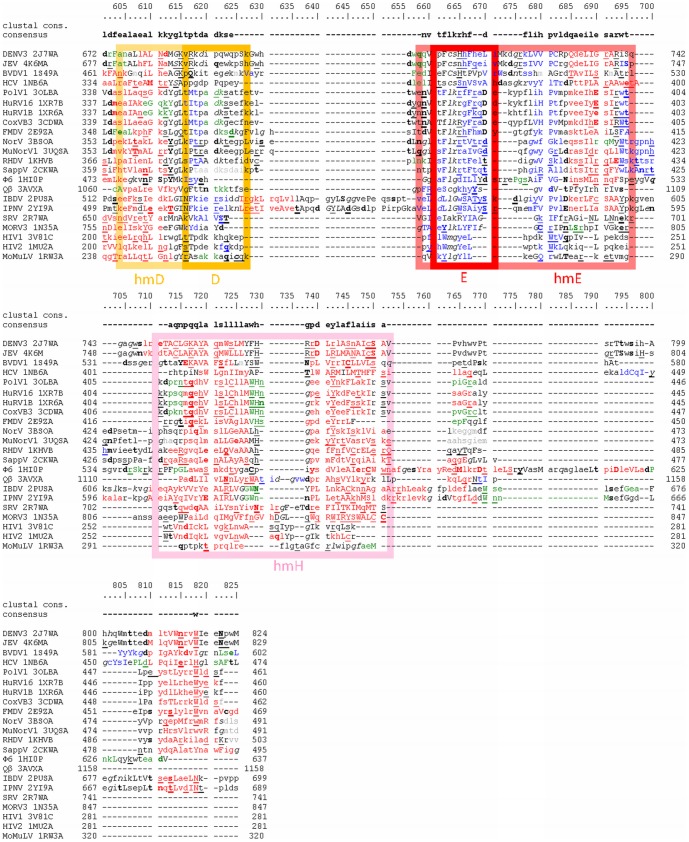
Figure 4. Structure based sequence alignment of vRdPs thumb subdomain.
Alignment of vRdPs is as in Figure 2 and 3. Amino acid residues in conserved sequence motifs D and E are highlighted by orange and red frames. Amino acid residues in the conserved structural homomorhps are highlighted the same but lighter colours. hmH homomorph is highlighted in pink.
Phylogenetic characterization of vRdPs
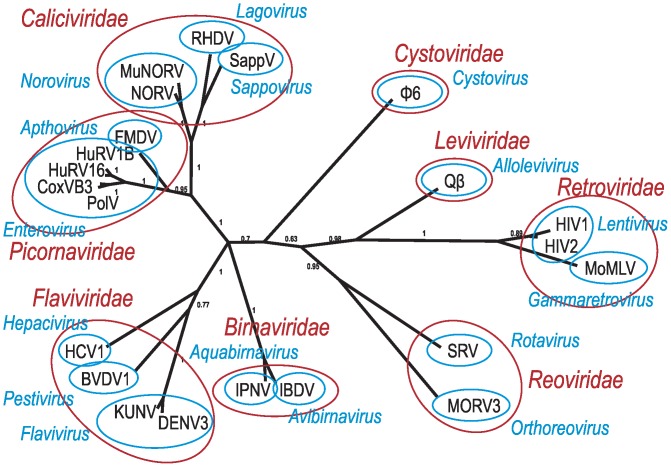
The evolutionary history of vRdPs was reconstructed using the Bayesian clustering analysis. Sequence (structure based sequence alignment) and structural (character matrix) information were used simultaneously in a unified analysis. Combination of these datasets was used to produce a phylogenetic tree with high Bayesian posterior probabilities for most branches (see Figure 5). Despite the high Bayesian support, one polytomy appeared concerning the position of Birnaviridae family.
Figure 5. Phylogenetic tree of vRdPs evolution.
Phylogenetic tree was calculated by an analysis unifying sequence and structure information. Only names of virus species coding vRdPs are listed in the tree. Individual virus species are grouped in genera (blue) and families (red) according actual ICTV virus taxonomy.
Our phylogenetic analysis classified all vRdPs into groups that correspond to the viral genera and families proposed by ICTV. RdDPs of RNA viruses replicating via DNA intermediate (Baltimore class VI) formed a clearly separated group of vRdPs. The RdRPs of +ssRNA and dsRNA viruses clustered together and did not form any separate groups. This suggests that dsRNA viruses evolved from +ssRNA viruses multiple times, and vice versa. The possible evolutionary scenarios of vRdP evolution and its impact on the reconstruction of RNA virus evolution will be discussed further.
Usage of each data set alone was less statistically powerful than the combined analysis (see Figure S3). Despite, our results rely mostly on sequence information incoming from a structure based sequence alignment. The 21-column character matrix served as a stabilizing element that properly placed ambiguous branches and prevent against long branch artifacts (compare Figure S3 panels A and B and Figure 5).
Discussion
Similarities among vRdPs
The vRdPs are an ancient and diversified enzyme group. They share only limited conservation in primary structure, however their protein structure [21], [24] and the mechanism of function [19], [23], [42] are very similar. The vRdPs adopt a conserved right hand conformation with three subdomains termed fingers, palm and thumb. Seven conserved sequence motifs were previously described in vRdPs [16], [17], [37]. Moreover, amino acid residues in these motifs adopt extremely conserved position in vRdPs' [24]. Herein, we described a novel conserved structural motif named homomorph H (hmH) formed by a conserved helix-turn-helix structure in the thumb subdomain of all vRdPs. Despite its high structure conservation, and hmH primary structure is slightly conserved. Function of hmH remains elusive and further biochemical studies will be needed to elucidate it.
Presence of vRdPs in all RNA virus species allows their use in phylogenetic analysis [7], [16], [25]–[31]. This approach was disputed by an extensive study showing the sequence conservation of vRdPs is too low to be successfully and meaningfully used for phylogenetic analysis employing classical methods [32]. The similarities among vRdPs may have evolved by convergent evolution [32], however these conclusions may be challenged by several arguments. 1) The vRdPs share seven conserved sequential collinearly arranged motifs; a phenomenon highly improbable via convergence [16]. 2) The right hand conformation is not the only fold that can be adapted by RNA-dependent polymerases. Cellular RdRPs participating in RNA interference accommodate totally different double barrel conformations [52]. 3) Modern bioinformatics approaches based on Bayesian analyses are more suitable for reconstruction of distant evolutionary relationships [53] than previously described statistical methods [32]. 4) Conserved protein tertiary structure of all vRdPs can supplement missing information in highly diverged protein sequences and allowing us to study the evolution of extremely distantly related proteins [13], [14].
Nevertheless, polymerases can adopt various conformations, changing their topology in response to bound template/incoming nucleotides, steps in polymerization cycle and artificially depending on crystallization conditions. We overcome this by selecting vRdPs' representatives crystallized under similar conditions (see Material and methods).
How did the vRdPs evolve?
Our phylogram shows the RdDP of Retroviridae forms a clearly separate group of RNA viruses replicating via the dsDNA intermediate (Baltimore class VI). This is caused by a series of specific interactions that occurs between template, product and protein, and differs significantly between RdDPs and RdRPs [54]. For example, RdDPs accommodates a conservative aromatic amino acid residue in motif B (alignment position 525 - Figure 3). This position is occupied by aspartate or asparagine interacting with aspartate in motif A (alignment position 416 - Figure 3) in RdRPs discriminating incorporation of dNTPs instead of NTPs [20]. Moreover, the structure of RdDPs is much simpler, many structural motifs are absent, and others are highly reduced [24].
RdRP of the +ssRNA bacteriophage Qβ is the closest relative of retroviral RdDPs. The Qβ polymerase already contains all motifs typical for RdRPs, but is still simpler having no additional structural motifs [55], [56]. As Qβ represents an ancient virus group [57], it is probable that the phylogram may be rooted between Qβ RdRP and retroviral RdRPs.
Rooting the evolutionary tree of vRdPs using cellular right handed polymerases as an outgroup shows, the root is positioned between bacteriophage Qβ RdRP and retroviral RdDPs (Černý et al, under submission). This is in concordance with RNA world theories and theories implicating viruses in the shift from RNA world to DNA world [58].
RdRPs of all RNA viruses are mixed together in our phylogram and they do not follow the Baltimore classification. For example RdRP of +ssRNA Qβ is closely related to the RdRPs of dsRNA viruses than to the RdRPs of other +ssRNA viruses and RdRP of dsRNA birnaviruses tends towards RdRPs of mammalian +ssRNA viruses. The RdRPs can easily replicate both ssRNA and dsRNA without any critical rearrangements in their structure. This is not surprising since picornaviral RdRP were shown to replicate dsRNA even without the aid of a helicase [59].
Primer dependence/independence also apparently evolved multiple times. RdRPs of viruses, which in our phylogram are closer to the expected root (Leviviridae, Reoviridae, Cystoviridae), do not require RNA or protein primer for reaction initialization [60]. This suggests that the original vRdPs were probably primer independent. De novo initiation is also typical for many cellular RdRPs [61].
Primer independent RdRPs of viruses from families Flaviviridae and Cystoviridae share remarkably large thumb subdomains of their RdRPs, allowing accurate positioning of the first incoming nucleotide and RNA polymerization initiation [62]. Despite that both proteins share similar interactions between enzyme, template and incoming nucleotide, the position of the priming motif is different [62].
Viruses from the family Birnaviridae and several other families encode cyclic permuted RdRP [31], [37]. It was suggested that birnaviral RdRPs represents an ancient group of polymerases that split from other polymerases before DdDPs, DdRPs, RdDPs and RdRPs were established as four distinct groups [31]. Our results indicate RdRPs with cyclic permutation are younger and they share a common evolutionary ancestor with RdRPs of +ssRNA virus RdRPs.
What does our model of vRdPs evolution tell us about the evolution of RNA viruses?
Virus evolution is an extremely complicated story. Viral genes and proteins evolve rapidly and relative proteins share only a low degree of homology [3]–[5], making virus phylogenetic reconstruction difficult. It is complicated to generate a proper alignment of selected proteins and the resulting phylograms usually do not have sufficient statistical support [32]. Therefore, a qualitative description of a set of virus features is used for reconstruction of distant phylogenetic virus relationships (capsid architecture, genome replication strategies, etc. [63],[64]). Nevertheless, this approach is sensitive to recombination events between virus and host, or between different viruses, and occurs quite often resulting in a mixture of different genes[65]–[68]. That is why, virus evolution nowadays is not considered as a linear process, but rather as a network [69].
Absence of any universal gene shared by all viruses makes reconstruction of virus evolution even more difficult, despite that some genes are shared among many viruses. An example of such a gene is a jelly-roll capsid protein that is typical for picorna-like viruses (+ssRNA genome), Microviridae, Parvoviridae (both ssDNA), Papylomaviridea, Polyomaviridae (both dsDNA), etc. [70], [71]. Jelly-roll capsid protein, however is an inappropriate candidate for a virus phylogenetic marker, since viruses sharing a jelly-roll capsid protein are only distantly related and protein is missing among closely related virus families.
Presence of the vRdPs in all RNA viruses [15] allowed to use the vRdPs as a marker for RNA virus evolution [28]. Nevertheless, their sequence similarity is too low to be used by classical phylogenetic approaches [32]. We overcome this using structure based homology of vRdPs. Our phylogram describing the evolutionary history of vRdPs may be understood as an evolutive phylogram of RNA viruses. Our results are in concordance with the actual concepts of virus evolution [63], [69] and depict the polyphyletic origin of dsRNA viruses. The first group is represented by Cystoviridae and Reoviridae families, while the second group is represented by the Birnaviridae family. Reoviridae and Cystoviridae share many common features. Both viral groups have similar multilayer capsid organization [72]. They replicate their genome by a conservative manner inside the inner virus capsid [73]. Viruses in Birnaviridae family are more similar to +ssRNA viruses. Their cyclically permuted RdRPs are similar to cyclically permuted RdRPs of +ssRNA viruses from Permutotetraviridae [31]. Moreover, birnaviruses replicate their genome in a semiconservative manner outside the virus capsid [74] using their guanylylated RdRP as a primer [75] that is similar to protein primed replication of picornavirus-like viruses [76], [77].
Mammalian +ssRNA viruses cluster together forming two monophyletic clades. The first is represented by viruses from the family Flaviviridae, while the second by viruses from families Caliciviridae and Picornaviridae. Regardless that the differences between them are smaller than in the case of dsRNA viruses, both these clades differ in the same biological aspect. Flaviviruses replicates their RNA by a primer independent manner [78], [79]. Their genome is either uncapped [80], [81] or capped by 7-methylguanosine cap [82]. Caliciviridae and Picornaviridae use vPg protein primer that also caps their genomes [83]. These similarities between mammalian +ssRNA viruses and Birnaviridae show they evolved from a common ancestor [31], [70], [84].
The last two groups of RNA viruses, families Leviviridae and Retroviridae, are distinctly separated. These two groups seem to be extremely ancient and they probably evolved from the last universal common ancestor of all life forms – even before the cell evolution [64], [85], [86]. This is in concordance with recent theories about evolution of ancient life forms, the transition from the RNA into the DNA word and cell evolution [58].
Only a limited number of vRdP protein structures are known now. Nevertheless, they come out from very diverse viral groups that can serve as representatives of other virus groups (Togaviridae and Coronaviridae would most probably follow Flaviviridae etc.). ThevRdPs with known protein structure come from viruses that are usually important as human or veterinary pathogens or represent important biological models. There is no known vRdP protein structure of any plant, protozoan or fungal virus. Moreover, no protein structure of any –ssRNA virus RdRP is known. Since RdRPs of –ssRNA viruses share many sequence motifs with other vRdPs [87]–[89], their structure will most probably be similar to the structure of other RNA viruses. Likewise, vRdPs structures of plant, protozoan and fungal viruses that are often closely related to animal viruses [68] will probably be similar.
Supporting Information
Linear organization of protein domains of vRdPs. The vRdP polymerase finger, palm and thumb subdomains are highlighted by blue, green and red. Remaining protein domains are colored by yellow. Conserved sequential and structural features are not shown. Diagram is in scale.
(TIF)
Protein structures of all vRdPs involved in analysis. Molecule positioning is the same as in Figures 1. Polymerase subdomains are highlighted as in the Figure S1: finger subdomain by blue, palm subdomain by green, thumb subdomain by red. Other protein domains are not visible. Molecular rendering in this figure were created with Swiss PDB Viewer.
(PDF)
Phylogenetic tree of vRdPs evolution based only on sequence or structure data. Phylogenetic trees were calculated using only sequence (A) or structure (B) borne information. Only names used for virus species coding vRdPs are listed in the tree.
(TIF)
Comparison of hmH and hmE. The RMSD of hmH and hmE were calculated for all individual couples of vRdPs and compared in table. Individual vRdP structures introduced by PBD code-strain are assigned to virus species. Row E shows RMSD values for hmE. Row H shows adequate values for hmH. It is apparent that RMSD values for hmH are comparable with values for hmE and they are often even lower.
(XLSX)
(DOCX)
Acknowledgments
We would like to express our thanks to Filip Husník and Martin Pospíšek for constructive criticism of our work and for interesting suggestions.
Funding Statement
This work was supported by the Czech Science Foundation (P502/11/2116 to DR and P302/12/2490 to LG., www.gacr.cz), by Grant Agency of University of South Bohemia (155/2013/P to LG and Z60220518 to DR, http://www.jcu.cz/research/gaju), by ANTIGONE (278976 to LG, http://www.antigonefp7.eu/ant/), by European Social Fund and the state budget of Czech Republic (CZ.1.07/2.3.00/30.0032 to JJV, http://www.msmt.cz/strukturalni-fondy/op-vpk-obdobi-2007-2013), by AdmireVet project (ED006/01/01 to DR, http://www.vri.cz/en/admirevet), and by the Czech Science Foundation (14-29256S to DR) and CIGA (20134311 to BCB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Steinhauer DA, Domingo E, Holland JJ (1992) Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene 122: 281–288. [DOI] [PubMed] [Google Scholar]
- 2. Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R (2006) Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabanillas L, Arribas M, Lázaro E (2013) Evolution at increased error rate leads to the coexistence of multiple adaptive pathways in an RNA virus. BMC Evol Biol 13: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith DB, McFadden N, Blundell RJ, Meredith A, Simmonds P (2012) Diversity of murine norovirus in wild-rodent populations: species-specific associations suggest an ancient divergence. J Gen Virol 93: 259–266. [DOI] [PubMed] [Google Scholar]
- 5. Pickett BE, Striker R, Lefkowitz EJ (2011) Evidence for separation of HCV subtype 1a into two distinct clades. J Viral Hepat 18: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krupovič M, Bamford DH (2010) Order to the viral universe. J Virol 84: 12476–12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koonin EV, Dolja VV (1993) Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol 28: 375–430. [DOI] [PubMed] [Google Scholar]
- 8. Illergård K, Ardell DH, Elofsson A (2009) Structure is three to ten times more conserved than sequence–a study of structural response in protein cores. Proteins 77: 499–508. [DOI] [PubMed] [Google Scholar]
- 9. Holm L, Sander C (1996) Mapping the protein universe. Science 273: 595–603. [DOI] [PubMed] [Google Scholar]
- 10. Wiens J (2004) The role of morphological data in phylogeny reconstruction. Syst Biol 53: 653–661. [DOI] [PubMed] [Google Scholar]
- 11. Nylander JA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey JL (2004) Bayesian phylogenetic analysis of combined data. Syst Biol 53: 47–67. [DOI] [PubMed] [Google Scholar]
- 12. McGowen MR, Spaulding M, Gatesy J (2009) Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol Phylogenet Evol 53: 891–906. [DOI] [PubMed] [Google Scholar]
- 13. Aravind L, Anantharaman V, Koonin EV (2002) Monophyly of class I aminoacyl tRNA synthetase, USPA, ETFP, photolyase, and PP-ATPase nucleotide-binding domains: implications for protein evolution in the RNA. Proteins 48: 1–14. [DOI] [PubMed] [Google Scholar]
- 14. Scheeff ED, Bourne PE (2005) Structural evolution of the protein kinase-like superfamily. PLoS Comput Biol 1: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baltimore D (1971) Expression of animal virus genomes. Bacteriol Rev 35: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poch O, Sauvaget I, Delarue M, Tordo N (1989) Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J 8: 3867–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruenn JA (2003) A structural and primary sequence comparison of the viral RNA-dependent RNA polymerases. Nucleic Acids Res 31: 1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gohara DW, Crotty S, Arnold JJ, Yoder JD, Andino R, et al. (2000) Poliovirus RNA-dependent RNA polymerase (3Dpol): structural, biochemical, and biological analysis of conserved structural motifs A and B. J Biol Chem 275: 25523–25532. [DOI] [PubMed] [Google Scholar]
- 19. Korneeva VS, Cameron CE (2007) Structure-function relationships of the viral RNA-dependent RNA polymerase: fidelity, replication speed, and initiation mechanism determined by a residue in the ribose-binding pocket. J Biol Chem 282: 16135–16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen JL, Long AM, Schultz SC (1997) Structure of the RNA-dependent RNA polymerase of poliovirus. Structure 5: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 21. Ferrer-Orta C, Arias A, Escarmís C, Verdaguer N (2006) A comparison of viral RNA-dependent RNA polymerases. Curr Opin Struct Biol 16: 27–34. [DOI] [PubMed] [Google Scholar]
- 22. Shatskaya GS, Dmitrieva TM (2013) Structural organization of viral RNA-dependent RNA polymerases. Biochemistry (Mosc) 78: 231–235. [DOI] [PubMed] [Google Scholar]
- 23. Ng KK, Arnold JJ, Cameron CE (2008) Structure-function relationships among RNA-dependent RNA polymerases. Curr Top Microbiol Immunol 320: 137–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang DM, Zemla AT, Zhou CL (2013) Highly similar structural frames link the template tunnel and NTP entry tunnel to the exterior surface in RNA-dependent RNA polymerases. Nucleic Acids Res 41: 1464–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolja VV, Carrington JC (1992) Evolution of positive-strand RNA viruses. Seminars in Virology. pp. 315–326.
- 26.Eickbush TH (1994) Origin and evolutionary relationships of retroelements. In: Morse SS, The evolutionary biology of viruses: Raven Press, 1185 Avenue of the Americas, New York, New York 10036-2806, USA. pp. 121–157. [Google Scholar]
- 27.Goldbach R, Wellink J, Verver J, van Kammen A, Kasteel D, et al. (1994) Adaptation of positive-strand RNA viruses to plants. Arch Virol Suppl 9: 87–97. [DOI] [PubMed]
- 28. Ward CW (1993) Progress towards a higher taxonomy of viruses. Res Virol 144: 419–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koonin EV (1991) The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol 72 (Pt 9): 2197–2206. [DOI] [PubMed] [Google Scholar]
- 30. Bruenn JA (1991) Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res 19: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorbalenya AE, Pringle FM, Zeddam JL, Luke BT, Cameron CE, et al. (2002) The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J Mol Biol 324: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zanotto PM, Gibbs MJ, Gould EA, Holmes EC (1996) A reevaluation of the higher taxonomy of viruses based on RNA polymerases. J Virol 70: 6083–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holm L, Rosenström P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38: W545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (2012) Virus taxonomy: classification and nomenclature of viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, USA. [Google Scholar]
- 35. Armougom F, Moretti S, Poirot O, Audic S, Dumas P, et al. (2006) Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acids Res 34: W604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrer-Orta C, Arias A, Perez-Luque R, Escarmís C, Domingo E, et al. (2004) Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J Biol Chem 279: 47212–47221. [DOI] [PubMed] [Google Scholar]
- 37. Pan J, Vakharia VN, Tao YJ (2007) The structure of a birnavirus polymerase reveals a distinct active site topology. Proc Natl Acad Sci U S A 104: 7385–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mizuguchi K, Deane CM, Blundell TL, Johnson MS, Overington JP (1998) JOY: protein sequence-structure representation and analysis. Bioinformatics 14: 617–623. [DOI] [PubMed] [Google Scholar]
- 39. Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22: 2577–2637. [DOI] [PubMed] [Google Scholar]
- 40. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 41. Meng EC, Pettersen EF, Couch GS, Huang CC, Ferrin TE (2006) Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinformatics 7: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gong P, Peersen OB (2010) Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A 107: 22505–22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- 44. Akaike H (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. [Google Scholar]
- 45. Schwarz G (1978) Estimating the Dimension of a Model. Annals of Statistics 6: 461–464. [Google Scholar]
- 47. Yang Z (1994) Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39: 306–314. [DOI] [PubMed] [Google Scholar]
- 48. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 49.Wilgenbusch JC, Warren DL, Swofford DL (2004) AWTY: A system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference. [DOI] [PubMed]
- 50. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, et al. (2000) The Protein Data Bank. Nucleic Acids Res 28: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrero D, Buxaderas M, Rodriguez JF, Verdaguer N (2012) Purification, crystallization and preliminary X-ray diffraction analysis of the RNA-dependent RNA polymerase from Thosea asigna virus. Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 1263–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salgado PS, Koivunen MR, Makeyev EV, Bamford DH, Stuart DI, et al. (2006) The structure of an RNAi polymerase links RNA silencing and transcription. PLoS Biol 4: e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 54. Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA (1992) Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256: 1783–1790. [DOI] [PubMed] [Google Scholar]
- 55. Kidmose RT, Vasiliev NN, Chetverin AB, Andersen GR, Knudsen CR (2010) Structure of the Qbeta replicase, an RNA-dependent RNA polymerase consisting of viral and host proteins. Proc Natl Acad Sci U S A 107: 10884–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takeshita D, Tomita K (2010) Assembly of Q{beta} viral RNA polymerase with host translational elongation factors EF-Tu and -Ts. Proc Natl Acad Sci U S A 107: 15733–15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Duin J, Tsareva N (2006) Single-stranded RNA phages. In: Calendar RL, The Bacteriophages (Second ed): Oxford University Press. [Google Scholar]
- 58. Forterre P (2002) The origin of DNA genomes and DNA replication proteins. Curr Opin Microbiol 5: 525–532. [DOI] [PubMed] [Google Scholar]
- 59. Cho MW, Richards OC, Dmitrieva TM, Agol V, Ehrenfeld E (1993) RNA duplex unwinding activity of poliovirus RNA-dependent RNA polymerase 3Dpol. J Virol 67: 3010–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Dijk AA, Makeyev EV, Bamford DH (2004) Initiation of viral RNA-dependent RNA polymerization. J Gen Virol 85: 1077–1093. [DOI] [PubMed] [Google Scholar]
- 61. Makeyev EV, Bamford DH (2002) Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell 10: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 62. Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI (2001) A mechanism for initiating RNA-dependent RNA polymerization. Nature 410: 235–240. [DOI] [PubMed] [Google Scholar]
- 63. Ahlquist P (2006) Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol 4: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bamford DH, Grimes JM, Stuart DI (2005) What does structure tell us about virus evolution? Curr Opin Struct Biol 15: 655–663. [DOI] [PubMed] [Google Scholar]
- 65. Scheel TK, Galli A, Li YP, Mikkelsen LS, Gottwein JM, et al. (2013) Productive homologous and non-homologous recombination of hepatitis C virus in cell culture. PLoS Pathog 9: e1003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith LM, McWhorter AR, Shellam GR, Redwood AJ (2013) The genome of murine cytomegalovirus is shaped by purifying selection and extensive recombination. Virology 435: 258–268. [DOI] [PubMed] [Google Scholar]
- 67. Pond SL, Murrell B, Poon AF (2012) Evolution of viral genomes: interplay between selection, recombination, and other forces. Methods Mol Biol 856: 239–272. [DOI] [PubMed] [Google Scholar]
- 68. Dolja VV, Koonin EV (2011) Common origins and host-dependent diversity of plant and animal viromes. Curr Opin Virol 1: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Koonin EV, Dolja VV (2012) Expanding networks of RNA virus evolution. BMC Biol 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koonin EV, Wolf YI, Nagasaki K, Dolja VV (2008) The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Microbiol 6: 925–939. [DOI] [PubMed] [Google Scholar]
- 71. Ravantti J, Bamford D, Stuart DI (2013) Automatic comparison and classification of protein structures. J Struct Biol 183: 47–56. [DOI] [PubMed] [Google Scholar]
- 72. Poranen MM, Bamford DH (2012) Assembly of large icosahedral double-stranded RNA viruses. Adv Exp Med Biol 726: 379–402. [DOI] [PubMed] [Google Scholar]
- 73. Lawton JA, Estes MK, Prasad BV (2000) Mechanism of genome transcription in segmented dsRNA viruses. Adv Virus Res 55: 185–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cortez-San Martín M, Villanueva RA, Jashés M, Sandino AM (2009) Molecular characterization of IPNV RNA replication intermediates during the viral infective cycle. Virus Res 144: 344–349. [DOI] [PubMed] [Google Scholar]
- 75. Dobos P (1995) Protein-primed RNA synthesis in vitro by the virion-associated RNA polymerase of infectious pancreatic necrosis virus. Virology 208: 19–25. [DOI] [PubMed] [Google Scholar]
- 76. Wimmer E, Hellen CU, Cao X (1993) Genetics of poliovirus. Annu Rev Genet 27: 353–436. [DOI] [PubMed] [Google Scholar]
- 77. Buck KW (1996) Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv Virus Res 47: 159–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Urcuqui-Inchima S, Patiño C, Torres S, Haenni AL, Díaz FJ (2010) Recent developments in understanding dengue virus replication. Adv Virus Res 77: 1–39. [DOI] [PubMed] [Google Scholar]
- 79. Rice CM (2011) New insights into HCV replication: potential antiviral targets. Top Antivir Med 19: 117–120. [PMC free article] [PubMed] [Google Scholar]
- 80. Wang C, Sarnow P, Siddiqui A (1993) Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J Virol 67: 3338–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pérard J, Leyrat C, Baudin F, Drouet E, Jamin M (2013) Structure of the full-length HCV IRES in solution. Nat Commun 4: 1612. [DOI] [PubMed] [Google Scholar]
- 82. Cleaves GR, Dubin DT (1979) Methylation status of intracellular dengue type 2 40 S RNA. Virology 96: 159–165. [DOI] [PubMed] [Google Scholar]
- 83. Goodfellow I (2011) The genome-linked protein VPg of vertebrate viruses - a multifaceted protein. Curr Opin Virol 1: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gorbalenya AE, Koonin EV (1988) Birnavirus RNA polymerase is related to polymerases of positive strand RNA viruses. Nucleic Acids Res 16: 7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koonin EV, Dolja VV (2006) Evolution of complexity in the viral world: the dawn of a new vision. Virus Res 117: 1–4. [DOI] [PubMed] [Google Scholar]
- 86. Holmes EC (2011) What does virus evolution tell us about virus origins? J Virol 85: 5247–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Poch O, Blumberg BM, Bougueleret L, Tordo N (1990) Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol 71 (Pt 5): 1153–1162. [DOI] [PubMed] [Google Scholar]
- 88. Müller R, Poch O, Delarue M, Bishop DH, Bouloy M (1994) Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J Gen Virol 75 (Pt 6): 1345–1352. [DOI] [PubMed] [Google Scholar]
- 89. Lukashevich IS, Djavani M, Shapiro K, Sanchez A, Ravkov E, et al. (1997) The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol 78 (Pt 3): 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ng KK, Cherney MM, Vazquez AL, Machin A, Alonso JM, et al. (2002) Crystal structures of active and inactive conformations of a caliciviral RNA-dependent RNA polymerase. J Biol Chem 277: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 91. Mastrangelo E, Pezzullo M, Tarantino D, Petazzi R, Germani F, et al. (2012) Structure-based inhibition of Norovirus RNA-dependent RNA polymerases. J Mol Biol 419: 198–210. [DOI] [PubMed] [Google Scholar]
- 92. Zamyatkin DF, Parra F, Alonso JM, Harki DA, Peterson BR, et al. (2008) Structural insights into mechanisms of catalysis and inhibition in Norwalk virus polymerase. J Biol Chem 283: 7705–7712. [DOI] [PubMed] [Google Scholar]
- 93. Fullerton SW, Blaschke M, Coutard B, Gebhardt J, Gorbalenya A, et al. (2007) Structural and functional characterization of sapovirus RNA-dependent RNA polymerase. J Virol 81: 1858–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yap TL, Xu T, Chen YL, Malet H, Egloff MP, et al. (2007) Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J Virol 81: 4753–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lu G, Gong P (2013) Crystal Structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PLoS Pathog 9: e1003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O'Farrell D, Trowbridge R, Rowlands D, Jäger J (2003) Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): structural evidence for nucleotide import and de-novo initiation. J Mol Biol 326: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 97. Choi KH, Groarke JM, Young DC, Rossmann MG, Pevear DC, et al. (2004) Design, expression, and purification of a Flaviviridae polymerase using a high-throughput approach to facilitate crystal structure determination. Protein Sci 13: 2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takeshita D, Tomita K (2012) Molecular basis for RNA polymerization by Qβ replicase. Nat Struct Mol Biol 19: 229–237. [DOI] [PubMed] [Google Scholar]
- 99. Ferrer-Orta C, Arias A, Pérez-Luque R, Escarmís C, Domingo E, et al. (2007) Sequential structures provide insights into the fidelity of RNA replication. Proc Natl Acad Sci U S A 104: 9463–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Love RA, Maegley KA, Yu X, Ferre RA, Lingardo LK, et al. (2004) The crystal structure of the RNA-dependent RNA polymerase from human rhinovirus: a dual function target for common cold antiviral therapy. Structure 12: 1533–1544. [DOI] [PubMed] [Google Scholar]
- 101. Gruez A, Selisko B, Roberts M, Bricogne G, Bussetta C, et al. (2008) The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol 82: 9577–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Graham SC, Sarin LP, Bahar MW, Myers RA, Stuart DI, et al. (2011) The N-terminus of the RNA polymerase from infectious pancreatic necrosis virus is the determinant of genome attachment. PLoS Pathog 7: e1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Garriga D, Navarro A, Querol-Audí J, Abaitua F, Rodríguez JF, et al. (2007) Activation mechanism of a noncanonical RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A 104: 20540–20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tao Y, Farsetta DL, Nibert ML, Harrison SC (2002) RNA synthesis in a cage—structural studies of reovirus polymerase lambda3. Cell 111: 733–745. [DOI] [PubMed] [Google Scholar]
- 105. Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, et al. (2008) Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure 16: 1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Das D, Georgiadis MM (2004) The crystal structure of the monomeric reverse transcriptase from Moloney murine leukemia virus. Structure 12: 819–829. [DOI] [PubMed] [Google Scholar]
- 107. Ren J, Bird LE, Chamberlain PP, Stewart-Jones GB, Stuart DI, et al. (2002) Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors. Proc Natl Acad Sci U S A 99: 14410–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Das K, Martinez SE, Bauman JD, Arnold E (2012) HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol 19: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear organization of protein domains of vRdPs. The vRdP polymerase finger, palm and thumb subdomains are highlighted by blue, green and red. Remaining protein domains are colored by yellow. Conserved sequential and structural features are not shown. Diagram is in scale.
(TIF)
Protein structures of all vRdPs involved in analysis. Molecule positioning is the same as in Figures 1. Polymerase subdomains are highlighted as in the Figure S1: finger subdomain by blue, palm subdomain by green, thumb subdomain by red. Other protein domains are not visible. Molecular rendering in this figure were created with Swiss PDB Viewer.
(PDF)
Phylogenetic tree of vRdPs evolution based only on sequence or structure data. Phylogenetic trees were calculated using only sequence (A) or structure (B) borne information. Only names used for virus species coding vRdPs are listed in the tree.
(TIF)
Comparison of hmH and hmE. The RMSD of hmH and hmE were calculated for all individual couples of vRdPs and compared in table. Individual vRdP structures introduced by PBD code-strain are assigned to virus species. Row E shows RMSD values for hmE. Row H shows adequate values for hmH. It is apparent that RMSD values for hmH are comparable with values for hmE and they are often even lower.
(XLSX)
(DOCX)