Abstract
cAMP is a pivotal second messenger that regulates numerous biological processes under physiological and pathological conditions, including cancer, diabetes, heart failure, inflammation and neurological disorders. In the past, all effects of cAMP were initially believed to be mediated by PKA and cyclic nucleotide-regulated ion channels. Since the discovery of EPAC proteins in 1998, accumulating evidence has demonstrated that the net cellular effects of cAMP are also regulated by EPAC. The pursuit of the biological functions of EPAC has benefited from the development and applications of a growing number of pharmacological probes targeting EPAC proteins. In this Perspective, we seek to provide a concise update on recent advances in the development of chemical entities including various membrane-permeable analogues of cAMP and newly discovered EPAC-specific ligands from high throughput assays and hit-to-lead optimizations.
1. Introduction
Cyclic adenosine monophosphate (cAMP, cyclic AMP or 3′–5′-cyclic adenosine monophosphate) is a pivotal second messenger derived from its precursor adenosine triphosphate (ATP). A wide variety of extracellular ligands bind to G-protein coupled receptors (GPCRs), activate adenylate cyclases (ACs) to catalyze the conversion of intracellular ATP to pyrophosphate and cAMP.1,2 cAMP regulates a number of key biological processes under physiological and pathological conditions, including neuronal signaling, gluconeogenesis, glycogenolysis, lipogenesis, cardiac and smooth muscle contraction, secretory processes, ion channel conductance, learning and memory.1
The local concentration and distribution of intracellular cAMP is regulated by ACs and the cyclic nucleotide phosphodiesterases (PDEs). Generally, numerous extracellular signals trigger a series of the conformational changes of GPCRs on the cell surface. Typically, Gs protein stimulates ACs to increase cAMP production inside the cell, whereas Gi protein inhibits ACs and lowers the level of cAMP.2–6 Meanwhile, the intercellular level of cAMP is degraded by PDEs which catalyze conversion of cAMP to 5′-AMP.7
In the past, all effects of cAMP were initially believed to be mediated by protein kinase A (PKA) and cyclic nucleotide-regulated ion channels.8–11 In 1998, two independent groups reported their findings that PKA-independent mechanism of cAMP action was regulated by a family of guanine nucleotide exchange factors (GEFs) called cAMP-GEFs which are also named as exchange protein directly activated by cAMP (EPAC).12,13 Since then, remarkable progress has been made on elucidating the molecular mechanism of EPAC proteins over the last fifteen years. Meanwhile, probing the biological functions of EPAC has been significantly facilitated by the development and applications of small-molecule EPAC ligands including various membrane-permeable analogues of cAMP and newly discovered EPAC-specific antagonists. Consequently, numerous additional biological functions of EPAC have been uncovered. This review briefly summarizes the structures of EPAC family members, EPAC signaling pathway and biological functions, and also provides a perspective on recent advances in the discovery of new chemical entities targeting EPAC proteins. In addition, these valuable pharmacological tools including cAMP analogues and EPAC antagonists have led to a greater understanding of the important role of EPAC proteins in different diseases, establishing EPAC proteins as novel molecular targets for new therapeutic strategies against various human diseases including cancer, diabetes, heart failure, inflammation and neurological disorders.
2. EPAC Family and EPAC2 Protein Structures
To date, two isoforms of EPAC have been identified, EPAC1 and EPAC2, which are also known as RAPGEF3 (cAMP-GEF-I) and RAPGEF4 (cAMP-GEF-II), respectively.12–14 As depicted in Figure 1, each EPAC family member composes an auto-inhibitory amino-terminal regulatory region and a carboxyl-terminal catalytic region for activation of Rap GTPase.14–18 The regulatory region contains a Dishevelled Egl-10 Pleckstrin (DEP) domain and at least one functional cyclic nucleotide binding domain (CNBD, one for EPAC1 and two for EPAC2). The carboxyl-terminal catalytic region consists of a Ras exchange motif (REM) domain and a Ras association (RA) domain as well as the CDC25-homology domain (CDC25-HD). The CDC25-homology domain is responsible for guanine nucleotide exchange activity and catalyzes the exchange of G-protein-bound GDP for GTP on the Ras-like small GTPases Rap1 and Rap2 isoforms.19,20
Figure 1.
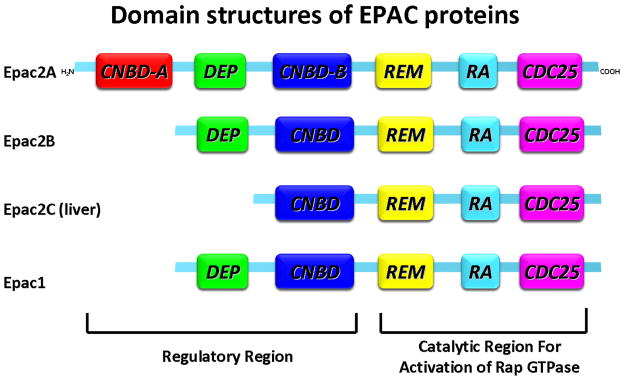
Domain structures of EPAC proteins. Each EPAC family member composes an auto-inhibitory amino-terminal regulatory region and a carboxyl-terminal catalytic region for activation of Rap GTPase. The regulatory region contains a Dishevelled Egl-10 Pleckstrin (DEP) domain and at least one functional cyclic nucleotide–binding domain (CNBD). The carboxyl-terminal catalytic region consists of a Ras exchange motif (REM) domain and a Ras association (RA) domain as well as the CDC25-homology domain (CDC25-HD). The CDC25-homology domain is responsible for guanine nucleotide exchange activity and catalyzes the exchange of G-protein-bound GDP for GTP on the Ras-like small GTPases Rap1 and Rap2 isoforms.
The two EPAC isoforms EPAC1 and EPAC2 are mostly expressed in both mature and developing tissues with different expression levels. EPAC1 is highly expressed in central nervous system, adipose tissue, blood vessels, kidney, ovary, and uterus, while EPAC2 is most detectable in the central nervous system, adrenal gland, and pancreas.21 EPAC2 has been found to exist as three splice variants. EPAC2A is expressed in cerebral cortex and pancreatic islets and has two cAMP-binding domains, whereas expression of EPAC2B is restricted to adrenal glands without cAMP-binding domain A. EPAC2C is found in the liver and lack of cAMP-binding domain A and DEP domain.22
The X-ray crystal structure determinations and analysis of two different conformations of EPAC2 protein have revealed in atomic detail that cAMP binding causes conformation change allowing the catalytic region to be available for the binding of Rap (Figure 2).15,16 NMR spectroscopy studies, peptide amide hydrogen/deuterium exchange mass spectrometry (DXMS) approach, small angle X-ray scattering, fluorescence resonance energy transfer (FRET) studies and molecular dynamics (MD) simulations further demonstrated the mechanism of autoinhibition and activation of EPAC2.23–32 In the absence of cAMP, the apo-EPAC2 protein exists in an autoinhibited conformation (inactive state), in which the cyclic nucleotide–binding domains sterically hinder the access of down-regulating effectors to the catalytic domain, effectively inhibiting the guanine nucleotide exchange activity. Upon binding of cAMP, a sequence of structural reorganizations within the cyclic nucleotide–binding domains allows the regulatory domain to move to the back side of the catalytic region, exposing the Rap-binding site of the catalytic region. It is worth noting that while X-ray structural analysis reveals no significant conformational changes between the CDC25-HD in inactive and active stages,15,16 DXMS studies indicate that in addition to relieving the steric hindrance imposed upon the catalytic lobe by the regulatory lobe, cAMP may also act as an allosteric modulator affecting the interaction between EPAC2 and Rap1 by inducing conformational changes at the ionic latch/hairpin structure, which is directly involved in Rap1 binding.32
Figure 2.
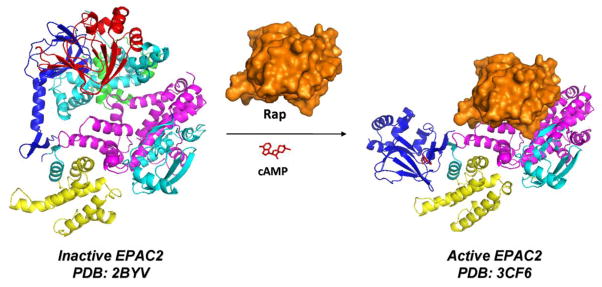
The X-ray crystal structures of inactive EPAC2 (the full-length apo-EPAC2, PDB: 2BYV) and active EPAC2 (EPAC2Δ305:Sp-cAMPS:Rap1B complex, PDB: 3CF6). CNBD-A, DEP domain, CNBD-B, REM domain, RA domain, and CDC25-HD are colored in red, green, blue, yellow, cyan, and magentas, respectively. Upon binding of cAMP, a sequence of structural reorganizations within the cyclic nucleotide–binding domains allows the regulatory domain to open and move to the back side of the catalytic region. This conformational change leads to the exposure of the catalytic region for binding of Rap GTPase to catalyze the exchange of GDP for GTP.
3. EPAC Signaling Pathway and Biological Functions
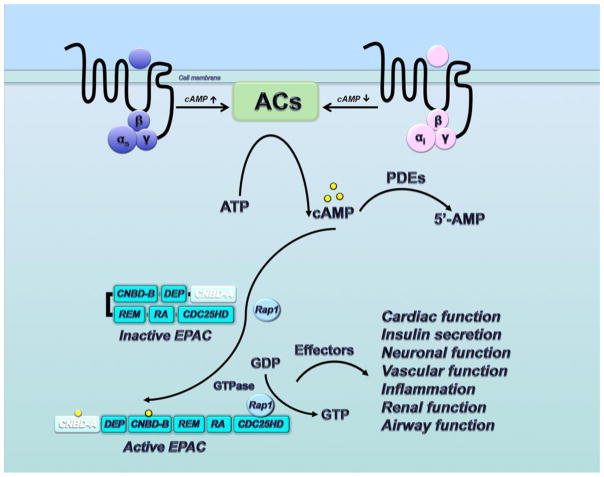
Cellular cAMP levels are modulated by hormones and neuromediators with specific effects on the homeostasis of multicellular organisms. The elevation in intracellular concentration of cAMP after Gs protein-coupled receptors activation is sufficient to activate EPAC. As a part of the downstream targets of cAMP, EPAC proteins as guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP on the small GTPases Rap1 and Rap2.14 Rap1 and Rap2 as members of the Ras family are involved in the network of multiple proteins to regulate cell adhesion and cell junction. EPAC proteins act as the critical upstream effectors, among many other Rap GEFs, controlling the Rap-mediated biological processes of cAMP.19,33 The signaling pathway of EPAC proteins is depicted in Figure 3. Accumulating evidence has demonstrated that EPAC signaling has a significant contribution in various biological processes, including cardiac function, insulin secretion, neuronal function, vascular function, inflammation, renal function, and airway function.34,35
Figure 3.
Signaling pathway of EPAC proteins. EPAC1 only contains one functional cyclic nucleotide binding domain (CNBD-B), while EPAC2 contains two CNBDs (CNBD-A and CNBD-B).
3.1. EPAC and Cardiac Function
In a collection of specialized muscle cells called cardiac myocytes, cAMP modulates a series of cardiac functions such as heart rate, cardiac contractility and relaxation.36,37 Sustained elevation of cAMP induces hypertrophy, cardiac dysfunction and eventually to the development of heart failure.38–43 Given the critical role of cAMP in cardiac function, more and more studies focused on the cardiac function of EPAC proteins, providing plenty of evidence that EPAC is a new regulator in cardiac physiology and pathophysiology.37,38,44–49 Overexpression of EPAC1 in left ventricular hypertrophy of animal models and patients demonstrated that EPAC1 contributes to the progression of heart failure.47,48 Very recent work using knockout mice for EPAC1, EPAC2, or both has revealed that β1-adrenergic receptor (β1-AR) activation causes EPAC2-mediated sarcoplasmic reticulum (SR) Ca2+ leak and arrhythmias via Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ)-dependent phosphorylation of ryanodine receptor 2 (RyR2)-S2814, indicating that this pathway contributes to β-AR-induced arrhythmias and reduced cardiac function.50 Thus, EPAC proteins represent novel therapeutic targets for the treatment of cardiac disorders.
3.2. EPAC in Insulin Secretion
EPAC2 is highly expressed in the insulin-producing pancreatic β-cells.51 A growing body of biological studies have demonstrated that EPAC2/Rap1 signaling pathway is essential in the regulation of the dynamics of insulin secretion.52 The initial studies including in vivo experiments in EPAC2 knockout mice indicated that the traditional antidiabetic drugs sulfonylureas might interact with EPAC2 to activate Rap1.53,54 Despite the controversial debate, this finding implicates that EPAC2 contributes to glucose-induced insulin secretion.55–57 Additional studies suggest that several intracellular signaling pathways with relation to insulin secretion are linked with EPAC2 such as recent discovered pathway EPAC2-Rim2-Rab3.58 The relevant genetic and pharmacological studies concerning EPACs’ involvement in glucose homeostasis and energy balance, through regulation of leptin and insulin signaling pathways, were discussed in a very recent review.59 The accumulating evidence supports that EPAC may represent a novel target to provide therapeutic potential for the treatment of diabetes and obesity.
3.3. Neuronal Function of EPAC
Both EPAC1 and EPAC2 are predominant in the brain including the hippocampus, striatum, and prefrontal cortex, cerebral cortex, cerebellum, olfactory bulb, thalamus, habenula, and pituitary.13,60 Compelling evidence has demonstrated that EPAC proteins have a significant contribution in the development and function of the nervous system.40,61 In addition, a recent study using EPAC knockout mice provided the direct in vivo evidence that EPAC1 and EPAC2 proteins synergistically regulated neurological functions in the brain for processing spatial learning and social interactions.62 Additional studies also showed that EPAC2-deficient mice exhibited robust deficits in social and communication behaviors, indicating potential contributions in brain disorders.63 Thus, targeting EPAC signaling pathway can be considered as a promising strategy for the treatment of various neurological disorders such as autism, anxiety and depression, schizophrenia and Alzheimer’s disease.63–73
3.4. EPAC and Vascular Function
The vascular endothelium regulates the extravasation of solutes, macromolecules, and cells between the circulating blood and the adjacent tissues. Accumulating evidence suggests that the EPAC signaling pathway controls cAMP-mediated hormones on endothelial function including cell adhesion, endothelial barrier function and gap junction formation in vascular endothelial cells.74–86 In addition, EPAC1-Rap signaling has been reported to regulate vascular permeability, intracellular responses, extracellular matrix adhesion and migration and inflammatory processes.87–89
3.5. EPAC in Inflammation
As mentioned above, EPAC1 modulates inflammatory processes by regulating the immune responses. During inflammation, leukocytes extravasate from the blood into the extravascular space to regulate leukocyte and transendothelial migration and integrin-mediated adhesion. Several studies have shown that EPAC1 activation modulates numerous inflammatory mediators in various leukocytes.33,79 In addition, EPAC1 enhances leukocyte adhesion and migration as a proinflammatory mediator.34,71,83,90–94 Intriguingly, recent studies using two mouse models of hyperalgesic priming provide new insights into the role of EPAC in chronic inflammatory pain. It has demonstrated that increasing the expression of GPCR kinase 2 (GRK2) in nociceptors or decreasing EPAC1 inhibited chronic hyperalgesia, suggesting that therapies targeted at tuning the balance between GRK2 and EPAC1 levels may have promise to prevent and treat chronic pain.95
3.6. Renal Function of EPAC
EPAC proteins are also involved in many physiological processes in the kidney including ion transport and cellular proliferation. EPAC1 and EPAC2 are highly expressed by all three segments of the proximal tubules and are enriched at the brush border membrane.96 Recent studies indicate that EPAC1 and/or EPAC2 represent a novel therapeutic target for diabetes-related diseases, particularly kidney failure. Although the exact mechanism of EPAC proteins -especially the contribution of EPAC1 and EPAC2 - to these diseases remains to be elucidated, the development of EPAC-specific activators or inhibitors as pharmacological probes will expedite the discovery process and biological characterization.97–101
3.7. Airway Function of EPAC
Because of the critical role of cAMP in modulating airway functions, it was anticipated that cAMP-induced regulation of these processes including airway smooth muscle contraction and proliferation could be mediated by EPAC. Recent studies in human airway smooth muscle cells showed that cigarette smoke extract reduced EPAC1 expression.71 Interestingly, the reduction of EPAC1 expression was also found in lung tissue from patients with chronic obstructive pulmonary disease (COPD).71 More and more findings have demonstrated that EPAC-mediated signaling represents a novel key pathway in airway fibroblasts and airway smooth muscle cells.102–104
4. Small Molecules Targeting EPAC
Since the discovery of the EPAC proteins in 1998, to better understand their functions and elucidate the EPAC-mediated signaling pathways under both physiological and pathological conditions, there is an increasing need to develop EPAC-specific ligands including agonists and antagonists as pharmacological probes and potential molecular therapeutics for human diseases. Several EPAC-selective and PKA-selective cAMP analogues have been identified as powerful pharmacological tools to distinguish the signaling pathways of EPAC and PKA in numerous biological processes.105,106 However, cAMP analogues could not discriminate the functions of EPAC1 and EPAC2 as two major isoforms share extensive sequence homology. Although some non-nucleotide small molecules such as brefeldin A were shown to inhibit EPAC function, there was lack of evidence to suggest that these compounds directly interact with EPAC.65,107–109 Early in 2012, Cheng’s research group reported a robust high throughput screening (HTS) assay by using a fluorescent cAMP derivative to identify EPAC-specific antagonists from compound libraries110–112. After that, Lezoualc’h and colleagues reported their validated fluorescence-based HTS assay on the basis of the ability of EPAC to regulate the nucleotide exchange activity of Rap1 for screening EPAC ligands113. Recently, by collaborating with Cheng, our team made substantial medicinal chemistry effort on hit-to-lead optimizations.114–116. The structure-activity relationships of cAMP analogues and newly discovered EPAC-specific antagonists, as well as the potential applications of these chemical probes for new therapeutic strategies will be discussed herein.
4.1. cAMP analogues as EPAC ligands
Early systematic studies focusing on structure-activity relationships of cAMP derivatives suggest that modification on 3′- and 5′-positions of the cyclophosphate ring of cAMP (1) with nitrogen or sulfur diminishes the affinity of cAMP analogues towards PKA (Figure 4). Further introduction of various substituents at these positions was found to be inappropriate.117,118 The cAMP binding to PKA is sensitive to most modifications of the cyclic phosphate ring, while modifications on the exocyclic phosphate-oxygen result in several more interesting findings. For example, replacement of the exocyclic phosphate-oxygen by sulfur generated two diastereoisomers, Sp-cAMPS (2) and Rp-cAMPS (3), respectively (Figure 4).119–121 Compound 2 is an activator of both PKA and EPAC, but has an affinity about 10-fold lower than cAMP. Compound 3 is a weak partial activator of PKA that acts as a competitive inhibitor at physiological PKA concentrations. It binds weakly to EPAC, where it acts as an inhibitor.122 Taken together, these results indicate that modification on the cyclic nucleotide is very sensitive for the activation properties.122–126 Further modification on 2 and 3 at the position 2 or 8 of the adenine base with different spacers has provided a set of valuable tools for chemical proteomics approaches.123
Figure 4.
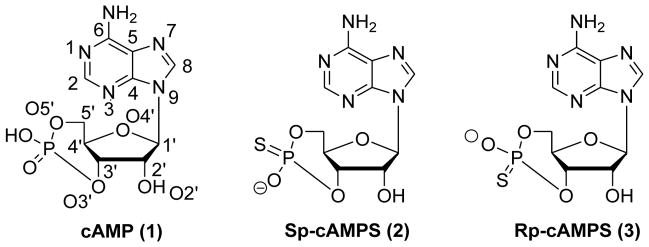
Chemical structures of cAMP molecule (1) and cAMP analogues with modified exocyclic oxygen atom (2 and 3).
More recently, there has been remarkable progress in understanding the molecular mechanism of PKA and EPAC activation. In particular, X-ray crystal structure determinations of PKA and EPAC2 have revealed the structural information of the amino acid residues around the cAMP binding site. From comparison of the primary amino acid sequences of the different cAMP-binding domains between PKA and EPAC, it was noticed that there is a highly conserved glutamate residue only present in the cAMP binding domains of the PKA.71,127,128 Insight into the structural basis indicated that 2′-OH on the ribose ring is essential for the binding to the conserved glutamate residue in the cAMP binding domain of PKA.
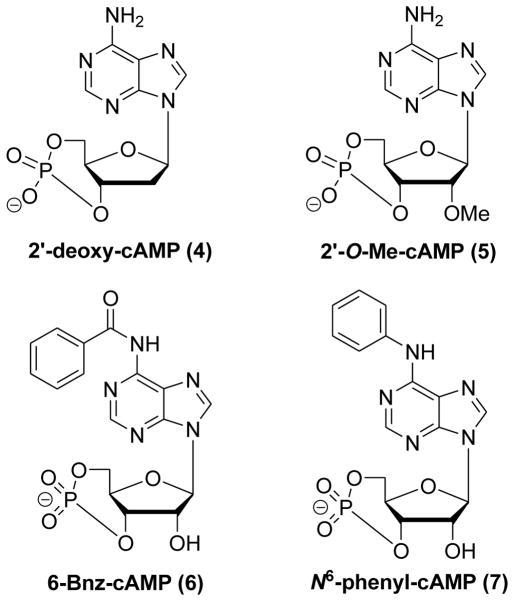
Several systematic structure–activity relationship investigations were carried out on 2′-OH on the ribose ring, the N6-position and the 8-position of the adenine moiety of cAMP. A variety of new cAMP analogues displayed the ability to selectively activate EPAC or PKA. 2′-deoxy-cAMP (4) with the 2′-OH moiety replaced by hydrogen selectively activated EPAC without any effect towards PKA (Figure 5).122,129 Despite compound 4 exhibiting some selectivity towards EPAC versus PKA, it is not an ideal EPAC activator due to a binding affinity >1000-fold less than cAMP itself.71 Further detailed evaluation showed that 2′-O-alkyl-modified cAMP analogues such as 2′-O-Me-cAMP (5) displayed more potent EPAC1 activation than cAMP and were unable to activate PKA, and these compounds displayed about 10 to 100-fold improved EPAC/PKA binding selectivity (Figure 5).64 These findings further suggest that the 2′-OH group is important for binding affinity and selectivity.64,106,127,128,130
Figure 5.
Chemical structures of 2′-deoxy-cAMP (4), 2′-O-Me-cAMP (5), 6-Bnz-cAMP (6) and N6-phenyl-cAMP (7).
In contrast, modifications on the N6-position of cAMP abolished the ability of cAMP analogues to activate EPAC but not PKA. For instance, 6-Bnz-cAMP (6) and N6-phenyl-cAMP (7) are full PKA activators and inefficient EPAC activators (Figure 5).64,122 This implies that suitably 6-substituted cAMP analogues may differentiate their selectivity between PKA and EPAC. It is worth mentioning that the generally low efficiency of the 6-modified analogues is likely not ascribed to the steric hindrance resulting from the introduction of bulky substituents. Some analogues with small groups, such as an oxygen atom at 6-position (like that in cGMP), are even less bulkier than that of cAMP and still have a lower affinity to EPAC.61
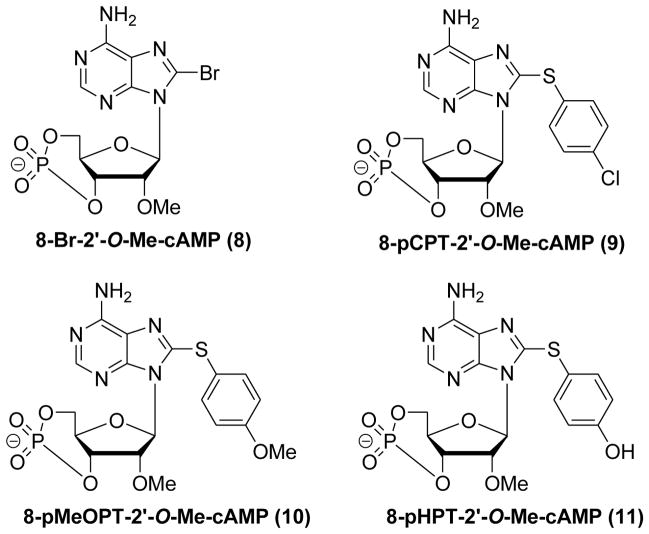
Systematical modifications on the 8-position of cAMP revealed that 8-substituted cAMP analogues possess differences in charge accommodation, hydrogen bonding and adaptability between cAMP homologous binding sites.129,131,132 In addition, the lipophilicity of cAMP analogues with the hydrophobic substitutions at 8-position has a major impact on their cell membrane permeability.106 As shown in Figure 6, several 2′-O-Me-cAMP analogues with modifications on the 8-position have been synthesized and their abilities to activate EPAC and PKA were compared. 8-Br-2′-O-Me-cAMP (8) and 8-pCPT-2′-O-Me-cAMP (9, a.k.a. 007) have been found not to activate PKA with an enhanced EPAC/PKA binding selectivity about three orders of magnitude. Detailed SAR studies have revealed that the S-phenyl ring on 8-position with para-substituted chloro (9), methoxy (10), or hydroxyl (11) can improve the binding affinity selectively towards EPAC (Figure 6).
Figure 6.
Chemical structures of 8-Br-2′-O-Me-cAMP (8), 8-pCPT-2′-O-Me-cAMP (9), 8-pMeOPT-2′-O-Me-cAMP (10) and 8-pHPT-2′-O-Me-cAMP (11).
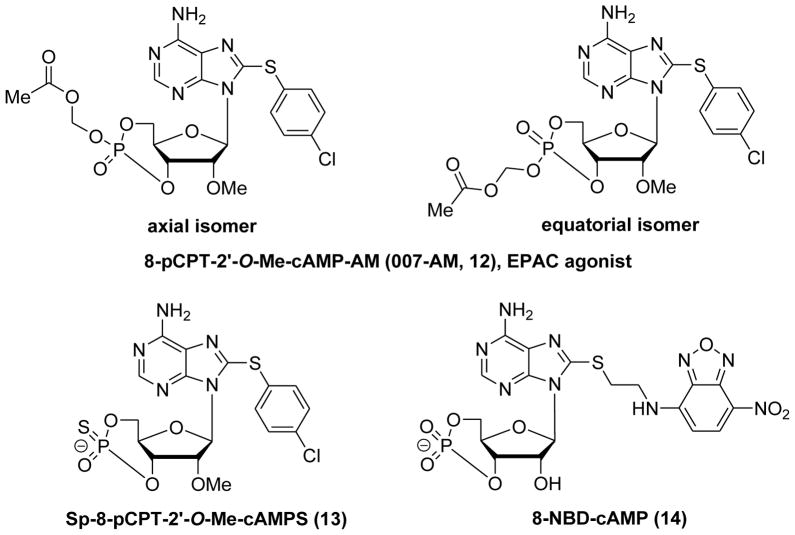
EPAC-selective cAMP analog 9 was administered in several animal models, demonstrating its biological applications under physiological circumstances. For instance, EPAC activation by intraplantar injection of 9 was found to induce hyperalgesia via a PKCε-dependent route.95,133 Intrarenal administration of 9 reduced renal failure in a mouse model for ischemia-reperfusion injury.134 Intrahippocampal injection of 9 improved fear memory retrieval in contextual fear conditioning through regulating hippocampal EPAC2 signaling.135 Although 9 was used as a handy pharmacological tool to elucidate the critical role of EPAC proteins in cAMP signaling pathway, the relatively low membrane permeability limited its further biological applications due to its negatively charged phosphate group. Introduction of an acetoxymethyl ester to 9 to mask the negatively charged, singly bonded oxygen on the phosphate group generated the labile ester 12 which consists of the equatorial and the axial isomers (Figure 7).136 This prodrug-like molecule is capable of penetrating cell membranes more efficiently and can be intracellularly hydrolyzed directly by cellular esterases to release the biologically active parent compound 9. 8-pCPT-2′-O-Me-cAMP-AM (12) has thus become a powerful tool in EPAC and PKA related research. Compound Sp-8-pCPT-2′-O-Me-cAMPS (13) has been further developed as a highly specific EPAC activator with high lipophilicity and membrane permeability, while it is not metabolized by phosphodiesterases (Figure 7).68,136,137 Despite significant progress on development of cAMP analogues as EPAC ligands, most of the selectivity studies with these cAMP analogues were based on EPAC1, none of which has been known to distinguish between the isoforms EPAC1 and EPAC2. In addition, the off-target effects of cAMP analogues including 9 to other targets such as PDEs are also an issue that needs to be addressed.4,7,137
Figure 7.
Chemical structures of 8-pCPT-2′-O-Me-cAMP-AM (12), Sp-8-pCPT-2′-O-Me-cAMPS (13) and 8-(2-[7-nitro-4-benzofurazanyl]aminoethylthio)adenosine-3′, 5′-cyclic monophosphate (14, 8-NBD-cAMP).
4.2. Non-nucleotide Small Molecules as EPAC Antagonists
As discussed above, although the EPAC-selective cAMP analog 9 as an EPAC activator has proven very useful for the elucidation of EPAC-mediated signaling pathways, there remains an urgent need to develop non-nucleotide small molecules as additional EPAC-selective and isoform-specific ligands (e.g. antagonists) for expediting the discovery of new biological roles and functions of EPAC in human diseases as well as potential therapeutic strategies.
Cheng and colleagues first reported their sensitive and robust HTS assay for identifying antagonists that directly compete with a fluorescent cAMP derivative 8-NBD-cAMP (14, Figure 7) in binding to purified full-length EPAC2.110 This assay was based on the finding that the fluorescent signal changed in a dose-dependent manner after binding of 8-NBD-cAMP to EPAC2. In addition, more than 100-fold increase of the fluorescent signal was altered under near saturating EPAC2 concentration. Furthermore, the addition of excess unlabelled cAMP decreased the fluorescent signal in a dose-dependent manner. Consistent with previous findings,138 alteration of the magnitude of fluorescence signal was only modest with a limited increase upon 8-NBD-cAMP binding to EPAC1 protein. Nevertheless, the significant reversible fluorescence change made it a remarkable readout for developing a sensitive and robust HTS assay for screening EPAC antagonists with high reproducibility.110
The pilot screening was preformed simply and effectively in 96-well format by using the NCI DTP (Developmental Therapeutics Program) diversity set library. Three compounds, NSC45576 (15), NSC119911 (16) and NSC686365 (17) (Figure 8) have been identified to significantly decrease the fluorescence signal with apparent IC50 values of 1.7 μM, 3.8 μM and 7.9 μM, respectively. Further validation demonstrated that these compounds not only inhibited EPAC2 GEF activity but also inhibited EPAC1-mediated Rap1 nucleotide exchange at 25 μM.110 The counter-screening assays measuring type I and II PKA holoenzyme activities were performed to test the specificity of these compounds. The results revealed that 15 and 17 do not block cAMP mediated PKA activation suggesting that these two compounds are EPAC specific antagonists that selectively inhibit cAMP-induced EPAC activation.
Figure 8.
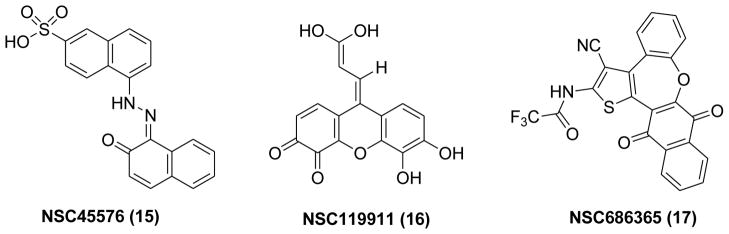
Chemical structures of identified EPAC inhibitors 15–17.
Further studies through a more extensive screen of the Maybridge Hitfinder compound library (14,400 compounds) under a 384-well format led to the discovery of seven HTS hits 18–24 (ESI-04 to ESI-10) that are able to inhibit EPAC2 activity completely at 25 μM in the presence of the equal concentration of cAMP.111
As ESI-06 (20) and ESI-08 (22) shared the same fragment of 5-cyano-6-oxo-1,6-dihydro-pyrimidine (Figure 9), further exploration of the structural determinants on this scaffold have been investigated.114 Extensive structure–activity relationship (SAR) analysis has resulted in the identification of two more potent EPAC antagonists HJC0197 (25) and HJC0198 (26) (Figure 10). Compound 26 with a cyclopropyl moiety and 25 with the 6-cyclopentyl moiety instead of the 6-cyclohexyl group displayed enhanced activity with the IC50 values of 4.0 μM and 5.9 μM, respectively. Further evaluation revealed that 25 also inhibits EPAC1-mediated Rap1-GDP exchange activity, while 26 is a little more specific for EPAC2.114
Figure 9.
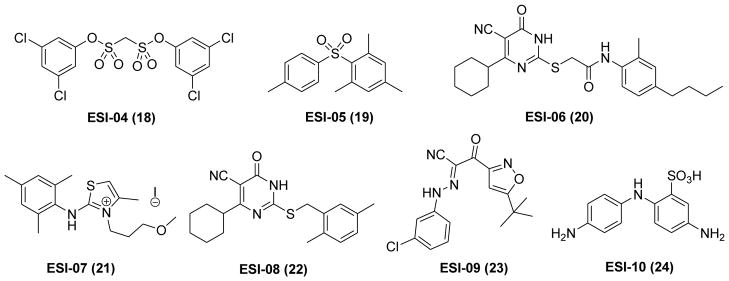
Chemical structures of HTS hits 18–24.
Figure 10.
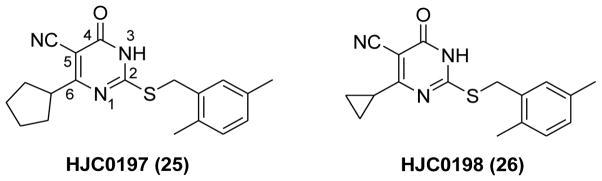
Chemical structures of 25 and 26.
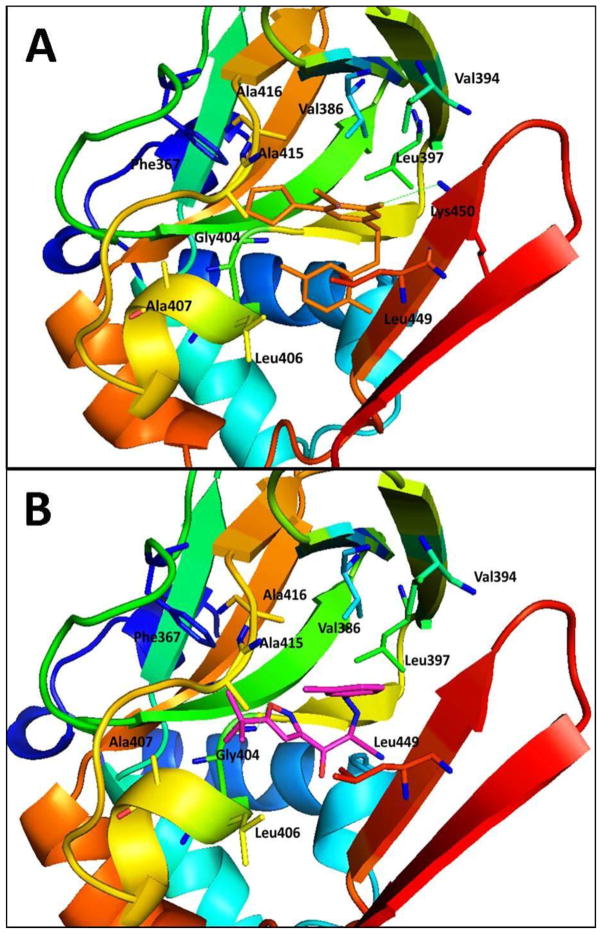
Molecular docking studies using the AutoDock Vina algorithm114,139,140 revealed that the hydrophobic groups of C-6 position and 2-position are critical for the binding affinity towards EPAC2. The SAR results and the representative docking data also indicated that optimization on C-6 position may lead to identification of more EPAC2-specific antagonists. As depicted in Figure 11A, the cyclopentyl group at the C-6 position interacts with the hydrophobic residues of Phe367, Ala415 and Ala416, while the hydrophobic S-benzyl moiety at 2-position forms interactions with Leu406 and Leu449. Further evaluation of 25 and 26 showed that both compounds did not alter cAMP-induced type I and II PKA holoenzymes activation at 25 μM, suggesting that both compounds are EPAC-specific inhibitors. The results in HEK293/EPAC1 and HEK293/EPAC2 cells have demonstrated that 25 and 26 can suppress EPAC1 and EPAC2 function in vitro.114
Figure 11.
Predicted binding mode and molecular docking of 25, and 23 into the cAMP binding domain B (CNBD-B) of EPAC2 protein. Important residues are drawn in sticks. Hydrogen bonds are shown as dashed green lines. (A) Binding mode of 25 (orange). The cyclopentyl group at the C-6 position interacts with the hydrophobic residues of Phe367, Ala415 and Ala416, while the hydrophobic S-benzyl moiety of 2-position forms interactions with Leu406 and Leu449. (B) Binding mode of 23 (pink). The tert-butylisoxazolyl moiety forms a hydrogen bond with the residue Gly404 and interacts with the hydrophobic residues of Phe367, Leu406, Ala407, and Ala415. Meanwhile, 3-chlorophenyl fragment forms hydrophobic interactions with residues Val386, Val394 and Leu397.
Further evaluation of cAMP-mediated EPAC1 GEF activity of seven hits (Figure 9) has revealed that compounds ESI-05 (19) and ESI-07 (21) are unable to inhibit EPAC1 GEF activity. Additional investigation on the specificity of 19 and 21 showed that neither of them displayed significant effects towards PKA, indicating that they are specific targeting EPAC2 protein. It is important to note that ESI-05 and ESI-07 inhibited EPAC2 activity with IC50 values of 0.4 ± 0.1 μM and 0.6 ± 0.1 μM, respectively, whereas cAMP showed an IC50 value of 40 ± 1 μM. Further studies demonstrated that 19 and 21 selectively modulated EPAC activation in living cells. Moreover, EPAC- and PKA-based fluorescence resonance energy transfer (FRET) sensor in living cells and Rap1-GTP pull-down assays further confirmed these results.111 Recent detailed biophysical study further confirmed 19 as a direct and selective inhibitor of EPAC2 with a binding affinity about 20-fold higher than cAMP.141
As 19 and 21 are exclusively EPAC2-specific antagonists with no effect toward EPAC1 protein, the potential mechanism of these small chemical probes was further investigated to analyze the effect of 21 binding to EPAC2 protein using deuterium exchange mass spectrometry (DXMS) approach. The resulting data indicated that EPAC2-specific antagonists may bind to the interface of CNBD-A and CNBD-B and lock the EPAC2 protein in its autoinhibitory conformation.111 Although other possible binding modes might not be excluded, the identification and characterization of these pharmacological probes capable of selectively targeting the EPAC2 protein represent a major milestone in the exploration of the complex cAMP signaling pathway. These EPAC2-specific antagonists have provided a new platform for further development.
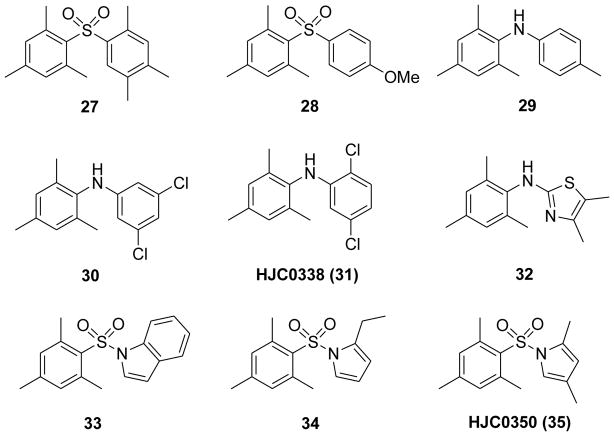
Previous SAR studies and the molecular docking results demonstrated that appropriate hydrophobic moieties of the molecules play an essential role for the specificity and potency towards EPAC2 protein. Based on the scaffold of 19, the 2,4,6-trimethylphenyl moiety was retained as a privileged fragment in the preliminary medicinal chemistry effort for hit-to-lead optimization. Consistent with the previous SAR studies, the 2,4,6-trimethylphenyl ring as an appropriate hydrophobic pharmacophore is important for the potency. For instance, compound 27 with such a privileged fragment and another appropriate hydrophobic group was also found to be a potent antagonist with an IC50 of 0.7 μM, while the methoxy compound 28 exhibited only moderate potency (Figure 12).115
Figure 12.
Chemical structures of representative diarylsulfones 27, 28 and N,N-diarylamines 29–32, and arylsulfonamides 33–35.
Based on the above SAR findings and the hit ESI-10 (24), a series of N,N-diarylamine derivatives have been designed, synthesized and characterized.115 Compound 29 showed an enhanced IC50 of 3.8 μM in comparison with 24. It is worth highlighting that 30 and HJC0338 (31) with the 3,5-dichloro or 2,5-dichloro displayed high potency with IC50 values of 0.9 and 0.4 μM, respectively (Figure 12). However, compound 32 containing a thiazole ring was found inactive with a dramatic loss of activity. SAR analysis of these N,N-diarylamine analogues further demonstrated that an appropriate hydrophobic phenyl moiety is preferable for competing with 8-NBD-cAMP binding to EPAC2.115
On the basis of the structural features of the preliminary SAR studies and the hit ESI-05, a series of arylsulfonamide derivatives as a novel chemical entity of EPAC2 antagonists have been designed and characterized (Figure 12).115 Compound 33 with an indole ring displayed a moderate potency with an IC50 of 1.2 μM. Compound 34 with the 2-ethylpyrrole moiety as a hydrophobic fragment showed higher potency with an IC50 of 0.5 μM, while 2,4-dimethylpyrrole derivative HJC0350 (35) displayed a significantly enhanced activity with an IC50 of 0.3 μM. The systematic and extensive SAR results have also revealed that appropriate hydrophobic pharmacophores are important for the potency of these EPAC ligands. Further validation studies have demonstrated that these three series including diarylsulfones, N,N-diarylamines, and arylsulfonamides (Figure 12) are EPAC2-specific antagonists. In addition, live cell imaging studies using EPAC1, EPAC2, or PKA FRET reporters also confirmed that compound 35 is an effective EPAC2-specific antagonist.115
3-(5-tert-Butyl-isoxazol-3-yl)-2-[(3-chlorophenyl)-hydrazono]-3-oxo-propionitrile (ESI-09, 23) has also been indentified and characterized as a novel non-cyclic nucleotide EPAC2 antagonist with an IC50 of 7.0 μM (Figure 9).112 Meanwhile, 23 inhibited cAMP-mediated EPAC1 GEF activity with an IC50 of 3.2 μM but no effect towards PKA, indicating that it is an EPAC-specific antagonist. Molecular docking studies based on the scaffold of 23 provided useful insight to design more potent antagonists. As shown in Figure 11B, the tert-butylisoxazolyl moiety forms a hydrogen bond with the residue Gly404 and interacts with the hydrophobic residues of Phe367, Leu406, Ala407, and Ala415. Meanwhile, 3-chlorophenyl fragment forms hydrophobic interactions with residues Val386, Val394 and Leu397. Further biological functional investigation showed that 23 specifically suppressed Akt phosphorylation, EPAC-mediated Rap1 activation and insulin secretion in pancreatic β cells. Using hit 23 as a pharmacological probe, the functional roles of EPAC1 in pancreatic cancer migration and invasion have been discovered, indicating that EPAC1 may represent an attractive therapeutic target for pancreatic cancer.112
Previous studies have revealed that activation of cAMP-EPAC pathway in hypothalamus induced multiple indices of leptin resistance, suggesting that EPAC may represent a novel pharmacological target for obesity.142 A more recent study demonstrated that resistance to high fat diet in EPAC1 knockout mice improved glucose tolerance, heightened leptin signaling and induced obesity. Compound 23 enhanced leptin signaling in organotypic hypothalamic slice culture system. Furthermore, administration of 23 in wild-type mice significantly reduced plasma leptin. These findings support the notion that EPAC1 may represent a novel therapeutic target for diabetes or obesity.143
Very recent studies showed that genetic EPAC1 knockout mice protected them from fatal rickettsiosis, nearly completely blocked rickettsial attachment and/or invasion into the endothelial cells.144 Importantly, pharmacological inhibition of EPAC1 in vivo using 23 completely recapitulates the EPAC1 knockout phenotype. Treatment with 23 in wild-type mice significantly protected them against rickettsial infection with much milder disease manifestations and dramatically improved survival, indicating that EPAC1 is a potential target for the prevention and treatment of fatal rickettsioses. 23 as the selective pharmacological probe has proven successful in unraveling the in vivo functions of EPAC1 to overcome potential limitations of knockout mouse models, and may provide potential novel therapeutics.144 Meanwhile, 23 showed excellent tolerability during the in vivo studies, indicating its low toxicity to animals.144 In addition, 23 displays no significant inhibitory effects on PDEs, as well as very weak inhibitory activities towards hERG and CYP450 enzymes (unpublished data). All these combined observations support that such non-nucleotide small molecules may have more advantages in terms of off-target effects and toxicities than cAMP analogs, although more extensive preclinical ADMET assessments remain to be explored for the clinical development.
Since compound 23 was identified as a novel EPAC-specific pharmacological probe to discern the physiologic functions of EPAC in vitro and in vivo, a practical and efficient method to readily prepare 23 at large scale was developed including a one-pot synthesis of isoxazole synthon, modified protocol for cyanomethyl ketone and a coupling step.116 The development of this efficient synthetic route for 23 will greatly facilitate the ongoing medicinal chemistry efforts of lead optimization and SAR studies in searching for new molecules with improved DMPK profiles. This is particularly important in light of a recent report by Rehmann suggesting that compounds 23 and 25 exhibit non-specific effects on protein stability when used at high concentrations above 50 μM.141 While more detailed characterizations are required to sort out the actual causes and mechanisms of such effects, the ability of 23 to recapitulate the genetic EPAC knockout phenotypes in multiple biological systems both in vitro and in vivo suggests that this class of compounds is specific to EPAC at lower concentrations or appropriate doses.112,143,144 Therefore, it is imperative to further optimize these lead compounds through rational drug design approaches to develop advanced candidates for IND-enabling studies.
Lezoualc’h and colleagues reported another functional fluorescence-based high throughput screening assay to identify EPAC inhibitor compounds from chemical libraries.113 This variant of the assay is on the basis of the ability of EPAC to catalyze the nucleotide exchange activity of Rap1.145 Tetrahydroquinoline CE3F4 (36) has been identified as EPAC1 inhibitor that blocked EPAC1-induced Rap1 activation both in cell-free systems and in intact cells and did not influence protein kinase A holoenzyme activity (Figure 13).113 Preliminary structure-activity study revealed that the formyl moiety on position 1 (37) and the bromo group on position 5 (38) of this class of analogues were essential for their activity targeting the EPAC1 (Figure 13). Although the additional data revealed that 36 and its analogues could also inhibit EPAC2, 36 and related compounds may serve as new chemical leads for further structural optimizations and useful pharmacological tools for determining the biological functions of EPAC1 in a wide range of biological processes.113,146 Further investigation on the activity of the (R)- and (S)-enantiomers of 36 revealed that (R)-CE3F4 (39) is a more potent cAMP antagonist than the racemic 36 and (S)-enantiomer (Figure 13). (R)-enantiomer 39 displays 10-fold selectivity for EPAC1 over EPAC2. The biochemical evaluation of tetrahydroquinoline analogues is very helpful in elucidating the structural features that are essential for high EPAC1 inhibitory activity.146
Figure 13.
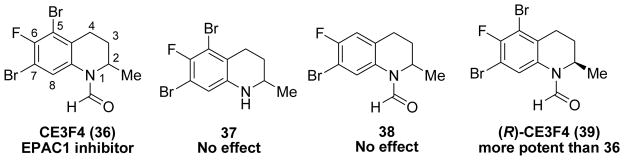
Chemical structures of tetrahydroquinoline analogues 36–39.
5. Conclusions and Future Directions
Since the discovery of EPAC proteins as the novel cAMP mediators in 1998, significant progress has been made by many pioneers in discriminating between PKA- and EPAC-mediated effects under physiological and pathophysiological circumstances.147 X-ray crystal structure determinations and NMR spectroscopy techniques have provided novel insights into the mechanism of cAMP activation of cyclic nucleotide binding domains. In particular, the development of the EPAC-selective cAMP analog 9 (8-pCPT-2′-O-Me-cAMP, 007) through rational design based on structure/sequence alignment analysis contributed to identification of new biological functions. Moreover, its prodrug 12 (8-pCPT-2′-O-Me-cAMP-AM, 007-AM) with improved cell permeability further delivered the reliable insights into the multiplicity of the biologic properties of EPAC. However, the potency, selectivity and specificity, as well as the off-target effects of cAMP analogues remain essential for further improvement.
Recent studies on development of the sensitive and robust HTS assays provided highly valuable non-nucleotide small molecules as EPAC-specific ligands (e.g. antagonists) for elucidating the signaling pathways and dissecting the physiological functions. Meanwhile, it is important to screen the specificity of new EPAC modulators using cells with deletion of EPAC1, EPAC2 or both, as well as their off-target effects to other proteins such as PDEs. Thus, developing new methodologies of chemical biology in this regard is also urgently needed. It is worthy to mention that the combination of genetic knockout animal models and small molecular EPAC-specific modulators has proven very useful to reveal the in vivo physiological functions of EPAC. Treatment with the EPAC-specific ligands with favorable in vivo bioavailability in wild-type animal models may overcome the potential shortcomings of the genetic knockout manipulations such as the secondary effects due to the complex signaling pathways and the possible physiological compensation.
It is the opinion of these authors that further HTS efforts to discover new chemical entities as EPAC-specific activators, and further medicinal chemistry efforts to optimize the currently available antagonists with enhanced potency, specificity to each of the isoforms, and drug-like properties, as well as the in vivo pharmacological evaluation in relevant disease models of lead candidates are highly anticipated to pave the way for the ultimate development of clinical applications. Given that EPAC proteins have been implicated to play important roles in major human pathological conditions such as cancer, neurological disorders, infectious disease, heart disease and diabetes, we believe that it is of great value in the development of pharmacological tools to probe the functions of EPAC in disease processes, and it is the hope that such efforts will eventually lead us to the development of advanced compounds into a clinical setting as potential mechanism-based therapies for those diseases.
Acknowledgments
This work was supported by grants R01 GM106218, R01 GM066170, R21 NS066510, P30 DA028821, and R21 MH093844 from the National Institutes of Health, R. A. Welch Foundation Chemistry and Biology Collaborative Grant from Gulf Coast Consortia (GCC) for Chemical Genomics, a training fellowship from the Keck Center for Interdisciplinary Bioscience Training of the GCC (NIGMS grant T32 GM089657), John Sealy Memorial Endowment Fund, and the Technology Development Foundation of Fuzhou University (Project Numbers 2013-XQ-8 and 2013-XQ-9).
ABBREVIATIONS USED
- cAMP
3′–5′-cyclic adenosine monophosphate or cyclic AMP
- ATP
adenosine triphosphate
- GPCRs
G-protein coupled receptors
- ACs
adenylate cyclases
- PDEs
phosphodiesterases
- PKA
protein kinase A
- GEFs
guanine nucleotide exchange factors
- EPAC
exchange protein directly activated by cyclic AMP
- CNBD
cyclic nucleotide binding domain
- DEP
Dishevelled Egl-10 Pleckstrin
- REM
Ras exchange motif
- RA
Ras association
- CDC25-HD
CDC25-homology domain
- Rap
Ras related protein
- DXMS
deuterium exchange mass spectrometry
- FRET
fluorescence resonance energy transfer
- MD
molecular dynamics
- β1-AR
β1-adrenergic receptor
- SR
sarcoplasmic reticulum
- CaMKIIδ
Ca2+/calmodulin-dependent protein kinase IIδ
- RyR2
ryanodine receptor 2
- GRK2
GPCR kinase 2
- COPD
chronic obstructive pulmonary disease
- HTS
high throughput screening
- SAR
structure-activity relationship
- PKCε
Protein kinase Cε
- CYP450
cytochrome P450 enzymes
- hERG
the human ether-à-go-go-related gene
- 8-NBD-cAMP
8-(2-[7-Nitro-4-benzofurazanyl]aminoethyl-thio) adenosine-3′, 5′-cyclic monophosphate
- DTP
Developmental Therapeutics Program
- ADMET
absorption, distribution, metabolism, excretion and toxicity
- DMPK
drug metabolism and pharmacokinetics
- IND
Investigational New Drug
Biographies
Haijun Chen received a dual degree (B.S. in chemistry and B.E. in bioengineering) from Fuzhou University, China in 2003. He earned his Ph.D. in medicinal chemistry from Shanghai Institute of Materia Medica, under the supervision of Dr. Fajun Nan in 2008. After graduation, he worked at ShangPharm for two years and then did his postdoctoral training at University of Texas Medical Branch (UTMB) under the direction of Dr. Jia Zhou. Currently, Dr. Chen is an Associate Professor at Fuzhou University. His research focuses on the rational drug design and synthesis of novel agents for the diagnosis and treatment of cancer and CNS disorders.
Christopher Wild studied biology and chemistry at the California State University, Northridge, where he received his Bachelor and Master of Science degrees under the direction of Dr. Gagik Melikyan. Subsequently, he worked as a program chemist for ChemicoMays, CA and a research chemist at Celenese, TX where he was a member of the acetyl catalyst development team under the tutelage of Dr. Michael Nutt. Currently, Christopher is a member of the chemistry faculty at San Jacinto College, TX, and is pursuing a Ph.D. as a Keck Research Fellow at UTMB under the supervision of Dr. Jia Zhou. His research focus is on the design and synthesis of small molecule inhibitors of EPAC and allosteric modulators of 5-HT2C receptor.
Xiaobin Zhou received his B.S. in pharmacy from South-Central University for Nationalities, China in 2012. He is currently pursuing his Ph.D. degree in medicinal chemistry at the College of Chemistry and Chemical Engineering, Fuzhou University. His main interest focuses on the design and synthesis of small molecules targeting EPAC proteins.
Na Ye earned her Ph.D. in medicinal chemistry from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, under the supervision of Dr. Ao Zhang in 2013. She is currently pursuing her postdoctoral training under the direction of Dr. Jia Zhou at UTMB. Her research topics focus on the target-based drug design and chemical synthesis of bioactive small molecules including EPAC-specific inhibitors as pharmacological probes and drug candidates for the treatment of CNS disorders, cancer, and other human diseases.
Xiaodong Cheng received a PhD in biochemistry and molecular biology from UTMB in 1994. After completing his postdoctoral fellowship at the University of California, San Diego in 1999, he returned to UTMB as a faculty member in the Department of Pharmacology and Toxicology. Currently, he is a Professor in the Department of Integrative Biology and Pharmacology, the University of Texas Health Science Center at Houston. His research has focused on applying multidisciplinary approaches to understand the structure and function of exchange protein directly activated by cAMP (EPAC) and oncogene KRAS. Recently, his research group has discovered novel small molecule inhibitors for EPAC proteins, established animal disease models, and is in the process of evaluating the therapeutic potential of targeting EPAC proteins.
Jia Zhou received his Ph.D. in organic chemistry in 1997 from Nankai University, China. Then he joined the chemistry faculty in the same university and was promoted to Associate Professor there. In 1999, he started his postdoctoral training in organic chemistry with Dr. Sidney M. Hecht at the University of Virginia. After further postdoctoral training in medicinal chemistry with Dr. Alan P. Kozikowski at Georgetown University, he has conducted research at Acenta Discovery, and PsychoGenics, Inc. as a Senior Principal Scientist for 7 years. Dr. Zhou is currently an Associate Professor (tenured) at the Chemical Biology Program, Department of Pharmacology and Toxicology at UTMB, leading a drug discovery research group. He is an author of more than 70 papers and an inventor of 9 patents.
Footnotes
CONFLICT OF INTERESTS
The authors declare no competing financial interest
References
- 1.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 2.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 5.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 6.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr Pharm Des. 2010;16:1114–1125. doi: 10.2174/138161210790963760. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 9.Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci US A. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biel M. Cyclic nucleotide-regulated cation channels. J Biol Chem. 2009;284:9017–9021. doi: 10.1074/jbc.R800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009;191:111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- 12.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 14.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 15.Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature. 2008;455:124–127. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- 16.Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- 17.Rehmann H, Prakash B, Wolf E, Rueppel A, de Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 18.Rehmann H, Rueppel A, Bos JL, Wittinghofer A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003;278:23508–23514. doi: 10.1074/jbc.M301680200. [DOI] [PubMed] [Google Scholar]
- 19.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 20.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Kilpinen S, Autio R, Ojala K, Iljin K, Bucher E, Sara H, Pisto T, Saarela M, Skotheim RI, Bjorkman M, Mpindi JP, Haapa-Paananen S, Vainio P, Edgren H, Wolf M, Astola J, Nees M, Hautaniemi S, Kallioniemi O. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 2008;9:R139. doi: 10.1186/gb-2008-9-9-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H, Seino S. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics. 2001;78:91–98. doi: 10.1006/geno.2001.6641. [DOI] [PubMed] [Google Scholar]
- 23.Mazhab-Jafari MT, Das R, Fotheringham SA, SilDas S, Chowdhury S, Melacini G. Understanding cAMP-dependent allostery by NMR spectroscopy: comparative analysis of the EPAC1 cAMP-binding domain in its apo and cAMP-bound states. J Am Chem Soc. 2007;129:14482–14492. doi: 10.1021/ja0753703. [DOI] [PubMed] [Google Scholar]
- 24.Das R, Chowdhury S, Mazhab-Jafari MT, Sildas S, Selvaratnam R, Melacini G. Dynamically driven ligand selectivity in cyclic nucleotide binding domains. J Biol Chem. 2009;284:23682–23696. doi: 10.1074/jbc.M109.011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper SM, Wienk H, Wechselberger RW, Bos JL, Boelens R, Rehmann H. Structural dynamics in the activation of Epac. J Biol Chem. 2008;283:6501–6508. doi: 10.1074/jbc.M707849200. [DOI] [PubMed] [Google Scholar]
- 26.Selvaratnam R, Chowdhury S, VanSchouwen B, Melacini G. Mapping allostery through the covariance analysis of NMR chemical shifts. Proc Natl Acad Sci US A. 2011;108:6133–6138. doi: 10.1073/pnas.1017311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvaratnam R, VanSchouwen B, Fogolari F, Mazhab-Jafari MT, Das R, Melacini G. The projection analysis of NMR chemical shifts reveals extended EPAC autoinhibition determinants. Biophys J. 2012;102:630–639. doi: 10.1016/j.bpj.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci US A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, Fan F, Flores SC, Mei F, Cheng X. Dissecting the mechanism of Epac activation via hydrogen-deuterium exchange FT-IR and structural modeling. Biochemistry. 2006;45:15318–15326. doi: 10.1021/bi061701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brock M, Fan F, Mei FC, Li S, Gessner C, Woods VL, Jr, Cheng X. Conformational analysis of Epac activation using amide hydrogen/deuterium exchange mass spectrometry. J Biol Chem. 2007;282:32256–32263. doi: 10.1074/jbc.M706231200. [DOI] [PubMed] [Google Scholar]
- 31.Tsalkova T, Blumenthal DK, Mei FC, White MA, Cheng X. Mechanism of Epac activation: structural and functional analyses of Epac2 hinge mutants with constitutive and reduced activities. J Biol Chem. 2009;284:23644–23651. doi: 10.1074/jbc.M109.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Tsalkova T, White MA, Mei FC, Liu T, Wang D, Woods VL, Jr, Cheng X. Mechanism of intracellular cAMP sensor Epac2 activation: cAMP-induced conformational changes identified by amide hydrogen/deuterium exchange mass spectrometry (DXMS) J Biol Chem. 2011;286:17889–17897. doi: 10.1074/jbc.M111.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 36.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 37.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 38.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Sand C, Jakobs KH, Michel MC, Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol. 2007;7:193–200. doi: 10.1016/j.coph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Laurent AC, Breckler M, Berthouze M, Lezoualc’h F. Role of Epac in brain and heart. Biochem Soc Trans. 2012;40:51–57. doi: 10.1042/BST20110642. [DOI] [PubMed] [Google Scholar]
- 41.Lee LC, Maurice DH, Baillie GS. Targeting protein-protein interactions within the cyclic AMP signaling system as a therapeutic strategy for cardiovascular disease. Future Med Chem. 2013;5:451–464. doi: 10.4155/fmc.12.216. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Hurtado G, Morel E, Dominguez-Rodriguez A, Llach A, Lezoualc’h F, Benitah JP, Gomez AM. Epac in cardiac calcium signaling. J Mol Cell Cardiol. 2013;58:162–171. doi: 10.1016/j.yjmcc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 43.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- 44.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G, Lezoualc’h F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 45.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 46.Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc’h F, Gomez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- 48.Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc’h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 49.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leech CA, Holz GG, Chepurny O, Habener JF. Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic beta-cells. Biochem Biophys Res Commun. 2000;278:44–47. doi: 10.1006/bbrc.2000.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 53.Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 54.Herbst KJ, Coltharp C, Amzel LM, Zhang J. Direct activation of Epac by sulfonylurea is isoform selective. Chem Biol. 2011;18:243–251. doi: 10.1016/j.chembiol.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsalkova T, Gribenko AV, Cheng X. Exchange protein directly activated by cyclic AMP isoform 2 is not a direct target of sulfonylurea drugs. Assay Drug Dev Technol. 2011;9:88–91. doi: 10.1089/adt.2010.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parnell E, Smith BO, Palmer TM, Terrin A, Zaccolo M, Yarwood SJ. Regulation of the inflammatory response of vascular endothelial cells by EPAC1. Br J Pharmacol. 2012;166:434–446. doi: 10.1111/j.1476-5381.2011.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehmann H. Epac2: a sulfonylurea receptor? Biochem Soc Trans. 2012;40:6–10. doi: 10.1042/BST20110640. [DOI] [PubMed] [Google Scholar]
- 58.Park JH, Kim SJ, Park SH, Son DG, Bae JH, Kim HK, Han J, Song DK. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic beta-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology. 2012;153:574–582. doi: 10.1210/en.2011-0259. [DOI] [PubMed] [Google Scholar]
- 59.Almahariq M, Mei FC, Cheng X. Cyclic AMP sensor EPAC proteins and energy homeostasis. Trends Endocrinol Metab. 2013 doi: 10.1016/j.tem.2013.10.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Wang ZH, Wu YY, Tang H, Tan L, Wang X, Gao XY, Xiong YS, Liu D, Wang JZ, Zhu LQ. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol Neurobiol. 2013;47:373–381. doi: 10.1007/s12035-012-8355-9. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Xu X, Tian Q, Zhang J, Qian K, Wang YX, Petralia RS, Tu W, Zhu LQ, Wang JZ, Lu Y. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron. 2012;73:774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srivastava DP, Jones KA, Woolfrey KM, Burgdorf J, Russell TA, Kalmbach A, Lee H, Yang C, Bradberry MM, Wokosin D, Moskal JR, Casanova MF, Waters J, Penzes P. Social, communication, and cortical structural impairments in Epac2-deficient mice. J Neurosci. 2012;32:11864–11878. doi: 10.1523/JNEUROSCI.1349-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 65.McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, Cooreman A, MacKenzie SJ. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem Soc Trans. 2005;33:1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- 66.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nijholt IM, Dolga AM, Ostroveanu A, Luiten PG, Schmidt M, Eisel UL. Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal. 2008;20:1715–1724. doi: 10.1016/j.cellsig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci US A. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, Esposito MF, Wand G, Kanes SJ, Abel T. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry. 2009;14:398–415. doi: 10.1038/mp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, Fagni L. Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol. 2009;587:101–113. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, Martinez A, Doskeland SO. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 72.Middeldorp CM, Vink JM, Hettema JM, de Geus EJ, Kendler KS, Willemsen G, Neale MC, Boomsma DI, Chen X. An association between Epac-1 gene variants and anxiety and depression in two independent samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:214–219. doi: 10.1002/ajmg.b.30976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivastava DP, Woolfrey KM, Jones KA, Anderson CT, Smith KR, Russell TA, Lee H, Yasvoina MV, Wokosin DL, Ozdinler PH, Shepherd GM, Penzes P. An autism-associated variant of Epac2 reveals a role for Ras/Epac2 signaling in controlling basal dendrite maintenance in mice. PLoS Biol. 2012;10:e1001350. doi: 10.1371/journal.pbio.1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 75.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 76.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 77.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 79.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768–776. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- 81.Schiermeier Q. Westernizing Eastern-bloc science. Nat Biotechnol. 2008;26:949–950. doi: 10.1038/nbt0808-949. [DOI] [PubMed] [Google Scholar]
- 82.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 83.Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc’h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 2010;459:535–546. doi: 10.1007/s00424-009-0747-y. [DOI] [PubMed] [Google Scholar]
- 85.Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol. 2011;300:L667–678. doi: 10.1152/ajplung.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eid AH. cAMP induces adhesion of microvascular smooth muscle cells to fibronectin via an Epac-mediated but PKA-independent mechanism. Cell Physiol Biochem. 2012;30:247–258. doi: 10.1159/000339061. [DOI] [PubMed] [Google Scholar]
- 87.Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 89.Yokoyama U, Minamisawa S, Quan H, Akaike T, Jin M, Otsu K, Ulucan C, Wang X, Baljinnyam E, Takaoka M, Sata M, Ishikawa Y. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2008;295:H1547–1555. doi: 10.1152/ajpheart.01317.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–284. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34:413–423. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol. 2006;80:1542–1552. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- 94.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–7370. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Heijnen CJ, van Velthoven CTJ, Willemen HLDM, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest. 2013 doi: 10.1172/JCI66241. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Konings IB, Zhao J, Price LS, de Heer E, Deen PM. Renal expression of exchange protein directly activated by cAMP (EPAC) 1 and 2. Am J Physiol Renal Physiol. 2008;295:F525–533. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- 97.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem. 2002;277:18598–18604. doi: 10.1074/jbc.M201868200. [DOI] [PubMed] [Google Scholar]
- 98.Yip KP. Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F882–890. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]
- 99.Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L710–L722. doi: 10.1152/ajplung.00486.2004. [DOI] [PubMed] [Google Scholar]
- 100.Aromataris EC, Roberts ML, Barritt GJ, Rychkov GY. Glucagon activates Ca2+ and Cl- channels in rat hepatocytes. J Physiol. 2006;573:611–625. doi: 10.1113/jphysiol.2006.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang SK, Xiao L, Li J, Liu F, Sun L, Kanwar YS. Role of guanine-nucleotide exchange factor Epac in renal physiology and pathophysiology. Am J Physiol Renal Physiol. 2013;304:F831–839. doi: 10.1152/ajprenal.00711.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Billington CK, Hall IP. Novel cAMP signalling paradigms: therapeutic implications for airway disease. Br J Pharmacol. 2012;166:401–410. doi: 10.1111/j.1476-5381.2011.01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26:112–120. doi: 10.1016/j.pupt.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dekkers BG, Racke K, Schmidt M. Distinct PKA and Epac compartmentalization in airway function and plasticity. Pharmacol Ther. 2013;137:248–265. doi: 10.1016/j.pharmthera.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 105.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2008;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 108.Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bala S, Pathak RK, Mishra V. Identification of EPAC (Exchange Protein Activated by cAMP) bioinformatically as a potential signalling biomarker in Cardiovascular Disease (CVD) and its molecular docking by a lead molecule. Bioinformation. 2011;6:176–178. doi: 10.6026/97320630006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsalkova T, Mei FC, Cheng X. A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS One. 2012;7:e30441. doi: 10.1371/journal.pone.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL, Jr, Cheng X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci USA. 2012;109:18613–18618. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Courilleau D, Bisserier M, Jullian JC, Lucas A, Bouyssou P, Fischmeister R, Blondeau JP, Lezoualc’h F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J Biol Chem. 2012;287:44192–44202. doi: 10.1074/jbc.M112.422956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H, Tsalkova T, Mei FC, Hu Y, Cheng X, Zhou J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg Med Chem Lett. 2012;22:4038–4043. doi: 10.1016/j.bmcl.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem. 2013;56:952–962. doi: 10.1021/jm3014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H, Ding C, Wild C, Liu H, Wang T, White MA, Cheng X, Zhou J. Efficient Synthesis of ESI-09, A Novel Non-cyclic Nucleotide EPAC Antagonist. Tetrahedron Lett. 2013;54:1546–1549. doi: 10.1016/j.tetlet.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jastorff B, Hoppe J, Morr M. A model for the chemical interactions of adenosine 3′:5′-monophosphate with the R subunit of protein kinase type I. Refinement of the cyclic phosphate binding moiety of protein kinase type I. Eur J Biochem. 1979;101:555–561. doi: 10.1111/j.1432-1033.1979.tb19750.x. [DOI] [PubMed] [Google Scholar]
- 118.Szentandrassy N, Harmati G, Farkas V, Horvath B, Hegyi B, Magyar J, Szenasi G, Marton I, Nanasi PP. Modified cAMP derivatives: powerful tools in heart research. Curr Med Chem. 2011;18:3729–3736. doi: 10.2174/092986711796642445. [DOI] [PubMed] [Google Scholar]
- 119.Van Haastert PJ, Van Driel R, Jastorff B, Baraniak J, Stec WJ, De Wit RJ. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984;259:10020–10024. [PubMed] [Google Scholar]
- 120.de Wit RJ, Hekstra D, Jastorff B, Stec WJ, Baraniak J, Van Driel R, Van Haastert PJ. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984;142:255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]
- 121.Botelho LH, Rothermel JD, Coombs RV, Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. doi: 10.1016/0076-6879(88)59017-1. [DOI] [PubMed] [Google Scholar]
- 122.Rehmann H, Schwede F, Doskeland SO, Wittinghofer A, Bos JL. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003;278:38548–38556. doi: 10.1074/jbc.M306292200. [DOI] [PubMed] [Google Scholar]
- 123.Bertinetti D, Schweinsberg S, Hanke SE, Schwede F, Bertinetti O, Drewianka S, Genieser HG, Herberg FW. Chemical tools selectively target components of the PKA system. BMC Chem Biol. 2009;9:3. doi: 10.1186/1472-6769-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Wit RJ, Hoppe J, Stec WJ, Baraniak J, Jastorff B. Interaction of cAMP derivatives with the ‘stable’ cAMP-binding site in the cAMP-dependent protein kinase type I. Eur J Biochem. 1982;122:95–99. doi: 10.1111/j.1432-1033.1982.tb05852.x. [DOI] [PubMed] [Google Scholar]
- 125.O’Brian CA, Roczniak SO, Bramson HN, Baraniak J, Stec WJ, Kaiser ET. A kinetic study of interactions of (Rp)- and (Sp)-adenosine cyclic 3′,5′-phosphorothioates with type II bovine cardiac muscle adenosine cyclic 3′,5′-phosphate dependent protein kinase. Biochemistry. 1982;21:4371–4376. doi: 10.1021/bi00261a028. [DOI] [PubMed] [Google Scholar]
- 126.Gjertsen BT, Mellgren G, Otten A, Maronde E, Genieser HG, Jastorff B, Vintermyr OK, McKnight GS, Doskeland SO. Novel (Rp)-cAMPS analogs as tools for inhibition of cAMP-kinase in cell culture. Basal cAMP-kinase activity modulates interleukin-1 beta action. J Biol Chem. 1995;270:20599–20607. doi: 10.1074/jbc.270.35.20599. [DOI] [PubMed] [Google Scholar]
- 127.Su Y, Dostmann WR, Herberg FW, Durick K, Xuong NH, Ten Eyck L, Taylor SS, Varughese KI. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science. 1995;269:807–813. doi: 10.1126/science.7638597. [DOI] [PubMed] [Google Scholar]
- 128.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 129.Schwede F, Christensen A, Liauw S, Hippe T, Kopperud R, Jastorff B, Doskeland SO. 8-Substituted cAMP analogues reveal marked differences in adaptability, hydrogen bonding, and charge accommodation between homologous binding sites (AI/AII and BI/BII) in cAMP kinase I and II. Biochemistry. 2000;39:8803–8812. doi: 10.1021/bi000304y. [DOI] [PubMed] [Google Scholar]
- 130.Bartsch M, Zorn-Kruppa M, Kuhl N, Genieser HG, Schwede F, Jastorff B. Bioactivatable, membrane-permeant analogs of cyclic nucleotides as biological tools for growth control of C6 glioma cells. Biol Chem. 2003;384:1321–1326. doi: 10.1515/BC.2003.148. [DOI] [PubMed] [Google Scholar]
- 131.Ogreid D, Ekanger R, Suva RH, Miller JP, Sturm P, Corbin JD, Doskeland SO. Activation of protein kinase isozymes by cyclic nucleotide analogs used singly or in combination. Principles for optimizing the isozyme specificity of analog combinations. Eur J Biochem. 1985;150:219–227. doi: 10.1111/j.1432-1033.1985.tb09010.x. [DOI] [PubMed] [Google Scholar]
- 132.Huseby S, Gausdal G, Keen TJ, Kjaerland E, Krakstad C, Myhren L, Bronstad K, Kunick C, Schwede F, Genieser HG, Kleppe R, Doskeland SO. Cyclic AMP induces IPC leukemia cell apoptosis via CRE-and CDK-dependent Bim transcription. Cell Death Dis. 2011;2:e237. doi: 10.1038/cddis.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stokman G, Qin Y, Genieser HG, Schwede F, de Heer E, Bos JL, Bajema IM, van de Water B, Price LS. Epac-Rap signaling reduces cellular stress and ischemia-induced kidney failure. J Am Soc Nephrol. 2011;22:859–872. doi: 10.1681/ASN.2010040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ostroveanu A, van der Zee EA, Eisel UL, Schmidt M, Nijholt IM. Exchange protein activated by cyclic AMP 2 (Epac2) plays a specific and time-limited role in memory retrieval. Hippocampus. 2010;20:1018–1026. doi: 10.1002/hipo.20700. [DOI] [PubMed] [Google Scholar]
- 136.Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. ChemBioChem. 2008;9:2052–2054. doi: 10.1002/cbic.200800216. [DOI] [PubMed] [Google Scholar]
- 137.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 138.Kraemer A, Rehmann HR, Cool RH, Theiss C, de Rooij J, Bos JL, Wittinghofer A. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J Mol Biol. 2001;306:1167–1177. doi: 10.1006/jmbi.2001.4444. [DOI] [PubMed] [Google Scholar]
- 139.Chen H, Yang Z, Ding C, Chu L, Zhang Y, Terry K, Liu H, Shen Q, Zhou J. Fragment-based drug design and identification of HJC0123, a novel orally bioavailable STAT3 inhibitor for cancer therapy. Eur J Med Chem. 2013;62:498–507. doi: 10.1016/j.ejmech.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen H, Wang CZ, Ding C, Wild C, Copits B, Swanson GT, Johnson KM, Zhou J. A combined bioinformatics and chemoinformatics approach for developing asymmetric bivalent AMPA receptor positive allosteric modulators as neuroprotective agents. ChemMedChem. 2013;8:226–230. doi: 10.1002/cmdc.201200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rehmann H. Epac-inhibitors: facts and artefacts. Sci Rep. 2013;3:3032. doi: 10.1038/srep03032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 2011;13:331–339. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I, Hao D, Stutz SJ, Dineley KT, Motamedi M, Hommel JD, Cunningham KA, Chen J, Cheng X. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol. 2013;33:918–926. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gong B, Shelite T, Mei F, Ha T, Hu Y, Xu G, Chang Q, Wakamiya M, Ksiazek TG, Boor PJ, Bouyer D, Popov V, Chen J, Walker DH, Cheng X. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1314400110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van den Berghe N, Cool RH, Horn G, Wittinghofer A. Biochemical characterization of C3G: an exchange factor that discriminates between Rap1 and Rap2 and is not inhibited by Rap1A(S17N) Oncogene. 1997;15:845–850. doi: 10.1038/sj.onc.1201407. [DOI] [PubMed] [Google Scholar]
- 146.Courilleau D, Bouyssou P, Fischmeister R, Lezoualc’h F, Blondeau JP. The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (EPAC1) Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2013.09.107. in press. [DOI] [PubMed] [Google Scholar]
- 147.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]