Abstract
The organization of the Golgi apparatus is determined in part by the interaction of Rab proteins and their diverse array of effectors. Here, we used multiple approaches to identify and characterize a small subset of effectors that mimicked the effects of Rab6 on Golgi ribbon organization. In a visual-based, candidate-protein screen, we found that the individual depletion of any of three Rab6 effectors, myosin IIA (MyoIIA), Kif20A, and Bicaudal D (BicD), was sufficient to suppress Golgi ribbon fragmentation/dispersal coupled to retrograde tether proteins in a manner paralleling Rab6. MyoIIA and Kif20A depletion were pathway selective and suppressed ZW10-dependent Golgi ribbon fragmentation/dispersal only while BicD depletion, like Rab6, suppressed both ZW10- and COG-dependent Golgi ribbon fragmentation. The MyoIIA effects could be produced in short term assays by the reversible myosin inhibitor, blebbistatin. At the electron microscope level, the effects of BicD-depletion mimicked many of those of Rab6-depletion: longer and more continuous Golgi cisternae and a pronounced accumulation of coated vesicles. Functionally, BicD-depleted cells were inhibited in transport of newly synthesized VSV-G protein to the cell surface. In sum, our results indicate small, partially overlapping subsets of Rab6 effectors are differentially important to two tether-dependent pathways essential to Golgi organization and function.
Keywords: Golgi apparatus, Rab6, Rab6 effectors, ZW10, COG, Golgi Homeostasis
Introduction
Small GTPases of the Rab family act as molecular switches to regulate diverse steps in membrane trafficking. Of the 60+ family members in humans, at least 12 are associated with the Golgi apparatus, the central organelle within the secretory pathway of eukaryotic cells. The major example is Rab6, the most abundant Golgi Rab protein (1), which in its GTP form is found in association with the membranes of the trans Golgi apparatus and trans Golgi network (TGN). Rab6 consists of two isoforms, Rab6a and Rab6a’, that differ in 3 amino acids and arise as alternate, equally common, splice products of the same gene. The two are often functionally redundant and are referred to collectively as Rab6. Like other Rab proteins, Rab6 acts through an array of effectors, in this case, 15 or more, to regulate a multitude of processes such as Golgi vesicle biogenesis (2), anterograde and retrograde vesicle trans-port from the trans Golgi/TGN to the plasma membrane or endoplasmic reticulum (ER) (3), and vesicle tethering. Rab proteins such as Rab6 (4,5) when overexpressed, especially in the GTP-restricted form, induce the large scale, retrograde redistribution of Golgi cisternal enzymes to the endoplasmic reticulum and, in essence, the disappearance of a juxtanuclear Golgi apparatus. The switch is of clear impact to Golgi membrane trafficking.
Rab6 is linked either directly or indirectly to both minus- and plus-end directed motors and coiled-coil proteins of the golgin family. Of these effectors, the motor proteins are likely crucial to Golgi organization. The juxtanuclear positioning of the mammalian Golgi ribbon is the balanced outcome of competing minus- and plus-end directed microtubule-dependent motor activity (for a recent review, see (6). However, contrary to this expectation, depletion of Rab6 itself has little, if any, effect on the juxtanuclear organization of the Golgi ribbon (7,8). Furthermore, depletion of the dynein linked Rab6 effector, bicaudal D (BicD), has little apparent effect on Golgi organization (9). Similarly, the knockdown of the Rab6 effector, myosin II (2,10), or the Rab6 linked kinesins: Kif1C, Kif5B (11), and Kif20A (present work) have moderate-to-small effects on the organization of juxtanuclear Golgi ribbon. Yet, in an epistatic, double knockdown experiment, Rab6 depletion does suppress the Golgi ribbon fragmentation and scattering induced by depletion of either the retrograde tether proteins ZW10/RINT-1 or COG3 (8). Moreover, when examined by the higher resolution approach of electron tomography rather than light microscopy, Rab6 depletion alone profoundly affects Golgi organization with an increase in cisternal number and the apparent merger of Golgi cisternal stacks to yield much greater Golgi cisternal continuity (10). This Golgi reorganization is accompanied by a large increase in trans-Golgi associated uncoated and coated vesicles (10). We hypothesize that Rab6 regulates Golgi organization through the recruitment of distinct effectors sets of specific for the respective tether-dependent trafficking pathways.
Here, we take a candidate protein approach to determine the subsets of Rab6 effectors crucial to ZW10- and COG-dependent Golgi organization. Using a visual screen, we assayed for the ability of effector depletion in double knockdown or chemical inhibition experiments to suppress Golgi ribbon fragmentation and dispersal. We scored the contribution of the four classes of known Rab6 effectors: i) microtubule-dependent motor linkers, ii) myosins (specifically MyoII), iii) Rab6-linked kinesins, and iv) Rab6-linked golgins. Depletion of the microtubule-dependent motor linkers, BicD1 and BicD2, strongly suppressed Golgi fragmentation and dispersal induced by the knockdown of both ZW10- and COG tethers. In striking contrast, MyoII and Kif20A depletion or chemical inhibition of MyoII through blebbistatin treatment resulted in a selective inhibition of ZW10- but not COG3-knockdown induced Golgi fragmentation. Epistatic knockdown of Kif1C and Kif5B or the golgins, golgin-97 and OCRL, had no significant suppressive effects on retrograde tether depletion induced Golgi reorganization. In sum, the effector sets for the two pathways overlapped at the level of BicD, but were otherwise distinct.
Results
We hypothesized that small and selective subsets of effectors are crucial to individual Rab6-dependent pathways contributing to Golgi organization. To illustrate and test this hypothesis, we assessed the effect of double knockdown of individual Rab6-linked candidate proteins on Golgi ribbon disruption induced by depletion of the retrograde Golgi tether proteins, ZW10 and COG3 (8, for review, (12)). As shown in Fig. 1A–C, depletion of the two tethers produced dissimilar Golgi ribbon disruption phenotypes. In the COG3 knockdowns, the Golgi fragments marked by GalNAcT2-GFP appeared as large, perinuclear fluorescent chunks rather irregular in shape. In the ZW10 knockdowns, the Golgi fragments were smaller, somewhat elongated, and clustered as a dispersed, perinuclear cloud of Golgi elements. In earlier work, we have shown that the somewhat elongated Golgi elements in ZW10-depleted cells align along microtubules (8). The candidate Rab6-linked proteins considered are listed in Table 1 as well as the siRNAs used. As cited in Table 1, the individual siRNAs have been previously validated.
Figure 1. BicD1/2 depletion suppressed COG- and ZW10-dependent Golgi ribbon fragmentation.
(A–C) HeLa cells stably expressing GalNAcT2-GFP were treated with siControl (A), siCOG3 (B) and siZW10 (C) RNAs and then fixed. Alternatively, (D–F), cells were incubated with siBicD1 (D), siBicD1+siCOG3 (E) and siBicD1+siZW10 (F) and then fixed. Depletion of BicD1 had little, if any effect, on the compact Golgi phenotype (D) whereas the co-depletion of BicD1 and COG3 or ZW10 effectively suppressed the Golgi ribbon dispersal induced by either COG3 (E) or ZW10 (F) depletion, respectively. (G–I) Similarly, the depletion of BicD2 had little, if any, effect on Golgi ribbon organization (G) while co-depletion of BicD2 and COG3 or ZW10 (H–I) resulted in a suppression of Golgi ribbon fragmentation. Total cells lysates from A–I were immunoblotted with anti-COG3, anti-ZW10, anti-BicD1, and anti-BicD2 antibodies. Top row (J,K) shows the high-level knockdown of ZW10 (J) and COG3 (K), respectively. Top row (L) shows the extensive knockdown of BicD1 protein and middle rows show the knockdown of COG3 and ZW10, respectively. Top row (M,N) shows the extensive knockdown of BicD2 and the middle rows show the knockdown of COG3 and ZW10, respectively. Tubulin was used as loading control. Total siRNA incubation time was 96 h. For protein depletion quantification, see Table 2.
Table 1.
List of Proteins Targeted
| Protein(s) | siRNA Sequence | Reference |
|---|---|---|
| Retrograde Golgi Tethers | ||
| COG3 | AGACUUGUGCAGUUUAACA | Zolov and Lupashin, 2005 |
| ZW10 | AAGGGTGAGGTGTGCAATATG | Hirose et al., 2004; Arasaki et al., 2006 |
| Rab6 | GAGAAGAUAUGAUUGACAU | Sun et al., 2007 |
| Rab6 Effectors | ||
| Golgins | ||
| Golgin-97 | siGENOME SMART pool | |
| OCRL | siGENOME SMART pool | |
| Motor Linkers | ||
| BicD1-1 | CCUUAAUGCCAUAAUCCGG | Splinter et al., 2010 |
| BicD1-2 | GCAAAGAGCCAAUGAAUAU | Splinter et al., 2010 |
| BicD2 | siGENOME SMART pool | |
| Non-Processive Motor | ||
| Myosin IIA | AGGAGUUUCGGCAGAGAGAUAUU | Miserey-Lenkei et al., 2010, Storrie et al., 2012 |
| Downstream Motor Proteins | ||
| Kif1C | siGENOME SMART pool | |
| Kif5B | UGAAUUGCUUAGUGAUGAA | Gupta et al., 2008 |
| Kif20A | siGENOME SMART pool | |
| Dynein (DHC1) | ACAUCAACAUAGACAUUCAUU | Gupta et al., 2008 |
MyoII and Kif20A Are Specific Motor Effectors for ZW10-Dependent Golgi Homeostasis While BicD Contributes to Both ZW10- and COG-Dependent Golgi Homeostasis
We first tested the importance of two related Rab6 effectors, BicD1 and BicD2, to the ZW10- and COG-dependent pathways. BicD1 and BicD2 are closely related proteins sharing about 67% sequence identity. They bridge vesicle associated Rab6 to various motor proteins including KIf5B and dynein (11). Assuming that the Golgi ribbon disruption induced by COG3- or ZW10-depletion is a consequence of decreased vesicle tethering leading to outward vesicle trafficking, we predicted that a decrease in vesicle bridging activity could lead to epistatic suppression. As shown qualitatively in Figure 1 and Supplemental Figure 1, co-depletion of either BicD1 or D2 suppressed Golgi dispersal into the small clustered Golgi elements induced by ZW10 depletion and the Golgi fragmentation induced by COG3 depletion. The extent of protein depletion is shown qualitatively for the siRNA treatments in the bottom portion of Figure 1 and quantitatively in Table 2.
Table 2.
Quantification of Protein Knockdown
| Protein(s) | % KD | % Knockdown COG3 | % Knockdown ZW10 | Golgi Ribbon Phenotype Suppression |
|---|---|---|---|---|
| COG3 | ||||
| Alone | 90 | N/A | ||
| ZW10 | ||||
| Alone | 90 | N/A | ||
| BicD1 | ||||
| Alone | 95 | - | - | N/A |
| +COG3 | 95 | 90 | Yes | |
| +ZW10 | 95 | - | 90 | Yes |
| BicD2 | ||||
| Alone | 90 | - | - | N/A |
| +COG3 | 90 | 85 | Yes | |
| +ZW10 | 85 | - | 80 | Yes |
| Myosin IIA | ||||
| Alone | 95 | - | - | N/A |
| +COG3 | 95 | 80 | No | |
| +ZW10 | 95 | - | 85 | Yes |
| Golgin-97 | ||||
| Alone | 85 | - | - | N/A |
| +COG3 | 80 | 80 | - | No |
| +ZW10 | 85 | - | 80 | No |
| OCRL | ||||
| Alone | 90 | - | - | N/A |
| +COG3 | 90 | 85 | No | |
| +ZW10 | 90 | - | 80 | No |
| Kif1C | ||||
| Alone | 90 | - | - | N/A |
| +COG3 | 90 | 85 | - | No |
| +ZW10 | 85 | - | 85 | No |
| Kif5B | ||||
| Alone | 90 | - | - | N/A |
| +COG3 | 90 | 85 | No | |
| +ZW10 | 85 | - | 80 | No |
| Kif20A | ||||
| Alone | 95 | |||
| +COG3 | 90 | 90 | - | No |
| +ZW10 | 95 | - | 90 | Yes |
With respect to Golgi ribbon organization, the extent of Golgi compaction and suppressed ribbon scattering was greater with the epistatic depletion of BicD2 (Table 3). In fact, the suppression observed was nearly equivalent to that of the Rab6 knockdown. At this level of resolution, BicD2 knockdown essentially mimicked the Rab6 knockdown phenotype. At lower levels of BicD1 depletion, a differential BicD effect was observed with ZW10 depletion induced Golgi fragmentation being preferentially suppressed (data not shown). This was particularly noticeable with a second siRNA directed against BicD1 that gave less extensive depletion of BicD1 (siBicD1-2). These results are consistent with earlier microinjection experiments in which we showed that ZW10 but not COG-dependent Golgi ribbon scattering is at least partially inhibited; 50% of cells expressing a competing C-terminal Bicaudal D fragment displayed inhibited Golgi fragmentation (8). We infer the previous failure in overexpression experiments to observe any suppression of COG3 depletion induced Golgi dispersal (8) was due to non-competitive levels of BicD C-terminal fragment expression.
Table 3. Quantification of Golgi area and fragmentation.
 values indicate strong suppression (≥50% decrease relative to Cog3 or ZW10 depletion, respectively),
values indicate strong suppression (≥50% decrease relative to Cog3 or ZW10 depletion, respectively),
 moderate suppression, and
moderate suppression, and
 strong enhancement.
strong enhancement.
| Protein(s) Depleted | Apparent Golgi Area (A.U) | Number of Golgi Fragments |
|---|---|---|
| Control | 1000±62 | 3.30±0.10 |
| COG3 | 1226±33 | 20.00±6.00 |
| ZW10 | 3752±126 | 19.85±1.75 |
| Rab6 | 715±120 | 5.85±0.40 |
| BicD1/2 | ||
| BicD1 | 1123±176 | 4.50±0.40 |
| BicD1+COG3 | 862±158 | 10.10±1.30 |
| BicD1+ZW10 | 1525±130 | 6.60±1.00 |
| BicD2 | 575±116 | 2.90±1.00 |
| BicD2+COG3 | 518±111 | 6.70±0.90 |
| BicD2+ZW10 | 671±168 | 5.45±0.70 |
| Myosin IIA | ||
| Myosin IIA | 865±106 | 7.00±1.00 |
| Myosin IIA+COG3 | 1016±22 | 22.50±6.25 |
| Myosin IIA+ZW10 | 1156±62 | 10.00±1.00 |
| Kif1C/Kif5B/Kif20A/Dynein | ||
| Kif1C | 1185±223 | 5.25±0.50 |
| Kif1C+COG3 | 1247±82 | 25.55±2.60 |
| Kif1C+ZW10 | 1602±180 | 19.50±6.20 |
| Kif5B | 1377±195 | 7.10±1.10 |
| Kif5B+COG3 | 1188±209 | 25.70±3.65 |
| Kif5B+ZW10 | 1575±186 | 15.00±0.25 |
| Kif20A | 1119±179 | 23.50±1.00 |
| Kif20A+COG3 | 1453±98 | 43.36±3.00 |
| KIF20A+ZW10 | 1578±70 | 12.45±0.80 |
| Dynein (DHC1) | 1851±106 | 28.40±0.90 |
| Dynein+COG3 | 1770±131 | 35.10±1.30 |
| Dynein+ZW10 | 2559±234 | 41.75±1.06 |
| Golgins | ||
| Golgin-97 | 666±103 | 2.90±0.45 |
| Golgin-97+COG3 | 1580±121 | 11.85±1.20 |
| Golgin-97+ZW10 | 1809±59 | 14.65±1.90 |
| OCRL | 1113±35 | 6.85±0.25 |
| OCRL+COG3 | 1251±236 | 16.10±3.25 |
| OCRL+ZW10 | 2199±137 | 28.20±3.85 |
Quantification of Golgi area and fragmentation based on the distribution of GalNAcT2-GFP fluorescence: Golgi area and fragmentation on an individual cellular basis using deconvolved images to sharpen the distinction between Golgi apparatus and general cytoplasm. Images were then segmented to demarcate the Golgi apparatus based on fluorescence intensity and Golgi area and the number of Golgi fragments was determined using iVision for Mac software. At least 30 cells from 4–5 image fields were analyzed per data point. Data are shown ± standard error of the mean. A.U - Arbitrary unit (1000 units = 4653 pixels).
As well as interacting with the minus-end-directed microtubule-dependent motor, cytoplasmic dynein 1, BicD1/D2 interact with the plus-end kinesin Kif5B (11). Moreover, two other kinesins, Kif1C and Kif20A, are either directly or indirectly dependent on Rab6. The Rab6 effector, BicDR-1, interacts with Kif1C (13) and Kif20A is itself a Rab6 effector (7,14,15). Hence, Rab6 is directly or indirectly linked to the recruitment of 3 different, plus-end directed, microtubule-dependent, processive motor proteins to Golgi-derived vesicles. To determine the relative contribution of these motors to Golgi organization within the context of our epistatic assay, we tested the effect of individual and epistatic Kif knockdowns on Golgi ribbon organization. Individual knockdown of Kif1C produced little to no Golgi ribbon disruption while that of Kif5B produced minor Golgi fragmentation (Fig. 2A,D,G; quantification Table 3). The epistatic knockdown of neither kinesin produced a significant suppressive effect in either a COG3- or ZW10-knockdown background (Fig. 2E,F,H,I; quantification Table 3). In brief, epistatic knockdown of Kif1C or Kif5B in a COG3 knockdown background gave a reduction in the size of the individual Golgi ribbon fragments (Fig. 2B,E,H) that was accompanied by a corresponding increase in fragment number (Table 3). In net, there was a slight enhancement in Golgi ribbon disruption. In contrast, an epistatic knockdown on a ZW10 knockdown background, produced little, if any, qualitative difference in Golgi ribbon organization (Fig. 2C,F,I) and, as revealed by segmentation analysis, the epistatic knockdown resulted in a decrease in apparent Golgi area with a variable but small effect on Golgi fragment number (Table 3).
Figure 2. Depletion of Kif20A selectively suppressed the siZW10 induced dispersal of the Golgi ribbon but not siCOG3 induced Golgi ribbon disruption.
(A–C) HeLa cells stably expressing GalNAcT2-GFP were treated with siControl (A) or siCOG3 (B) and siZW10 (C) and then fixed. Alternatively, (D–F), cells were incubated with siKif1C (D), siKif1C+siCOG3 (E) and siKif1C+siZW10 (F). siKif1C treated cells showed a compact Golgi ribbon phenotype (D) and in double knockdown siKif1C+siCOG3 /or siZW10, the Golgi ribbon was fragmented with little, if any, suppression apparent (E,F). Kif5B depletion had little, if any, effect on Golgi ribbon organization while in co-knockdown experiments with COG3 (H) or ZW10 (I) little, if any, suppression of Golgi ribbon fragmentation was noted. Kif20A-dpleted cells showed an apparently normal Golgi ribbon in many cases and in cells with a pronounced failure in cytokinesis long Golgi ribbon strands were observed (arrowheads, J). In epistatic knockdowns, Kif20A and COG3 co-depletion produced enhanced Golgi ribbon fragmentation (K) while Kif20A and ZW10 co-depletion produced an epistatic suppression of Golgi ribbon fragmentation (L). Total cell lysates from (A–L) were immunoblotted with antibodies directed against COG3, ZW10, Kif1C, Kif5B and Kif20A respectively. Top row (M,N) shows the high level knockdowns of Kif1C protein, COG3 (M), and ZW10 (N), respectively. Top row (O,P) shows the extensive knockdown of Kif5B protein and middle row the similar extensive knockdowns of COG3 (O) and ZW10 (P), respectively. Top row (Q,R) showing the extensive knockdowns of Kif20A protein and middle row those of COG3 (Q) and ZW10 (R) respectively. Anti-tubulin was used as loading control. Total siRNA incubation time was 96 h. For protein depletion quantification, see Table 2.
As the final kinesin candidate, we tested the role of Kif20A, a processive motor protein implicated in cytokinesis (16) and in interphase cells in Rab6-dependent, retrograde Golgi trafficking (14). As expected, Kif20A knockdown inhibited cytokinesis leading to the accumulation of multinucleate cells in which the Golgi ribbon consisted of long networked strands (Figure 2J, arrowheads). In epistatic double knockdown experiments, a strong suppression of Golgi dispersal induced by ZW10 depletion was observed. However, Golgi fragmentation induced by COG3 depletion was not altered (Figure 2 and Table 3). In summary, neither epistatic knockdown of Kif1C nor Kif5B had a strong effect on tether-knockdown induced Golgi fragmentation and dispersal. In striking contrast, Kif20A partially mimicked the suppressive effects produced by Rab6-, MyoIIA- or BicD-depletion with a selective suppression of ZW10- but not COG3-knockdown induced Golgi ribbon scattering (Figure 2 and Table 3). These results selectively place Kif20A as an upstream component of a ZW10-dependent trafficking pathway, presumably involved in outward vesicle transport from the Golgi apparatus.
Next, we considered the actin-based motor, Myosin IIA (MyoIIA), a known Rab6 effector. MyoII is a non-processive motor that affects vesicle release (2) and transport (17). MyoIIA promotes the pinching off of vesicles at the trans Golgi/trans Golgi network (TGN) in a Rab6-dependent manner (2). These vesicles are non-coated (10). Here we took both a siRNA approach and a more acute drug inhibitor approach. Epistatic MyoIIA knockdown had a reproducible, differential effect, strongly suppressing the Golgi fragmentation and dispersal induced by ZW10-knockdown, but failed to suppress the COG3-knockdown phenotype (Fig. 3,4). These differential effects were found at myosin knockdown levels exceeding 90% (Table 2). Using blebbistatin, a reversible, drug inhibitor of myosin II, we observed the same differential phenotypic suppression effect. As shown in Figure 4, brief blebbistatin treatment of GalNAcT2-GFP HeLa cells had no apparent effect on the organization of the Golgi ribbon. However, consistent with the differential epistatic effect of MyoIIA knockdown on Golgi ribbon fragmentation induced by ZW10 versus COG3 knockdown, we found that blebbistatin significantly inhibited ZW10-depletion-induced Golgi disruption (Fig. 3B,C,E), but had no significant effect on COG3-depletion induced Golgi disruption (Fig. 4). Importantly, the suppressive effect of blebbistatin was almost fully reversed after a 3 h drug washout (Fig. 3C,E,F). Together, these results strongly indicate that Golgi ribbon scattering and concomitant Golgi dispersal is the outcome of a dynamic resetting of a Golgi organizational equilibrium that can be rapidly shifted in one direction or the other, by variation in MyoII activity. We hypothesize the variations in vesicle scission/transport are responsible. We suggest that MyoII is a starting point for the ZW10-dependent pathway based on its known activity in vesicle scission.
Figure 3. Myosin IIA depletion and blebbistatin treatment suppressed siZW10 induced Golgi ribbon fragmentation.
HeLa cells stably expressing tGalNAcT2-GFP were incubated with siControl (A) and siMyosin IIA (B) and then fixed. Depletion of Myosin IIA (B) had little, if any, effect on the Golgi ribbon. Alternatively, cells were incubated with siZW10 (C) and siMyosin IIA+siZW10 (D) and fixed. The epistatic double knockdown of Myosin IIA+ZW10 (D) produced a compact Golgi ribbon phenotype. (E,F) Blebbistatin was added to a final concentration of 25 μM for 6 h in cells incubated with siZW10 for 90 h and then cells were fixed. Blebbistatin suppressed the apparent increase in Golgi ribbon area and fragmentation induced by siZW10 (E). The effect of blebbistatin was fully reversible with a 3 h drug washed out; the siZW10 induced clustered Golgi element phenotype reappeared when the drug was washed out (F). Total cells lysates from A–D were immunoblotted with anti-ZW10, anti-Myosin IIA antibodies. Top row (G) shows the extensive knockdown of Myosin II and the middle row (G) shows that of ZW10, respectively. Total siRNA incubation time was 96 h. (A,C,E,F) Apparent Golgi area and number of fragments were quantified and plotted as a bar graph (H). Data presented as mean +/− SEM. WO stands for wash out. For protein depletion quantification, see Table 2.
Figure 4. Neither Myosin IIA depletion nor blebbistatin treatment suppressed the COG-dependent Golgi ribbon fragmentation.
HeLa cells stably expressing GalNAcT2-GFP were incubated with siControl (A) for 90 h before adding blebbistatin to a final concentration of 25 μM for 6 h (B) and then cells were fixed. Blebbistatin did not impact Golgi ribbon organization in cells treated with siControl (B). Alternatively, (C,D), cells were treated with siMyosin IIA (C), siCOG3 (D) and then fixed. Depletion of Myosin IIA alone (C) had little, if any, effect on the Golgi ribbon and failed to suppress the Golgi dispersal induced by COG3-depletion (D). (E,F) Cells were treated with siMyosin IIA+siCOG3 (E) or siCOG3 to which blebbistatin was added to a final concentration of 25 μM for 6 h (F) and then fixed. Neither siRNA induced Myosin IIA knockdown nor acute inhibition of Myosin II by blebbistatin suppressed COG-dependent Golgi ribbon fragmentation (E,F). Total cells lysates from A,C,D and E were immunoblotted with anti-Myosin IIA anti-, COG3 antibodies. Top row (G) shows the extensive knockdown of Myosin IIA and the middle row (G) shows the knockdown of COG3 respectively. Total siRNA incubation time was 96 h. (A,B,D,F) Apparent Golgi ribbon area and the number of Golgi ribbon fragments was quantified and plotted as a bar graph (H). Data presented as mean +/− SEM. For protein depletion quantification, see Table 2.
Finally, we tested the effect of cytoplasmic dynein 1 (DHC1) depletion, i.e., loss-of-function, as validation of the relative role of minus-end and plus-end directed motor protein in Golgi ribbon organization particularly with respect to ZW10, a protein proposed by some to be a linker between dynein and organelle membranes in interphase cells (for review, (18)). Our fundamental prediction was that if ZW10 or COG3 acted primarily as tether proteins then epistatic knockdown of cytoplasmic dynein 1 the Golgi ribbon would be, if anything, more dispersed. We opted to knockdown DHC 1 using a previously published siRNA (19). As expected, the knockdown of DHC 1 alone significantly disrupted the Golgi ribbon with individual Golgi elements being dispersed outward from the nucleus (Supplemental Fig. 2, quantification, Table 3). In epistatic double knockdown experiments with COG3 or ZW10, an epistatic enhancement in Golgi repositioning was observed (Supplemental Fig. 2; quantification Table 3). This is the expected outcome if both proteins are acting as tethers. This result is particularly important with respect to the interpretation of the ZW10 knockdown phenotype and its suppression. The epistatic enhancement of Golgi dispersion with a dynein 1 knockdown was, in fact, stronger in the ZW10- than the COG3-knockdown background. This is a significant phenotypic indicator that ZW10 acts a tether rather than as a dynein anchor.
Golgin Class Rab6 Effectors, Golgin-97 and ORCL, Make Little to No Contribution to ZW10- and COG-Dependent Golgi Homeostasis
The last class of Rab6 effectors considered was the golgins. We took the golgins, golgin-97 and OCRL, as test cases. Golgins are long coiled-coil proteins that have been implicated both in linking Golgi cisternae together into a Golgi stack and in the long-range tethering of incoming vesicles. Individual knockdowns of either golgin-97 or OCRL had small to negligible effects on Golgi ribbon organization in our assay (compare, Fig. 5A,D,G; for quantification see Table 3). In the case of the golgin-97 knockdown, the Golgi ribbon was somewhat more compact (Table 3) while in the case of the OCRL knockdown there was negligible quantitative change in Golgi ribbon area with the segmentation analysis revealing the ribbon to be slightly more fragmented (Table 3). in an epistatic knockdown experiment, golgin-97 depletion failed to suppress COG3- depletion induced Golgi fragmentation (Fig. 5E). However, the fragments did appear larger. Quantitatively, by light microscopy, apparent Golgi area was greater than that observed in the GOG3 single knockdown and the number of Golgi fragments was decreased (Table 3). Overall, the results are consistent with a small coalesce of the Golgi to produce larger fragments. Likewise, epistatic knockdown of OCRL produced weak effects with the quantification indicating no consistent trends in apparent Golgi area versus Golgi fragment number (Table 3). We conclude that neither of these golgins are a crucial Rab6 effector with respect to Golgi ribbon fragmentation and, in particular, the ZW10 and COG retrograde tether pathways.
Figure 5. Depletion of neither Golgin-97 nor OCRL suppressed COG3- or ZW10- dispersal dependent Golgi ribbon fragmentation.
(A–C) HeLa cells stably expressing GalNAcT2-GFP were treated with siControl (A) or siCOG3 (B) and siZW10 (C) and then fixed. Alternatively, (D–F), cells were incubated with siGolgin-97 (D), siGolgin-97+siCOG3 (E) and siGolgin-97+siZW10 (F) and then fixed. Golgin-97−depleted cells showed a compact Golgi ribbon phenotype (D) and in double knockdown experiments, the Golgi ribbon was fragmented (E,F). (G–I) Cells were incubated with siOCRL (G), siOCRL+siCOG3 (H) and siOCRL+siZW10 (I) then fixed. OCRL-depleted cells showed a compact Golgi ribbon phenotype (G) and in double knockdown experiments, the Golgi ribbon was fragmented (H,I). Total cells lysates from A–I were immunoblotted with anti-COG3, anti-ZW10, anti- Golgin-97 and anti-OCRL antibodies. Top row (J,K) shows the extensive knockdown of golgin-97 protein and middle row those of ZW10 (J) and COG3 (K), respectively. (L) Knockdown of OCRL, COG3 and ZW10 respectively. Tubulin was used as loading control. Total siRNA incubation time was 96 h. For protein depletion quantification, see Table 2.
BicD2 Depletion Mimics Much of the Effects of Rab6-depletion on Golgi Cisternal Organization
Of the individual candidate proteins screened, BicD1/2 depletion most closely mimicked the suppressive effects of an epistatic Rab6 knockdown on Golgi ribbon organization. At the resolution of the light microscope, quantitative segmentation analysis of the BicD2 knockdown revealed a juxtanuclear Golgi ribbon that was more compact than that produced by Rab6-depletion and like Rab6 loss-of-function suppressed both ZW10 and COG3-knockdown induced Golgi ribbon fragmentation. To further assess the extent to which this single Rab6 effector can produce the full phenotypic effects of Rab6 on Golgi positioning and organization, we used electron microscopy to resolve Golgi cisternae and Golgi proximal vesicles in GalNAcT2-GFP HeLa cells. For electron microscopy, thin-sectioned samples were prepared from high-pressure frozen, freeze-substituted cells. As shown in Figure 6A and quantified in Table 4, the Golgi apparatus in control cells consisted of Golgi cisternal stacks of ~700 nm in length containing 3–4 cisternae and surrounded by a small number of vesicles (arrows). In striking contrast with BicD2 knockdown, the Golgi cisternal stacks were longer, on the average ~1350 nm in length, and the frequency of Golgi-associated, coated vesicles was 3-fold higher (Fig. 6B arrows, Table 4). Increased cisternal continuity and the accumulation of Golgi-associated, coated vesicles are both prominent effects produced by Rab6-depletion (10). Furthermore, as shown in Figure 7, BicD depletion did slow cargo transport from the Golgi apparatus to the plasma membrane presumably at least in part as a consequence of effects on Golgi cisternal organization. In total, BicD depletion mimics much but not all of the Rab6 phenotype seen by electron microscopy; however, no increase in cisternal number was observed.
Figure 6. BicD2 depletion resulted in Golgi proximal accumulation of coated vesicles and elongated Golgi cisterna.
HeLa cells stably expressing GalNAcT2-GFP were incubated with siControl (A) or siBicD2 (B) for 96 h followed by high pressure freezing, freeze substitution and embedding for electron microscopy. Arrows point to vesicles along the Golgi ribbon in siControl (A) and siBicD2 treated cells (B). Vesicle accumulation appeared much less common in the Control than the BicD2-depleted cells. Results are quantified in Table 4.
Table 4.
Quantification of Golgi Morphology in siControl and siBicD2 Transfected Cells.
| Experiment 1
|
|||||
|---|---|---|---|---|---|
| siControl Golgi
|
|||||
| Cell | Number of stacks per Golgi rich area | Average number of Golgi-associated vesicles per stack | Average maximum cisternae length (nm) | Average Golgi stack width (nm) | Average number of cisternae per stack |
| 1 | 3 | 7 | 608 | 260 | 3 |
| 2 | 3 | 6 | 714 | 214 | 3 |
| 3 | 3 | 7 | 675 | 333 | 4 |
| 4 | 4 | 7 | 848 | 304 | 3.5 |
| 5 | 3 | 6 | 637 | 304 | 3 |
| Average ± SEM siBicD2 Golgi |
3.2 ± 0.2 | 6.5 ± 0.8 | 696 ± 42 | 283 ± 21 | 3.3 ± 0.1 |
| 1 | 3 | 17 | 1498 | 550 | 3.6 |
| 2 | 3 | 17 | 1369 | 428 | 4 |
| 3 | 2 | 31 | 1249 | 392 | 3.5 |
| 4 | 2 | 11 | 1321 | 196 | 3 |
| 5 | 2 | 28 | 1589 | 321 | 3.5 |
| 6 | 3 | 16 | 1654 | 357 | 3.3 |
| Average ± SEM | 2.5 ± 0.2 | 20 ± 2 | 1447 ± 80 | 374 ± 37 | 3.5 ± 0.2 |
|
| |||||
| Experiment 2
|
|||||
| siControl Golgi
|
|||||
| 1 | 3 | 7 | 653 | 321 | 3.6 |
| 2 | 3 | 8 | 742 | 363 | 3 |
| 3 | 4 | 8 | 870 | 370 | 3 |
| 4 | 3 | 8 | 758 | 230 | 4 |
| 5 | 3 | 6 | 542 | 333 | 3.3 |
| 6 | 3 | 6 | 600 | 298 | 3 |
| Average ± SEM siBicD2 Golgi |
3.2 ± 0.2 | 7.1 ± 0.4 | 694 ± 40 | 319 ± 20 | 3.3 ± 0.1 |
| 1 | 3 | 13 | 1283 | 249 | 3.3 |
| 2 | 2 | 15 | 1435 | 262 | 3 |
| 3 | 2 | 18 | 1338 | 391 | 3.5 |
| 4 | 2 | 25 | 1359 | 323 | 3.5 |
| 5 | 2 | 15 | 970 | 303 | 4 |
| 6 | 3 | 21 | 1382 | 452 | 3.3 |
| Average ± SEM | 2.3 ± 0.2 | 17.8 ± 1.8 | 1248 ± 82 | 330 ± 27 | 3.4 ± 0.1 |
|
| |||||
|
Overall Average
|
|||||
| siControl Golgi | 3.2 | 6.8 | 695.0 | 301.0 | 3.3 |
| siBicD2 Golgi | 2.4 | 18.9 | 1347.5 | 352.0 | 3.4 |
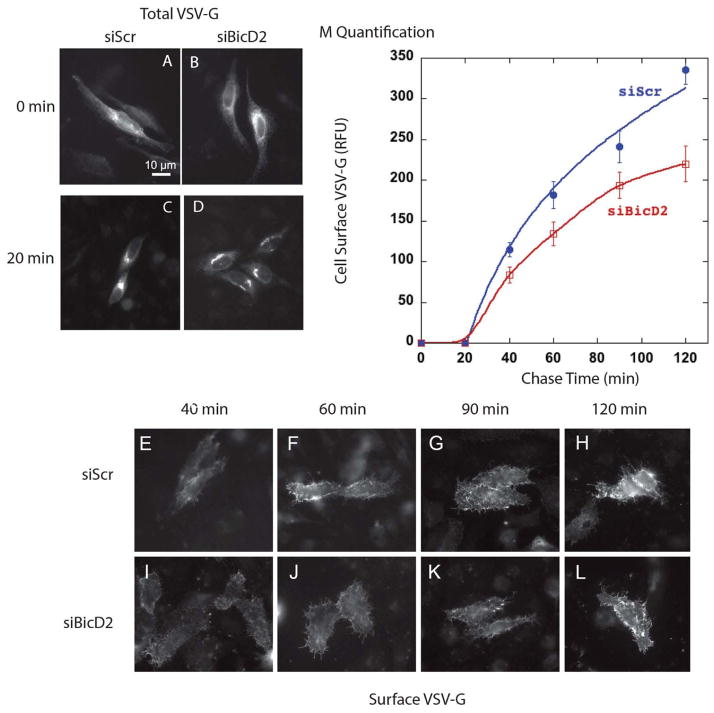
Figure 7. Depletion of BicD2 inhibited VSV-G transport from Golgi to cell surface but not from the ER to Golgi apparatus.
WT HeLa cells were incubated with either siControl or siBicD2 RNAs for 4 days and then transfected with temperature sensitive plasmid DNA encoding tsO45 GFP-tagged VSV-G protein. Cells were incubated overnight at 39.5°C, a non-permissible temperature for VSV-G transport. At the end of expression at 39.5°C, cells (A,B) were fixed and VSV-G was located in ER by wide field light microscopy (63x/1.40 numerical aperture objective) in siControl (A) and siBicD2 (B) treated cells. Cells (E–L) were then shifted to permissive condition, 32°C, and fixed at various chase times. At the end of 20 min chase there was equivalent juxtanuclear Golgi accumulation of VSV-G in both siControl (C) and siBicD2 (D). At later chase times 60, 90 and 120 min siControl cells (F–H) had significantly higher cell surface accumulation of VSV-G than the siBicD2 cells (J–L). Cell surface accumulation of VSV-G was quantified and plotted (M). Data presented as mean +/− SEM.
Discussion
The goal of these studies was to establish which subsets of Rab protein effectors regulated retrograde tether-dependent trafficking pathways important to the organization and homeostasis of the mammalian Golgi apparatus. We chose as our example Rab, Rab6, the most abundant Golgi associated Rab protein (1), and its effectors, and as the example retrograde Golgi tether complexes, ZW10/RINT1 and COG. Depletion of either tether complex disrupts both Golgi apparatus function and structure (e.g., (8)) and provides a read-out for different aspects of retrograde Golgi trafficking, ZW10 being important to trafficking from the Golgi apparatus to the ER (8) and COG to intra-Golgi retrograde trafficking (20,21). Taking a visual, candidate protein approach, we found that the Rab6 motor protein effectors, MyoII and Kif20A, were selectively required for ZW10-dependent Golgi ribbon organization/homeostasis while BicD was required for both ZW10- and COG-dependent Golgi organization/homeostasis. Surprisingly, we failed to find any Rab6 effector that was selective for the COG-dependent Golgi homeostasis even though the screen included multiple examples from the four known functional classes of Rab6 effectors. In net, a small, motor-rich, subset of Rab6 effectors is central for retrograde tether dependent Golgi organization.
Little consideration has been given previously to how Rab6 and its effectors regulate Golgi ribbon organization. Neither overexpression of GDP-restricted Rab6a (22) nor the knockdown of Rab6a/a’ (7,8), the two major splicing isoforms of Rab6 in HeLa cells that differ by 3 amino acids from one another, significantly alters the juxtanuclear organization of the Golgi ribbon. Typically, the Golgi ribbon is slightly more compact and marginally more fragmented than normal (e.g., (7,8) and present work). Likewise, the knockdown of individual Rab6 effectors, in particular those with known links to motor proteins, has little effect on juxtanuclear organization of the Golgi ribbon. For example, knockdown of MyoII, an effector that links Rab6 to actin and the biogenesis and transport of Golgi-derived vesicles involved in both anterograde and retrograde trafficking, has little effect on the juxtanuclear organization of the Golgi ribbon with the overall ribbon distribution being slightly more compact ((2,10) and present work). Similarly, dominant-negative mutant overexpression and/or knockdown of BicD, a Rab6 effector implicated in linking Rab6 to dynein (23) and also recruitment of plus-end directed motor proteins, has little negative effect on the Golgi ribbon ((8,9,24) and present work). However, when Rab6-knockdown cells are examined at the higher resolution of electron microscopy and especially when visualized by electron tomography, major reorganization of Golgi cisternal stacks occurs in Rab6-depleted cells with the cisternae being longer and more numerous per stack. Additionally, the TGN is dilated with a profound increase in the number of Golgi proximal COPI- and clathrin-coated vesicles and trans-Golgi/TGN associated arrested COPI- and clathrin-coated budding/fusion figures (10). Similarly, at the individual effector level, electron microscopy revealed here that BicD depletion mimicked many of the effect of Rab6 knockdown including increased cisternal length and coated vesicle accumulation. Furthermore, as found in epistatic, double knockdown experiments, Golgi ribbon organization is indeed highly dependent on Rab6 effectors when considered in the context of individual trafficking pathways. As shown here specific subsets of Rab6 effectors are required for the Golgi fragmentation induced by depletion of the retrograde tether proteins, COG3 or ZW10. Rab6 and its effectors are indeed important to Golgi organization.
We propose a working model in which the two Rab6 effectors, MyoII and Kif20A, act sequentially through their known roles in Golgi vesicle formation and transport to support selectively ZW10 depletion-induced Golgi fragmentation (see, Fig. 8). In so doing, we place both of these effectors upstream on ZW10 and suggest that neither interacts directly with the retrograde tether protein ZW10. MyoII has been implicated in Golgi vesicle scission at the TGN (2,10) and subsequent vesicle transport (17). Kif20A, originally identified as Rabkinesin-6 (14), has been implicated in the retrograde transport of Golgi-derived vesicles to the ER. Inhibition of either vesicle formation or transport through MyoII or Kif20A depletion would result in peri-Golgi vesicle accumulation and hence stabilize the apparent Golgi ribbon observed by light microscopy. Certainly, that is the case for MyoII (10) and present work. As previously reported (20), COG complex dependent Golgi fragmentation is accompanied by the accumulation of vesicles that are coated by the COPI complex. Likely, the fission/release of most, if not all, of the COG complex dependent vesicles is the result of a COPI-dependent process.
Figure 8. Schematic depiction of the contribution of distinct sets of Rab6 effectors to ZW10- and COG-dependent Golgi homeostasis.
Control Golgi ribbon organization is shown on the left at the level of fluorescence microscopy and that in ZW10- or COG3 knockdown (KD) cells on the right. The pathway specific contributory roles of Myo II, Kif20A and BicD to the respective pathways (arrows) are indicated by the red inhibition symbol.
The previous data indicating that the Rab6 effectors, BicD1/D2, serve to recruit dynein to the trans Golgi network (TGN) and to support retrograde Golgi-to-ER transport (24,25) provide contradictory evidence regarding the role of BicD in Golgi organization. If dynein recruitment were the major function, then failure to recruit this minus-end directed motor should result in Golgi ribbon fragmentation because plus-end directed motors would then predominant. On the other hand, BicD and/or BICD related proteins are also implicated in the Rab6-dependent recruitment of plus-end directed motors, Kif1C and Kif5B (3,11,13) and this could counterbalance any dynein effect. However, with individual or double Kif knockdown, the Golgi ribbon failed to become more compact (present work; Majeed and Storrie, unpublished observations). As summarized by Yadav and Linstedt (6), the cumulative data from previous knockdown or overexpression experiments provides little evidence of Rab6 or its effectors having a significant dynein-dependent effect on Golgi organization. We show BicD depletion mimicked that of Rab6 in producing a juxtanuclear Golgi ribbon that was more compact. Taken at face value, the epistatic suppression of ZW10- and COG3-induced Golgi fragmentation/dispersion with BicD1/D2 depletion observed here strongly indicates that BicD has an important upstream role in Golgi ribbon homeostasis. We propose that this outcome could be explained by drawing an analogy with LIS1. The Rab6 effector, LIS1 has been demonstrated to hold cytoplasmic dynein in an idling complex that depresses dynein motor activity (26). If there were mechanistic parallels between LIS1 and BicD, then the depletion of BicD might actually release dynein inhibitory effects.
In conclusion, we have identified MyoII, Kif20A, and BicD as key Rab6 effectors that contribute significantly to the juxtanuclear organization of the Golgi ribbon in mammalian cells. In epistatic assays, MyoII, Kif20A, and BicD and in the case of MyoII chemical inhibition also stabilize the juxtanuclear organization of the Golgi ribbon relative to retrograde tether induced Golgi fragmentation and scattering. These effects were selective with MyoII and Kif20A acting upstream of the ZW10 tether complex and BicD acting upstream of both ZW10 and COG (Fig. 8). Mechanistically, MyoII may act by affecting vesicle scission and/or transport (2,17) and Kif20A by supporting Golgi to ER trafficking. One explanation for the role of BicD in both pathways could be that the protein acts a negative regulator of dynein activity in parallel with recent data on LIS1 (26). Other tested Rab6 effectors such as the kinesins, Kif1C and Kif5B, make little contribution to the observed phenotypes and the golgin effectors, OCRL and golgin-97, appeared to either individually or in epistatic experiments to have little role in Golgi ribbon homeostasis. We conclude that a small set of Rab6 motor protein effectors appear to be most crucial to Golgi organization/homeostasis.
MATERIALS AND METHODS
Cell culture and chemicals
WT and stably expressing GalNAcT2-GFP HeLa cells (27) were grown in DMEM supplemented with 10%fetal bovine serum in a humidified incubator at 37°C and 5% CO2. Geneticin, G-418, was added for maintenance of transfected GalNAcT2-GFP. All cell culture media, sera and other cell culture associated reagents were procured from either Invitrogen (Carlsbad, CA), Sigma/Aldrich (St. Louis, MO) or Atlas Biologicals, Inc. (Fort Collins, CO). Blebbistatin was purchased from Cayman Chemical (Ann Arbor, MI).
Antibodies
Most antibodies were purchased from commercial sources. Goat antibodies directed against Kif5B and OCRL were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Mouse monoclonal beta-tubulin antibody was purchased from Sigma/Aldrich (St. Louis, MO). Rabbit antibodies directed against BicD2 was purchased from Bethyl Laboratories Inc. (Montgomery, TX). Rabbit antibodies directed against C-terminal of human Myosin IIA was purchased from Covance (Dallas, TX). Rabbit antibodies directed against Kif1C and BicD1 were purchased from Abcam (Cambridge, MA). The mouse monoclonal anti human Golgin-97 antibody was purchased from Invitrogen Molecular Probes (Grand Island, NY). ZW10 antibody was purchased from MyBiosource (San Diego, CA). Mouse monoclonal COG3 antibodies were a gift of Dr. Vladimir Lupashin, UAMS, Little Rock, AR and used as described earlier (8). Monoclonal antibodies directed against an ectodomain of VSV-G protein were a gift from Dr. Harvey Lodish (Department of Biology, Massachusetts Institute of Technology).
RNA interference
All siRNAs were manufactured by Dharmacon RNA Technologies, a division of Thermo Fisher (Lafayette, CO). A complete list of targeted proteins and siRNAs is given in Table 1. siRNAs were transfected at a final concentration of 100 nM, using Oligofectamine (Invitrogen, Carlsbad, CA) in absence of FBS according to previously described protocols (7,8). In brief, for visual analysis, 70,000 cells were seeded per 35 mm tissue culture dish containing 12 mm diameter cover glasses (Fisher Scientific) one day prior to siRNA transfection. Successive cycles of siRNA transfections were performed on day 0 and day 1. 96 hours (4 days) post initial transfection cells were fixed with 3% formaldehyde. Cell lysates were prepared for Western blotting from individual tissue culture dishes for subsequent quantitative assessment of protein depletion.
Blebbistatin treatment
70,000 GalNAcT2-GFP HeLa cells were seeded in a 35 mm tissue culture dish containing 12 mm diameter cover glasses. Two successive cycles of siRNA transfections on day 0 and 1 were performed using siCOG3, siZW10 and siControl one day after seeding the cells. 90 hours post initial transfection, cells were incubated with blebbistatin to a final concentration of 25 μM for 6 hours. In most experiments, cells were then fixed with 3% formaldehyde. In some experiments, blebbistatin was rapidly washed away by rinsing with OptiMEM and the cells incubated in DMEM for 3 h before fixation.
Western blot analysis
HeLa cell lysates were prepared in 2% SDS. Western blotting was done as described previously (8,28). Western blots were quantified using a LI-COR Odyssey system (LI-COR Biosciences, Lincoln, NE).
VSV-G transfection and chase
70,000 WT HeLa cells were seeded on coverslips in 35 mm dishes a day prior to siRNA transfections. After 80 hours incubation with either siControl or siBicD2 WT HeLa cells were transfected with plasmids encoding a GFP-tagged variant of the tsO45 mutant of VSV-G protein using the FuGene HD transfection reagent (Promega, Madison, WI). Post-transfection cells were incubated overnight at 39.5°C, non-permissive temperature for the transport of VSV-G from the ER to Golgi apparatus. Cells were then shifted to 32°C, permissive temperature for tsO45 transport, in the presence of cycloheximide to prevent further protein synthesis. Cells were incubated at 32°C for various chase times: 0 min, 20 min, 40 min, 60 min, 90 min and 120 min. Cells were then fixed with 3% formaldehyde and cell surface stained for VSV-G. Under these conditions, the total siRNA treatment time was 4 days. Quantitative results are the mean of 3 independent experiments.
Fluorescence microscopy and image processing
To assess the effect of protein knockdown on Golgi phenotype, HeLa cells stably expressing Golgi marker GalNAcT2-GFP were grown on coverslips and transfected with the respective siRNAs as described above. 96 hours post siRNA transfection, cells were fixed with 3% formaldehyde. GalNAcT2-GFP fluorescence were visualized using a BD CARVII spinning disk confocal microscopy accessory mounted on a Zeiss 200M inverted microscope controlled by iVision Mac™ software (BioVision Technologies) to give confocal image stacks. Images were collected with 63x/1.40 numerical aperture objective and subsequently processed using iVision-Mac™ software. For the analysis of GalNAcT2-GFP fluorescence distribution from which Golgi fragment number and area was determined, images were first deconvolved using the Huygens Professional 4.3.0p3 software to sharpen the distinction between Golgi apparatus and general cytoplasmic fluorescence. Deconvolved images were segmented between Golgi fragments/elements and general cytoplasm on the basis of the intensity of GalNAcT2-GFP fluorescence using iVision Mac™ software. Golgi fragment number were then determined based on segmentation using iVision Mac™ software. Total Golgi area per cell was computed as the summed area of the Golgi segments within an individual cell. Reported results are the average across 30 or more cells.
Quantification of total VSV-G distribution between juxtanuclear Golgi and ER was done exactly as described previously (10). Briefly, VSV-G-GFP expressing cells were imaged with wide field optics and then juxtanuclear VSV-G-GFP and total cellular G proteins were determined by outlining the appropriate areas manually and the mean pixel intensity in the juxtanuclear Golgi area and the total was determined with iVision Mac™ software. The cell surface VSV-G quantification was done by immune- labeling for an extracellular VSV-G epitope. Stained cells were manually outlined and mean pixel intensity per cell was determined with iVision Mac™ software. All pixel intensities were corrected for background fluorescence as determined by outlining the non cellular areas within the images. A total of at least 30 cells were quantified for both total VSV-G accumulation and cell surface labeling for VSV-G.
High-pressure freezing, freeze-substitution and electron microscopy
Cells were seeded on carbon coated, 3 mm circular sapphire discs (Leica Microsystems) for subsequent high-pressure freezing. HeLa cells expressing GalNAcT2-GFP cultured on sapphire discs were transfected with siBicD2, siBicD2+siMyosin IIA or non-targeting siControl duplexes as above. High-pressure freezing was performed with 2% Type IX ultra-low temperature gelling agarose (Sigma-Aldrich, St. Louis, MO) in PBS supplemented with 2% FBS as cryoprotectant as described previously (29,30) using a Leica EM PACT2 RTS high-pressure freezing unit (Leica Microsystems). All solutions and sample holders (Swiss Precision Instruments) for high-pressure freezing were pre-warmed to 37°C. All manipulations were carried out on an inverted heating block warmed to 37°C under a dissecting microscope (Leica Microsystems). Post high-pressure freezing, discs were stored in liquid nitrogen.
Specimens were freeze-substituted with anhydrous acetone containing 2% OsO4/0.1% glutaraldehyde/1%H2O at −90°C for 16–22 h using a Leica AFS unit (Leica Microsystems). After specimens were warmed to 0°C over 2 days, they were immediately moved to the cold room (4°C), and incubated for 1 hour each with 1% tannic acid/1% H2O in acetone followed by 1% OsO4/1% H2O in acetone, respectively. Disks were rinsed with acetone before and after incubation. Samples were warmed to room temperature and plastic embedded essentially as described previously (10,31).
Serial thin sections (50 nm) cut with a diamond knife on a Leica UltraCut7-UCT microtome and were collected and post-stained with aqueous uranyl acetate and Reynold’s lead citrate (Electron Microscopy Sciences) to enhance contrast. Images depicting representative Golgi regions were taken using a FEI Tecnai TF20 intermediate-voltage electron microscope operated at 80 keV.
Supplementary Material
Supplemental Figure 1: Golgi ribbon fragmentation induced by COG3 depletion as shown at the individual cell level was suppressed by BicD depletion. HeLa cells stably expressing GalNAcT2-GFP were incubated with siControl (A), siCOG3 (B), siBicD1 (D), siBicD1+siCOG3 (E), siBicD2 (G) and siBicD2+siCOG3 (H) RNAs. (C,F and I) show by COG3 antibody staining at the individual cell level that depletion of the protein was extensive under all conditions and suppression was achieved in cells in little, if any, COG3 staining was apparent: siCOG3 (C), siBicD1+siCOG3 (F) and siBicD2+siCOG3 (I), respectively. Total siRNA incubation time was 96 h.
Supplemental Figure 2: Depletion of cytoplasmic dynein heavy chain 1 (DHC1) produces enhanced Golgi ribbon dispersal/fragmentation in COG3- or ZW10-depleted cells. (A–C) HeLa cells stably expressing GalNAcT2-GFP were treated with siControl (A) or siCOG3 (B) and siZW10 (C) and then fixed. Alternatively, (D–F) cells were incubated with siDynein (D), siDynein+siCOG3 (E) and siDynein+siZW10 (F) and then fixed. Epistatic double knock of siDynein and siCOG3 or ZW10 resulted in epistastic enhancement of Golgi ribbon fragmentation (E, F). Total siRNA incubation time was 96 h.
Acknowledgments
We thank Vladimir Lupashin, Department of Physiology and Biophysics, UAMS, for discussions on the COG complex and Laura MacDonald, Department of Physiology and Biophysics, for discussions on the wording of the manuscript. We thank Jeffrey A. Kamykowski for technical assistance with electron microscopy. This work was supported in part by NIH grant GM 92960.
References
- 1.Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12:645–654. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- 3.Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Martinez O, Antony C, Pehau-Arnaudet G, Berger EG, Salamero J, Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci US A. 1997;94:1828–1833. doi: 10.1073/pnas.94.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- 6.Yadav S, Linstedt AD. Golgi positioning. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young J, Stauber T, del Nery E, Vernos I, Pepperkok R, Nilsson T. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A’. Mol Biol Cell. 2005;16:162–177. doi: 10.1091/mbc.E04-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Shestakova A, Hunt L, Sehgal S, Lupashin V, Storrie B. Rab6 regulates both ZW10/RINT-1 and conserved oligomeric Golgi complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18:4129–4142. doi: 10.1091/mbc.E07-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumoto K, Hoogenraad CC, Kikuchi A. GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006;25:5670–5682. doi: 10.1038/sj.emboj.7601459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storrie B, Micaroni M, Morgan GP, Jones N, Kamykowski JA, Wilkins N, Pan TH, Marsh BJ. Electron tomography reveals Rab6 is essential to the trafficking of trans-Golgi clathrin and COPI-coated vesicles and the maintenance of Golgi cisternal number. Traffic. 2012;13:727–744. doi: 10.1111/j.1600-0854.2012.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, Demmers J, Fornerod M, Melchior F, Hoogenraad CC, Medema RH, Akhmanova A. Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 2010;8:e1000350. doi: 10.1371/journal.pbio.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Storrie B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Shepherd IT, Akhmanova A, Hoogenraad CC. Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J. 2010;29:1637–1651. doi: 10.1038/emboj.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 15.Echard A, el Marjou A, Goud B. Expression, purification, and biochemical properties of rabkinesin-6 domains and their interactions with Rab6A. Methods Enzymol. 2001;329:157–165. doi: 10.1016/s0076-6879(01)29076-4. [DOI] [PubMed] [Google Scholar]
- 16.Hill E, Clarke M, Barr FA. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 2000;19:5711–5719. doi: 10.1093/emboj/19.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M, Malhotra V. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 2012;31:3976–3990. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallee RB, Varma D, Dujardin DL. ZW10 function in mitotic checkpoint control, dynein targeting and membrane trafficking: is dynein the unifying theme? Cell Cycle. 2006;5:2447–2451. doi: 10.4161/cc.5.21.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta V, Palmer KJ, Spence P, Hudson A, Stephens DJ. Kinesin-1 (uKHC/KIF5B) is required for bidirectional motility of ER exit sites and efficient ER-to-Golgi transport. Traffic. 2008;9:1850–1866. doi: 10.1111/j.1600-0854.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 20.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith RD, Willett R, Kudlyk T, Pokrovskaya I, Paton AW, Paton JC, Lupashin VV. The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic. 2009;10:1502–1517. doi: 10.1111/j.1600-0854.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang S, Storrie B. Cisternal rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol Biol Cell. 2005;16:2586–2596. doi: 10.1091/mbc.E04-10-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, Visser P, Grosveld F, Galjart N. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 2001;20:4041–4054. doi: 10.1093/emboj/20.15.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 25.Short B, Preisinger C, Schaletzky J, Kopajtich R, Barr FA. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12:1792–1795. doi: 10.1016/s0960-9822(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 26.Yamada M, Kumamoto K, Mikuni S, Arai Y, Kinjo M, Nagai T, Tsukasaki Y, Watanabe TM, Fukui M, Jin M, Toba S, Hirotsune S. Rab6a releases LIS1 from a dynein idling complex and activates dynein for retrograde movement. Nat Commun. 2013;4:2033. doi: 10.1038/ncomms3033. [DOI] [PubMed] [Google Scholar]
- 27.Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shestakova A, Zolov S, Lupashin V. COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic. 2006;7:191–204. doi: 10.1111/j.1600-0854.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 29.McDonald K, Schwarz H, Muller-Reichert T, Webb R, Buser C, Morphew M. “Tips and tricks” for high-pressure freezing of model systems. Methods Cell Biol. 2010;96:671–693. doi: 10.1016/S0091-679X(10)96028-7. [DOI] [PubMed] [Google Scholar]
- 30.Verkade P. Moving EM: the Rapid Transfer System as a new tool for correlative light and electron microscopy and high throughput for high-pressure freezing. J Microsc. 2008;230:317–328. doi: 10.1111/j.1365-2818.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 31.Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci US A. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–1278. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arasaki K, Taniguchi M, Tani K, Tagaya M. RINT-1 regulates the localization and entry of ZW10 to syntaxin 18 complex. Mol Biol Cell. 2006;17:2780–2788. doi: 10.1091/mbc.E05-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Golgi ribbon fragmentation induced by COG3 depletion as shown at the individual cell level was suppressed by BicD depletion. HeLa cells stably expressing GalNAcT2-GFP were incubated with siControl (A), siCOG3 (B), siBicD1 (D), siBicD1+siCOG3 (E), siBicD2 (G) and siBicD2+siCOG3 (H) RNAs. (C,F and I) show by COG3 antibody staining at the individual cell level that depletion of the protein was extensive under all conditions and suppression was achieved in cells in little, if any, COG3 staining was apparent: siCOG3 (C), siBicD1+siCOG3 (F) and siBicD2+siCOG3 (I), respectively. Total siRNA incubation time was 96 h.
Supplemental Figure 2: Depletion of cytoplasmic dynein heavy chain 1 (DHC1) produces enhanced Golgi ribbon dispersal/fragmentation in COG3- or ZW10-depleted cells. (A–C) HeLa cells stably expressing GalNAcT2-GFP were treated with siControl (A) or siCOG3 (B) and siZW10 (C) and then fixed. Alternatively, (D–F) cells were incubated with siDynein (D), siDynein+siCOG3 (E) and siDynein+siZW10 (F) and then fixed. Epistatic double knock of siDynein and siCOG3 or ZW10 resulted in epistastic enhancement of Golgi ribbon fragmentation (E, F). Total siRNA incubation time was 96 h.