Abstract
Schedule-induced polydipsia (SIP) is generated by subjecting a highly motivated animal to a sub-optimal rate of food reinforcement while also providing access to a fluid. SIP is one of several adjunctive (or displacement) behaviors that are expressed in an exaggerated form that is deemed ‘excessive’. This feature makes SIP an attractive model for studying an excessive ethanol drinking phenotype in rodents. Multiple experimental variables are crucial for the full manifestation of adjunctive drinking, including the degree of food deprivation, the inter-pellet interval selected, and the size of the food reward offered. Although these variables were extensively studied and optimized for water polydipsia in rats, a similarly customized approach to ethanol SIP and application of the procedure in mice have largely been curtailed in favor of the default variable values historically used for water SIP in rats. Further, ethanol SIP also requires careful consideration of variables such as taste and ethanol concentration. Investigation of the stress axis and neurochemical systems such as dopamine and serotonin in mediating adjunctive drinking stemmed from two leading hypotheses regarding the underlying mechanisms of SIP generation: 1) SIP as a coping strategy to mitigate stress associated with the aversive environmental condition, and 2) SIP as a displacement of reward in a highly motivated animal. Ethanol SIP is a powerful model of excessive intake because it can generate an ethanol-dependent state and sustain frequent and intoxicating levels of blood ethanol with voluntary oral consumption. The required food deprivation and the loss of the excessive drinking phenotype following removal of the generator schedule are the two main limitations of the model. Future utility of ethanol SIP will be enhanced by more fully dissecting the underlying hormonal and neurochemical mechanisms and optimizing experimental variables for ethanol SIP on a per species and strain basis.
Keywords: schedule-induced polydipsia, adjunctive drinking, stress, genetics, dependence, pharmacotherapy
Introduction
The term ‘poly-dipsia’ is of Greek origin and translates as ‘much thirst’. Polydipsia (excessive water intake) is clinically observed as an initial symptom of diabetes insipidus (Moeller, Rittig, & Fenton, 2013) and is observed occasionally in psychotic patients with schizophrenia or related mental illness (Goldman, 2010; Moreno & Flores, 2012). Experimentally, Lester (1961) was the first investigator to document schedule-induced polydipsia (SIP) in rats provided access to ethanol, and this represented a rapid translation from seminal work published by Falk (1961) regarding SIP with water earlier that same year. These investigators discovered that rats will consume excessive quantities of fluid amounting to either half their body weight or 10-fold baseline levels in 3 h if food-restricted and placed under a schedule of intermittent food reinforcement. In a typical SIP experiment, subjects are food-restricted to 80–90% of their free-feeding body weight, and then are placed into an operant chamber for daily sessions of 30–180 min in duration. The operant chambers minimally contain a fluid source and a food pellet dispenser and receptacle (see Fig. 1). Key to the generation of polydipsia is the schedule of intermittent food delivery, which is often referred to as the ‘generator schedule’. Historically, multiple types of interval schedules have been used, including fixed interval (FI), variable interval (VI), and fixed time (FT). The FI and VI schedules require animals to press a lever for food access. For example, following a 1-min or an on-average 1-min interval, respectively, the first lever response is reinforced and then another 1-min delay is imposed before the subject is again eligible for reinforcement, and so on. When an animal is under schedule control (see section I.f below), bursts of fluid intake occur immediately after each pellet delivery and this culminates in the consumption of large volumes throughout the session. The earliest investigations with SIP employed a variable interval 1-min (VI-1) schedule (Falk, 1961; Lester, 1961). However, this response contingency was later found not to be a crucial factor for the manifestation of SIP in its fully exaggerated form (Falk, 1969), and most contemporary applications omit response requirements from the procedure and allow pellets to be delivered automatically according to a FT interval (notice absence of lever in Fig. 1).
Figure 1. Apparatus for schedule-induced polydipsia procedure.
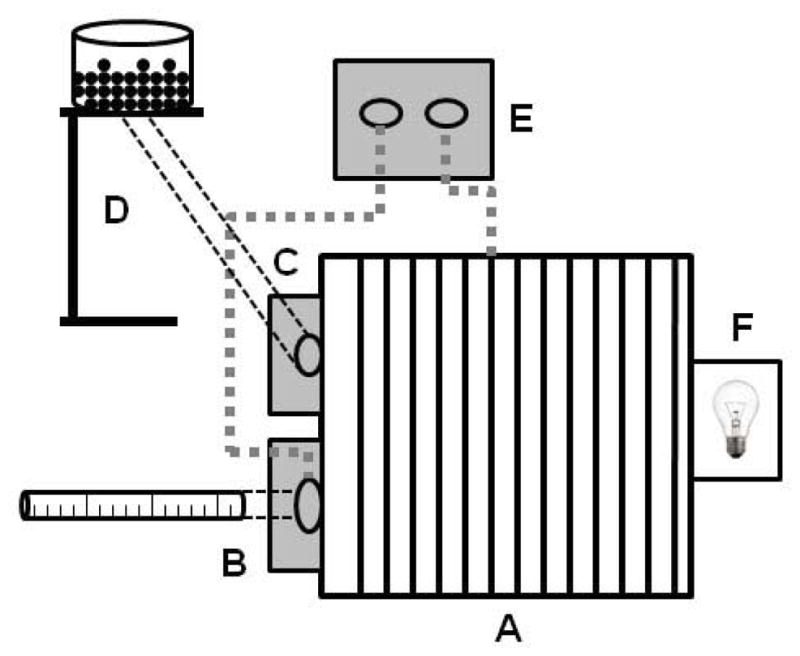
The operant chamber configuration in our laboratory includes a stainless-steel rod floor (A), graduated drinking sipper attached to a stationary mount (B), food receptacle (C), food pellet dispenser (D), lickometer circuit (E) with electrical connections (dotted gray lines) to metal sipper and rod floor, and house light (F). Apparatus is positioned within a sound-attenuating cabinet and a fan is used to ventilate the chamber and mask external noise. Iterations to this configuration and its components are discussed in the text.
SIP is classified as an adjunctive (or displacement) behavior along with other scheduled-induced behaviors such as aggression, escape, wheel running, and air licking (see Falk, 1971 for review). By definition, adjunctive behaviors are excessive in nature and are purportedly derived from thwarting conditions (sub-optimal food reinforcement rate) that dramatically increase the probability of the animal engaging in other possibilities within the environmental context provided, and this exerted effort is viewed as both evolutionarily advantageous and successful adaptation (Armstrong, 1950; Falk, 1971). Because of its key feature of excessiveness, the phenomenon of adjunctive drinking has been tailored to address excessive ethanol intake to model alcoholism in humans. Multiple comprehensive reviews of adjunctive behavior (Falk, 1971, 1998; Wallace & Singer, 1976), polydipsia for the study of pathologic behavior (Moreno & Flores, 2012), and the implementation of SIP to study excessive ethanol consumption (Falk & Samson, 1975; Falk & Tang, 1988; Falk, Zhang, Chen, & Lau, 1994; Meisch, 1975; Meisch & Thompson, 1972; Mello, 1975) have been previously published, and the current review does not attempt to exhaustively replicate these earlier efforts. This work strives to identify crucial features of SIP in rodents that have culminated from decades of investigation and to specifically outline procedural aspects of significance that pertain to the examination of excessive ethanol intake in rats and mice. In reviewing the literature, it was apparent that ethanol SIP studies in rodents have largely retained the procedural parameters that were initially characterized for the investigation of water SIP in rats. The optimization of these experimental variables to account for species differences (rats vs. mice) and fluid properties (water vs. ethanol) will likely enhance the future utility of ethanol SIP as a model of excessive intake. Identification and discussion of these key procedural variables are presented next.
I. Distinguishing features and experimental variables of SIP procedure that engender excessive ethanol drinking
a) Food deprivation
Since a strong feeding response is required to keep animals actively engaged during schedule exposure, food deprivation is a necessary prerequisite for the manifestation of polydipsia. Falk (1969) explored the relationship between adjunctive water drinking and level of food deprivation in one of his early seminal experiments characterizing the SIP phenomenon. In brief, rats were maintained at 80% of their free-feeding body weight under a 1.5-min interval schedule until water SIP fully developed, and then over a 3-week period the animals were permitted to slowly gain weight to approximately 105%. No appreciable difference in water intake was observed as rats surpassed 90%, but between 90% and 105% a linear decline in adjunctive drinking was observed. Although these findings were later recapitulated for water SIP in gerbils placed under a FT 3-min schedule (Porter, 1983), a comparable evaluation of food deprivation level and ethanol SIP has yet to be determined. This ends up being more than just an academic exercise, as it is not known whether the caloric value of ethanol would appreciably influence the relationship between these two variables (see additional discussion in section III below).
It is unclear from the food deprivation study mentioned above (Falk, 1969) whether restriction to 80% was necessary for acquisition of adjunctive drinking, though 90% was apparently sufficient for maintenance of behavior in its exaggerated form. In the vast majority of water and ethanol SIP applications it is standard practice for rodents to be maintained at 80–85% of their free-feeding body weight. The pertinent question is whether this degree of deprivation is necessary. The answer may be dependent upon the interval schedule selected (see below), because a more frequent delivery of pellets (i.e., 0.5 min) may require a greater level of deprivation to keep subjects vigilant and engaged in adjunctive drinking throughout a session. A simple measure of vigilance is whether or not animals consume all of the pellets that are delivered, and engagement can be assessed by whether drinking occurs after each pellet delivery (see ‘schedule control’ below). This point is illustrated by some of our recent work with male C57BL/6 and DBA/2 mice that were restricted to 90% of their free-feeding body weight and then serially examined for ethanol SIP with FT intervals ranging from 0.5 to 20 min during 1-h sessions. Mice were provided access to 5% v/v ethanol for 10 sessions at each FT interval, and food pellets remaining in the chamber at the conclusion of each session were tallied. The percentage of pellets consumed during the 10th and final session at each FT interval per strain is shown in Figure 2 (see legend for additional details). A two-way repeated measures ANOVA detected significant main effects of strain [F(1,9) = 9.69; p < 0.05] and interval [F(1,9) = 182.63; p < 0.001] as well as a strain × interval interaction [F(9,81) = 3.68; p < 0.001] for the percentage of pellets consumed. The percentage of pellets consumed was significantly greater than baseline (FT-0) values with 1-min and longer intervals in both strains (all p’s < 0.001). Both strains consumed approximately 100% of pellets offered when under FT intervals of 2-min and longer. Thus, in our hands a deprivation of 90% was sufficient to maintain vigilance in mice under a FT-2 schedule. This is consistent with the observation that ethanol drinking was under strong schedule control with intervals of 2-min and greater, as indicated by licking after each pellet delivery (Ford, Steele, McCracken, Finn, & Grant, 2013; also see Fig. 4B below). Ideally, food restriction should be adjusted to the least degree that is necessary to sustain a fully developed polydipsia.
Figure 2. Vigilance in pellet consumption as a product of fixed time interval length.
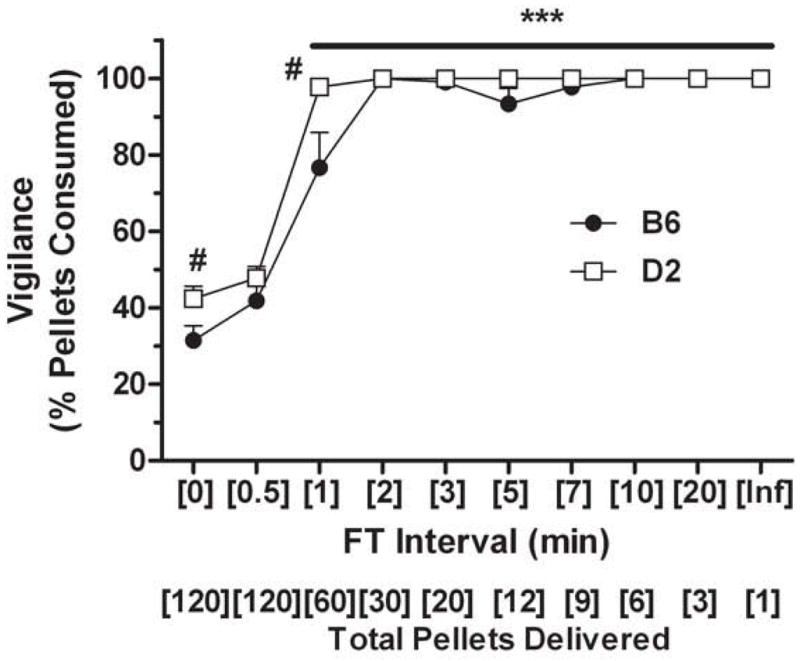
Ethanol-preferring (C57BL/6J; B6) and non-preferring (DBA/2; D2) mice that were provided access to a 5% v/v ethanol solution are shown. The total number of pellets (20 mg) presented to the mice during the 1-h sessions is noted below the X-axis. FT-0 was a massed feeding schedule whereby all pellets were presented in the food hopper at session onset (i.e., no schedule) whereas FT infinity (Inf) involved the delivery of one pellet at session onset with no additional reinforcement provided. Data plotted are the mean ± SEM percentage of pellets consumed during the final (10th) session at each interval for n = 5–6 mice/strain. ***p < 0.001 versus respective baseline (FT-0) value (all intervals ≥ 1 min for both strains); #p < 0.05 between strains within the noted interval. See text for additional experimental details.
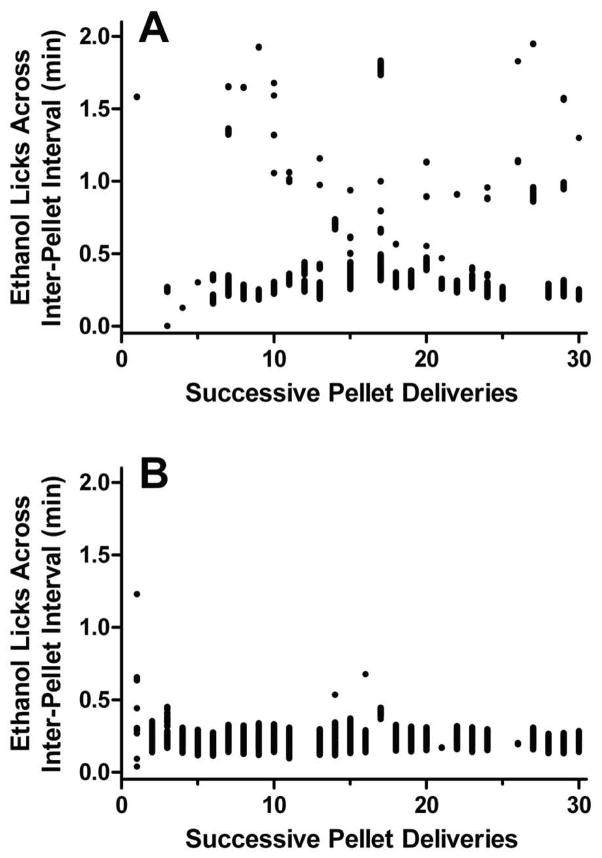
Figure 4. Schedule control throughout the inter-pellet interval.
The distribution of licks on a sipper containing 5% v/v ethanol is shown for a representative D2 mouse. The mouse was exposed to a FT-2 min schedule during 1-h sessions for 10 days. Drinking throughout the inter-pellet interval is depicted on the Y-axis, with 0 min being immediately post-pellet delivery. The initial (panel A) and final (panel B; 10th) session are presented to demonstrate the shift toward greater schedule control with increased experience with the interval as well as the increased number of total licks (1799 vs. 2233; 24% increase). See text for additional experimental details.
b) Inter-pellet interval
One crucial variable is that of interval length between successive pellet deliveries. The earliest work with water polydipsia in rats demonstrated that, like all adjunctive behaviors, the quantity of behavior generated relied upon a bitonic function as a factor of inter-pellet interval, with short and long intervals resulting in modest intakes and intermediate intervals giving rise to excessive intake (Falk, 1966a, 1969; Flory, 1971). The results of these studies were highly replicable, and rats showed maximal water intakes with intervals of 2 or 3 min. A similar relationship between inter-pellet interval and water SIP was determined in rhesus and java macaques (Allen & Kenshalo, 1976, 1978; Grant & Johanson, 1988). Somewhat surprisingly, a parametric analysis to determine the optimal interval for generating the greatest amount of ethanol SIP in rats has yet to be carried out, and contemporary investigations have used only a narrow range of intervals (0.5–2 min). Rarely has more than one interval been examined in the same study. Until recently, a similar knowledge gap existed for mice concerning both water and ethanol SIP. We recently assessed ethanol (5% v/v) and water intakes in mice across a broad range of FT intervals ranging from 0.5 to 20 min to capture the entire bitonic function (Ford et al., 2013). In both ethanol-preferring C57BL/6 and non-preferring DBA/2 mouse strains, FT intervals of 2–5 min generated the greatest volume of ethanol intake. These ‘peak’ FT intervals for ethanol are consistent with earlier work reported for water SIP in rats, suggesting that the bitonic function that describes the relationship between FT interval and volume consumed may be conserved across rodent species and fluid types.
c) Reward magnitude
The magnitude of the reward presented is closely related to the inter-pellet interval in that they both serve to govern the rate at which food is acquired by food-restricted animals. Falk (1967) reported that rats provided with two 45-mg pellets per delivery consumed significantly less total water than rats given only a single pellet. In particular, consumption was found to decline during the second half of the session when two pellets were offered as reward, a finding uncharacteristic for the typically stable water polydipsia observed throughout entire sessions when a smaller reward size is presented. Additional studies from a second laboratory found that rats which received two pellets per delivery consumed more water per interval, but less total water throughout the session when compared to a single-pellet condition (Flory, 1971; Yoburn & Flory, 1977). Because the total number of pellets delivered in the two conditions was constant and the two-pellet sessions allowed only half the number of drinking opportunities, it is conceivable that a decline in intake levels similar to that reported by Falk (1967) would have occurred with a longer session. Because food satiation throughout the session likely affects drinking outcomes, it is important to match choice of reward size with appropriate session duration. Unfortunately, no information is available regarding the influence of reward magnitude on ethanol SIP in rodents. This may be due in part to the standardized sizing of pellets that is commercially available for rats (45 mg) and mice (20 mg). Given the 10-fold or greater difference in body weights between species, it is worth noting that the 20-mg pellets for mice are probably larger than ideal for use in the SIP procedure. If a 5- or 10-mg pellet was commercially available then it might serve to slow the food acquisition rate throughout the session and yield even greater intake volumes in mice. In addition to pellet size, the presentation of food in a densely packed form is important, as intermittent delivery of granulated food results in the animals spending more time engaging the food bin receptacle area and less time drinking (Mumby & Beck, 1988).
d) Ethanol concentration
Early demonstrations of ethanol SIP in rats reported a 5% v/v ethanol concentration as yielding the greatest g/kg intakes (Falk, Samson, & Winger, 1972; Kulkosky, 1979; Roehrs & Samson, 1980). Rats apparently titrate their intake volumes when provided access to higher ethanol concentrations (6–10%), as these solutions yielded g/kg intakes comparable to those observed with 5%. Together, these early findings contributed to the standardized use of 5% ethanol in nearly all ethanol SIP experiments in rodents up to the current day. One notable exception is a recent study in which ethanol concentrations ranging from 2–32% w/v were serially evaluated in an ethanol SIP procedure with three pairs of rat lines selected for ethanol preference (Gilpin, Badia-Elder, Elder, & Stewart, 2008). Consistent with earlier observations of outbred rat strains (i.e., Holtzman, Long Evans), the non-preferring lines (NP, LAD1, and LAD2) tended to reduce their intake volume in a manner that stabilized g/kg intakes across ethanol concentrations. In contrast, both the P and HAD2 ethanol-preferring rat lines exhibited approximately 2-fold increases in g/kg intakes as concentrations were incrementally heightened from 2 to 16% (Gilpin et al., 2008). Examination of an identical range of ethanol concentrations in Sprague-Dawley rats determined that g/kg intakes peaked with the 16% concentration, although high variability between subjects was observed and intakes varied with the number of presentations at each ethanol concentration (Meisch & Thompson, 1972). Not only do these findings point to the importance of genetic background in studying ethanol SIP (see additional discussion below), but they also suggest that customizing ethanol concentration on a per rat strain/line basis would be prudent if generating excessive g/kg intake is germane to the experimental goals.
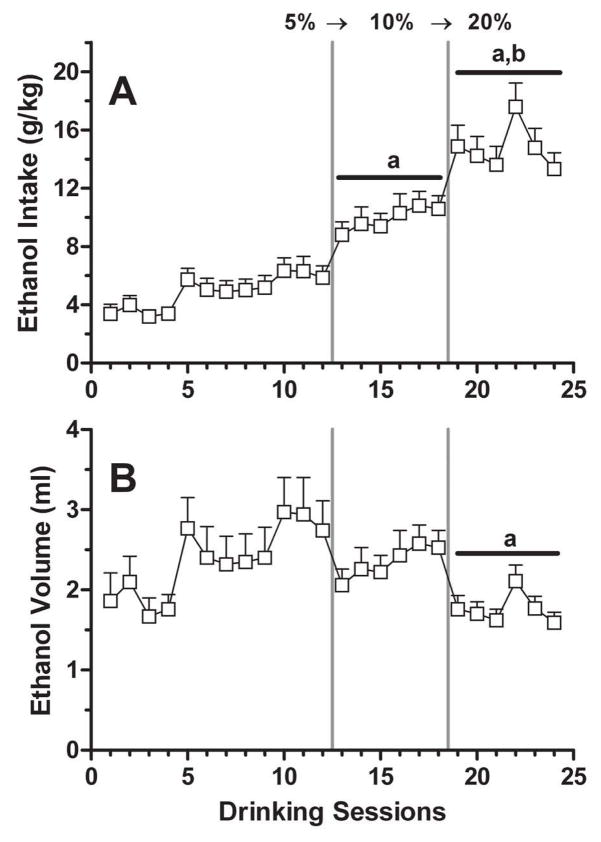
Parametric examination of ethanol concentration in mice undergoing an ethanol SIP procedure has not been extensively explored. In one study, C57BL/6 (B6) and DBA/2 (D2) mice acquired ethanol SIP in the presence of 5% ethanol over twenty 1-h sessions, and then were given four additional sessions each with concentrations of 10 and 20% ethanol (Mittleman, Van Brunt, & Matthews, 2003). Consistent with most previously published work with rats, both mouse strains titrated their g/kg intakes by progressively reducing volume of intake with increasing ethanol concentration. We recently conducted a study assessing the effects of ethanol concentration manipulation in the same two strains of mice. In brief, male B6 and D2 mice were food-restricted to 90% of their free-feeding body weight and then tested in 16-h daily sessions. Each session included six 60-min exposures to a FT-3 schedule of intermittent pellet (20 mg) delivery interspersed by 120-min timeouts. While mice had continuous access to an ethanol solution, water was provided via a retractable sipper tube only during the timeout periods. Ethanol SIP was established over an initial 12 sessions with a 5% v/v solution, followed by six additional sessions each with 10% and then 20% ethanol (Fig. 3). One-way repeated measure ANOVAs determined significant main effects of concentration on g/kg [F(2,14) = 61.68; p < 0.001] and volume [F(2,14) = 6.60; p < 0.001] intakes (see Fig. 3 legend for additional details of analyses). Introduction of 10% and then 20% ethanol resulted in g/kg intakes that were significantly increased by 2- to 3-fold (p’s < 0.01) when compared to 5% ethanol intakes. Intake volumes declined significantly (by approximately 30%) when the 20% ethanol solution was provided (p < 0.01). This relationship between ethanol concentration and g/kg intake was similar to that reported in P and HAD2 rats (see above). It is worth noting that ethanol concentration manipulations may yield different results in the same strains depending on the experimental design used (i.e., single versus multiple FT schedule exposures per day) and the extent of experience with the procedure. Additional studies on the influence of ethanol concentration are clearly needed to explain the disparity in findings documented in both rat and mouse models.
Figure 3. Relationship between ethanol concentration and intake.
Each data point represents the mean ± SEM of g/kg intake (panel A) or ethanol volume (panel B) consumed by n = 8 male C57BL/6 mice during each 16-h session. The vertical gray lines signify transitions in the ethanol concentration provided (5→10→20% v/v). For analysis, the average of the final six sessions with 5% ethanol was compared to the six-session means with 10% and 20% ethanol. ap < 0.01 versus 5% ethanol; bp < 0.01 versus 10% ethanol. See text for additional experimental details.
e) Taste
In two-bottle choice drinking procedures, addition of a sweetener such as saccharin can significantly enhance the g/kg ethanol intake across a large range of ethanol concentrations (for example, see Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008). Because ethanol SIP already generates excessive intakes with unadulterated solutions, there has been little exploration of tastant effects. Ethanol SIP in rats provided access to a 5% ethanol solution was modestly increased when 0.20–0.25% saccharin was added (Gilbert, 1978; Samson & Falk, 1974b). It is unclear with either of these observations whether additional experience with ethanol SIP, rather than necessarily the addition of saccharin, contributed to the changes in ethanol intake. However, the supplementation of a range of ethanol concentrations with either 3 or 9% glucose markedly augmented ethanol SIP in rats when compared to an ethanol-only control group (Kulkosky, 1979). The fact that rats will maintain and even modestly increase adjunctive water drinking with the addition of low (0.1–0.9%), but not higher (≥ 1.2%), concentrations of sodium chloride (Falk, 1966b) further suggests that taste factors are relevant to the non-regulatory fluid intake represented by SIP.
f) Acquisition of SIP and demonstration of schedule control
Rodents require exposure to multiple SIP sessions before expressing adjunctive drinking in its most exaggerated form (Falk, 1984). This latency to achieve stable drinking patterns is convincingly depicted by Mittleman and colleagues (2003) with their demonstration of water or ethanol SIP acquisition in both B6 and D2 mouse strains over a series of daily, 1-h sessions. Comparable acquisition curves for water SIP in rats under similar schedule conditions and over a similar time course has also been previously illustrated (Lopez-Crespo, Rodríguez, Pellón, & Flores, 2004; López-Grancha, Lopez-Crespo, Sanchez-Amate, & Flores, 2008; Platt, Beyer, Schechter, & Rosenzweig-Lipson, 2008). Regardless of species/strain examined or fluid type offered, the excessive drinking tends to fully develop after 10 or more sessions of cumulative experience with an FT interval schedule. It is feasible that longer daily sessions (and hence greater amounts of schedule experience) would enable subjects to acquire SIP in fewer sessions.
Two measurements have historically been used to assess the degree of schedule control over adjunctive drinking: 1) the temporal proximity of drinking following pellet delivery, and 2) the percentage of inter-pellet intervals in which consumption occurs throughout the entire session. In the earliest work, it was noted that a burst of water consumption occurred immediately following the delivery of each pellet (Falk, 1961), and a serial evaluation across a range of intervals revealed that the latency to initiate drinking post-pellet increased monotonically as the interval was lengthened (Segal, Oden, & Deadwyler, 1965). The advent and incorporation of lickometer devices with the drinking sippers (see Fig. 1) have allowed for in-depth analysis of this temporal relationship, with one study of short intervals between 15 and 90 seconds and water SIP in rats being particularly impressive (Lopez-Crespo et al., 2004). This work demonstrated that for a short interval schedule (i.e., 15 sec), licks occur throughout the interval, but with a longer interval of 90 seconds an initial burst of licking is observed within the initial 10-sec post-pellet, followed by a trailing decline in licks until the 60-sec mark, and then very low probability of licking for the remainder of the interval. It is believed that the initial burst of fluid intake post-pellet is not a specific response to meal completion, but rather is a characteristic of adjunctive behaviors in general. The observation that adjunctive wheel running is initiated immediately after pellet delivery (Levitsky & Collier, 1968) would lend support to this notion. Regarding the second measurement, Segal and colleagues (1965) reported a bitonic function for the percentage of inter-pellet intervals in which water drinking occurred as interval length was varied. The highest percentage (i.e., peak) was found with intervals of 2- to 4-min, a relationship that closely mirrors the peak in volume consumption across the bitonic function. Unfortunately, measures of schedule control are seldom assessed in conjunction with the standard reporting of intake volume, and we were unable to find an example of an ethanol SIP study that documented these variables.
From the same mouse experiment described in section I.a (above) in which male mice were serially exposed to incrementally larger FT interval schedules in the presence of 5% ethanol, we assessed drinking patterns for evidence that ethanol intake also falls under schedule control. Cumulative lick records were captured with lickometer circuits, using the configuration illustrated in Figure 1. Specifically, the transition from a FT-1 to a FT-2 schedule was the focus of our inquiry. The lick distributions within the inter-pellet intervals were examined during the initial (1st) and final (10th) session on the FT-2 schedule (see representative D2 mouse shown in Fig. 4). During the initial session, 83% of the inter-pellet intervals contained ≥3 licks whereas this measure was enhanced to 93% by the final session. Further, 10 sessions of experience with the FT-2 schedule resulted in ethanol drinking that occurred almost exclusively during the initial 0.5 min of the inter-pellet interval (Fig. 4). Together, the repetitive drinking after each pellet delivery and the temporal focus immediately post-pellet are indicative of strong schedule control. Thus, measures of schedule control seem to apply to both water and ethanol SIP, and progressive shifts in schedule control with additional experience under an interval schedule may explain why numerous sessions are required for the acquisition and full expression of excessive drinking (for a prototypical example, see Mittleman et al., 2003).
II. Significant biological and environmental factors that contribute to drinking outcomes
a) Mediation by stress hormones
Many studies have shown that the environmental conditions that generate adjunctive behaviors also produce activation of the hypothalamic-pituitary-adrenal (HPA) axis (Brett & Levine, 1979, 1981; Dantzer & Mormède, 1981; Dantzer et al., 1988a; López-Grancha et al., 2006; Mittleman, Jones, & Robbins, 1988; Tazi, Dantzer, Mormede, & Le Moal, 1986). Several stress mediators such as corticosterone (CORT), epinephrine, and norepinephrine are elevated in rodents under the interval schedule conditions that support SIP. Increased concentrations of the pituitary hormones prolactin in rats (Dantzer et al., 1988a) and adrenocorticotropic hormone (ACTH) in monkeys (Helms et al., 2013) were similarly reported. The significance of stress hormones in the behavioral processes underlying SIP was further validated by both surgical (adrenalectomy) and pharmacological (CORT synthesis inhibitor metyrapone) manipulations that resulted in an attenuated acquisition or maintenance of adjunctive water drinking (Levine & Levine, 1989; Mittleman, Blaha, & Phillips, 1992). Collectively, these observations have given rise to the hypothesis that adjunctive drinking is a ‘coping’ strategy to mitigate the stressful conditions imposed by intermittent reinforcement at a sub-optimal rate. Perhaps the strongest piece of evidence in support of this hypothesis is that CORT levels in rats decline throughout a SIP session when the rats engage in adjunctive water drinking (Brett & Levine, 1981; Mittleman et al., 1988), but this benefit does not occur when the animals are exposed to the interval schedule and access to the water bottle is omitted (Dantzer et al., 1988a; Tazi, Dantzer, Mormede, & Le Moal, 1986). Curiously, the effect of adjunctive water drinking on CORT concentrations appears to be a special case, as the plasma catecholamines noradrenaline and adrenaline were unaltered by fluid consumption (Dantzer et al., 1988a), and engagement in the alternate adjunctive behavior of wheel running led to elevated levels of plasma CORT in rats (Tazi et al., 1986). It is tempting to speculate that a special relationship between excessive fluid intake and CORT levels exists whereby common steroid precursors for CORT and aldosterone are shunted away from CORT and toward aldosterone in response to stress (i.e., ACTH stimulation) and challenge to fluid homeostasis (i.e., reflexive sodium retention).
Surprisingly, previous reports exploring stress axis involvement in adjunctive drinking have focused exclusively on water intake, and so no information is available for ethanol SIP in rodents. To address this gap in knowledge, our laboratory group recently completed work in B6 and D2 mice that evaluated the relationship between plasma CORT and adjunctive ethanol drinking across multiple interval schedules that captured the bitonic function of behavioral output (Ford et al., 2013). We found that both strains displayed a bitonic function of g/kg ethanol intake and blood ethanol concentration that peaked at or near a 5-min interval, but also observed that corresponding CORT levels rose monotonically with incremental lengthening of the inter-pellet interval regardless of the strain examined or fluid type (5% ethanol or water) offered. Further, no correlations between g/kg ethanol intake and plasma CORT were found for either strain, regardless of whether this relationship was analyzed with each interval separately or all intervals combined. Although this latter finding contrasts with earlier work in rats in which a significant negative correlation between water consumed and plasma CORT was observed (r = −0.85; Mittleman et al., 1988), it is consistent with the recent work in cynomolgus macaques in which neither ACTH nor cortisol levels were related to the ethanol dose consumed under a FT 5-min schedule (Helms et al., 2013). At least in mice engaged in adjunctive ethanol drinking, CORT likely reflects the aversive aspects of increasing intervals between food reinforcement rather than engagement in adjunctive drinking per se.
b) Neurochemical systems
Another prominent hypothesis under intense scrutiny to explain adjunctive drinking is that of polydipsia as a displacement activity. From an adjunctive drinking perspective, displacement activity is defined as “the interruption of a consummatory behavior (food intake) in an intensely motivated animal (food deprived) that induced the occurrence of another behavior (fluid intake) immediately following the interruption (proximal portion of inter-pellet interval) which is facilitated by environmental stimuli (interval schedule of food reinforcement)” (Falk, 1971). Given the essential role of reward processes in this explanation of adjunctive drinking generation, it is not surprising that investigation of neurochemical systems has primarily focused on brain reward circuitry (dopaminergic projections) and those neurotransmitter systems (serotonin, glutamate, GABA, and opioid) known to modulate its tone. Pharmacological manipulations of dopamine neurotransmission have been extensively tested for effects on water SIP, and are summarized elsewhere (Moreno & Flores, 2012). For example, D2 receptor antagonism with haloperidol dose-dependently reduced water intake (Mittleman, Rosner, & Schaub, 1994) and was associated with declines in the time spent drinking and increases in striatal dopamine (Todd, Beck, & Martin-Iverson, 1992). These findings are consistent with the more recent observations that both amphetamine and cocaine pretreatments, which would be expected to elevate extracellular dopamine concentrations, led to reductions in water drinking in rats exhibiting excessive intake (Lopez-Grancha et al., 2008). Destruction of dopamine terminals within the mesolimbic dopamine circuitry (i.e., nucleus accumbens and olfactory tubercle) in rats via bilateral 6-hydroxydopamine lesions prevented acquisition of water SIP (Robbins & Koob, 1980). Mice with a genetically engineered deletion of the dopamine transporter (DAT) exhibited a diminished acquisition of ethanol SIP with access to a 5% solution (Mittleman et al., 2011), although earlier work with a two-bottle choice procedure showed that neither male nor female DAT knockout mice displayed differences in ethanol consumption versus wild-type controls when a comparable 4% solution was offered (Hall, Sora, & Uhl, 2003). The apparent disparity between these observations may reflect the differential roles of dopaminergic signaling in the context of non-regulatory (SIP) versus regulated (two-bottle choice) ethanol consumption, similar to differences noted for adjunctive versus deprivation-induced water drinking following destruction of dopamine terminals within the mesolimbic pathway (Robbins & Koob, 1980). Rapid scan voltammetry of dopamine in the rat nucleus accumbens core during water SIP acquisition revealed that DA steadily increased across the 30-min session and peaked shortly after schedule termination, but DA efflux was found to be unrelated to drinking response, as a similar result was obtained when the water bottle was removed (Weissenborn, Blaha, Winn, & Phillips, 1996). Collectively, these findings suggest that enhancement of dopaminergic tone diminishes established adjunctive drinking, but that dopamine signaling is essential for acquisition of SIP. At this time it is unclear whether dopamine’s role in adjunctive drinking differs for water versus ethanol intake, as most studies to date have been conducted with water SIP and further investigation specific to ethanol consumption is needed.
Similar to the impact of DAT deletion in mice, treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine in rats attenuated adjunctive water drinking (Hogg & Dalvi, 2004; Woods et al., 1993). Other pharmacological manipulations of serotonergic neurotransmission have proven equally effective. For example, the 5-HT2C agonist WAY-163909 reduced water SIP, and this effect was reversed by pretreatment with the 5-HT2C antagonist SB215505 (Rosenzweig-Lipson et al., 2007). Additional evidence for serotonergic involvement in SIP comes from a comparison between Roman High Avoidance (RHA) and Roman Low Avoidance (RLA) rat strains. RHA rats display an enhanced acquisition of water SIP, and this strain difference is partly attributable to a serotonergic (5-HT) hyperactivity in the nucleus accumbens and striatum of RHA rats under basal conditions when compared to RLA rats (Moreno et al., 2010). Although ethanol SIP has not been tested in these strains, the RHA strain is known for its increased propensity to self-administer ethanol and other drugs of abuse (Giorgi, Piras, & Corda, 2007; Martin & Baettig, 1981). Overall, the influence of serotonergic transmission over adjunctive drinking is less clear, with pharmacological interventions thought to elevate extrasynaptic serotonin levels (fluoxetine) or augment the post-synaptic excitatory action of serotonin (WAY-163909), culminating in reduced consumption whereas an inherently elevated serotonergic tone in RHA rats is associated with enhanced drinking. Given that RHA rats also express significant differences in dopaminergic and GABAergic tone when compared to RLA rats (Giorgi et al., 1994), it seems reasonable to conclude that serotonin may not be the predominant regulator of adjunctive drinking in these strains.
As alluded to above for dopamine, only a limited number of studies have explored the neurochemical bases of ethanol SIP through pharmacological intervention. Mittleman and colleagues have been true pioneers in this regard. In a mouse model of ethanol SIP, treatment with acamprosate, naltrexone, memantine, or amantadine revealed that each drug significantly reduced ethanol consumption, but also exhibited efficacy in decreasing the regulatory drinking of water following a prolonged period of fluid deprivation and/or adjunctive water drinking (Escher, Call, Blaha, & Mittleman, 2006; Escher & Mittleman, 2006). These findings led to the overall conclusion that the treatments tested were non-selective in their reduction of adjunctive ethanol intake. Clearly, additional studies are warranted as only a limited number of therapeutic targets have been tested to date with one mouse strain (B6) and one ethanol concentration (5%) under a single set of SIP conditions (FT-1; 40-min session).
c) Individual variability, genetic background, and strain/line effects
Significant individual variability in the expression of water SIP has been previously noted by numerous lab groups employing outbred rat strains (Sprague-Dawley, Long-Evans, Wistar, etc.). Some subjects appear to show little to no increase in fluid intake over baseline levels when exposed to a FT interval schedule of food reinforcement, although in some cases this may be the consequence of only a single interval being tested. Experimentally, intake differences are handled by parsing the subjects into SIP-negative versus SIP-positive groups (Dantzer et al., 1988a, b), low-drinker versus high-drinker groups (Lopez-Grancha et al., 2008), or drinker versus non-drinker groups (Mittleman et al., 1988). Water intake differences between these groupings following an adequate number of SIP acquisition sessions typically ranged from 3- to 4.5-fold depending on session length (30–60 min). Investigation of parallel experimental procedures in conjunction with these individual differences in adjunctive water consumption has revealed that SIP-positive rats also exhibit drinking in response to electrical stimulation of the lateral hypothalamus (Mittleman & Valenstein, 1984) and display a greater neurochemical (i.e., greater forebrain dopamine system sensitivity to drug and physical stressors [Mittleman, Castañeda, Robinson, & Valenstein, 1986]) and behavioral (i.e., more rapid active avoidance learning [Dantzer et al., 1988b]) reactivity to aversive stimuli. The relationship between these individual differences in neurochemistry and behavior has yet to be explicitly demonstrated for inter-subject variability in adjunctive ethanol drinking.
In general, documented differences in two-bottle choice ethanol drinking between rodent strains or lines are also observed under ethanol SIP conditions. This has proven true for comparisons of B6 versus D2 mice (Ford et al., 2013; Mittleman et al., 2003) and for RHA versus RLA rats (Martin & Baettig, 1981), Fischer 344 versus Lewis rats (Stöhr et al., 2000), P versus NP rats and HAD2 versus LAD2 rats (Gilpin et al., 2008). These observations suggest that these two drinking traits may be genetically correlated, and that overlapping sets of genes contribute to both regulated and adjunctive ethanol drinking. Preliminary evidence from recombinant BXD inbred mouse strains exposed to ethanol SIP indicated that adjunctive drinking as a trait was approximately 27–31% heritable, depending on the drinking measure evaluated (Goldowitz et al., 2006). A comparable realized heritability of 32% was reported in a short-term selection study for preference of a 10% ethanol solution in mice (Belknap, Richards, O’Toole, Helms, & Phillips, 1997).
d) Gender and age
Several studies have indicated that female rats (Sprague-Dawley, Fischer 344) acquire water SIP faster and express greater levels of adjunctive drinking than their male counterparts (Katovic, Gresack, & Spear, 1999; Stohr et al., 2000). However, this does not seem to be universally observed, as no sex differences were reported for adjunctive water drinking in Lewis rats (Stohr et al., 2000). Although evidence is more limited for ethanol SIP, one study found that females of both the RHA and RLA lines outperformed males during acquisition and maintenance of excessive drinking (Martin & Baettig, 1981). For each of these studies, a single interval schedule was examined (FT-1 min), so the possibility remains that the optimal interval length for male and female rodents may differ, and that the chosen interval more often favored a greater expression of excessive intake in females. Although additional male-female comparisons with SIP are needed before definitive conclusions can be drawn, the observation of sex differences is consistent with the preponderance of evidence suggesting that female rodents tend to consume more than males within multiple models of ethanol self-administration (Almeida et al., 1998; Middaugh, Kelley, Bandy, & McGroarty, 1999). Within other excessive intake models, a sex difference in mice was observed with an intermittent-access procedure (Hwa et al., 2011), but not in an examination of the alcohol deprivation effect (Tambour, Brown, & Crabbe, 2008).
A limited number of studies have explored the effect of age on acquisition and expression of water SIP. One report found that 24-month-old male Sprague-Dawley rats exhibited difficulty in acquiring water SIP, as a greater degree of food restriction (70% versus the standard 80% free-feeding body weight) was required to induce polydipsia when compared to historical controls that were 4 months old or younger (McCaffrey, Pavlik, & Allen, 1981). The authors interpreted this finding as a reduction in motivational set point in aged rats. A second study found no difference in water SIP among rat groups ranging from 30- to 120-days old after accounting for differences in body weight (Reynolds, Kenny, & Wright, 1977). It would be of interest to characterize a similar age span for ethanol SIP, especially given the documentation of enhanced vulnerability to and greater consumption of ethanol in adolescent when compared to adult animals (McBride, Bell, Rodd, Strother, & Murphy, 2005; Spear, 2004).
III. Advantages and limitations of ethanol SIP
Multiple animal models of excessive ethanol intake employ scheduled conditions to generate an escalation in self-administration. Drinking in the dark (DID; limited access), the alcohol deprivation effect (ADE), chronic intermittent access (every other day), and SIP are some prominent examples (for review, see Becker, 2013). These procedures share common features, such as intake that is orally consumed, voluntary, and expressed in a quantity and pattern that often results in blood ethanol concentrations (BECs) synonymous with binge-like drinking and legal intoxication (≥ 0.80 mg/mL; NIAAA, 2004). Ideally, excessive intake models would also support the development of ethanol dependence and allow for repeated or sustained self-intoxication throughout the day; signs reminiscent of observations in alcoholics. These latter qualities are what most clearly differentiates ethanol SIP from other excessive intake models (although, see discussion of limitations below). DID, ADE, and chronic intermittent access can produce acute ethanol intoxication, but these effects are typically short-lived and limited to the period immediately following the reintroduction of ethanol access. In contrast, experimental parameters to generate a state of ethanol dependence have been extensively studied for the ethanol SIP procedure in rats (Falk et al., 1972; Falk & Tang, 1988; McMillan, Leander, Ellis, Lucot, & Frye, 1976; Samson & Falk, 1975). Further, documentation of withdrawal signs (i.e., hyperactivity, tonic-clonic seizures following key shaking) in these studies would seemingly confirm that dependency is achieved. An essential parameter of dependency induction is the number and total duration of schedule exposure periods throughout a 24-h period. While two 1-h exposures were insufficient for the development of physical dependence (Samson & Falk, 1975), six such sessions equally interspersed throughout the day were successful (Falk et al., 1972). The latter procedural variation sustained BECs at or above 1 mg/mL throughout most of the day and supported peak concentrations between 1.5–3.0 mg/mL. This level of continual or repeated exposure seems to be critical, as an investigation of chronic intermittent ethanol exposure via vapor inhalation determined that a sustained experience with BECs of 1.75 mg/mL or greater was essential for escalations in withdrawal-induced drinking in mice (Griffin, Lopez, Yanke, Middaugh, & Becker, 2009). The use of ethanol SIP to establish ethanol dependence in mice has received far less attention. In the one known study, female outbred mice were given four 1-h exposures per 24 h over 7 days with access to either 6 or 10% ethanol (Ogata, Ogato, Mendelson, & Mello, 1972). Although this procedure yielded a comparable range of BECs (70–279 mg/mL) as a similar procedure in rats (above), overt signs of physical withdrawal were not detected. Multiple factors could explain this negative result: an insufficient number of schedule exposures throughout the day (six exposures were needed for rats), the absence of a robust measure for assessing withdrawal severity (such as handling-induced convulsions), or the use of a mouse strain resistant to ethanol withdrawal seizures. Additional work in mice is clearly needed to determine whether the development of ethanol dependence with SIP generalizes to other rodent species beyond rats.
Ethanol SIP in rodents appears to have construct validity in modeling alcohol abuse and alcoholism. First, consistent with the notion of adjunctive drinking as a displacement behavior, adjunctive ethanol drinking in humans can be induced by a fixed interval schedule. Subjects demonstrated excessive ethanol consumption when provided both monetary reinforcements via a slot machine game on either a 30- or 90-sec interval schedule and unlimited access to beer during a 30-min session (Doyle & Samson, 1988). The 90-sec interval generated significantly greater ethanol intakes than the 30-sec interval, and consumption patterns revealed that drinking fell under schedule control in the majority of subjects tested (i.e., highest drinking observed immediately after reinforcement). Second, another leading theory to explain the manifestation of adjunctive drinking is that of a coping strategy to deal with aversive environmental conditions (see above). Farber, Khavari, and Douglass (1980) found that self-reported reasons for drinking can be empirically classified into either negative reinforcement (‘escape’) drinking or positive reinforcement (‘social’) drinking. Escape drinking is known to occur when either no alternate coping strategies are available due to set circumstances or an individual has a limited repertoire of coping to begin with (Timmer, Veroff, & Colten, 1985). Escape drinkers scored higher on alcohol consumption indices, and in a cohort of 133 alcoholics, 93% fell into the category of ‘escape’ drinking. Escape drinking also predicts abuse status, even after correcting for overall consumption (Polich & Orvis, 1979). Another study reported that drinking to cope with stress was a stronger predictor of overall consumption and heavy consumption (binge) episodes when compared to social drinking (Abbey, Smith, & Scott, 1993). In fact, relapse has been linked to the use of similar coping strategies by alcoholics when they are challenged with aversive conditions. In a study of university students, escape drinking was found to be the only positive direct predictor of binge drinking and the development of risky alcohol consumption patterns (Williams & Clark, 1998). Collectively, these findings suggest that humans engage in drinking as both displacement and escape behaviors when faced with aversive environmental conditions, and that in both cases this consumption is oftentimes excessive.
A significant limitation of ethanol SIP is that maintenance of excessive intake dissipates with the discontinuation of the generator schedule. Tang, Brown, and Falk (1982) demonstrated that removal of the schedule conditions even after extensive experience with a single 3-h exposure per day resulted in a return to ethanol intake levels commensurate with a massed feeding condition in rats. This finding was similar regardless of the cumulative experience with the daily 3-h schedule exposures (29 vs. 109 days) or the number of times the schedule was removed (up to 11 times). This effect is not unique to ethanol SIP, as a similar reduction in excessive water intake is found in mice upon schedule removal (Palfai, Kutscher, & Symons, 1971; Tang et al., 1982). One caveat is that the impact of schedule removal has not been exhaustively explored in rodents. Work with multiple animal models has identified key procedural variables that are now recognized as important for generating excessive intake: 1) experiencing ethanol as a positive reinforcer prior to dependence induction (Roberts, Cole, & Koob, 1996), 2) permitting the association of ethanol drinking with the alleviation of adverse symptoms that accompany withdrawal (Becker & Lopez, 2004), 3) providing intermittent rather than continuous ethanol exposure throughout dependence induction (Becker & Lopez, 2004; O’Dell, Roberts, Smith, & Koob, 2004), and 4) exposing animals to repeated cycles of chronic exposure and subsequent withdrawal (Finn et al., 2007; Griffin et al., 2009; Lopez & Becker, 2005). Earlier work with the suspension of schedule conditions did not account for these experimental contingencies. First, ethanol dependence does not consistently develop with exposure to only one schedule exposure per day; multiple episodic peaks in blood ethanol concentrations are required (Falk et al., 1972; Samson & Falk, 1975). Second, the animals were not permitted to experience ethanol as a positive reinforcer prior to onset of intermittent food delivery. Third, the animals were not ethanol-deprived prior to schedule removal, and so they were unfamiliar with associating ethanol drinking with the relief of withdrawal symptoms. Therefore, the inability to achieve sustained, excessive consumption following schedule removal in earlier work may be due to the absence of key procedural factors. Additional studies will be required to investigate this possibility.
It is noteworthy that a modified ethanol polydipsia procedure in non-human primates culminates in rates and patterns of consumption under schedule that are predictive of a heavy drinking phenotype when open access to ethanol is provided in the absence of the generator schedule (Grant et al., 2008). This modified procedure importantly takes advantage of the negative reinforcing effects of ethanol via ‘escape’ drinking (see above); it rewards subjects that drink a set dose of ethanol rapidly with an earlier termination of the FT interval schedule. This is in contrast to rodent applications of SIP that include exposures to a FT interval schedule during sessions of fixed duration and function primarily to establish the positive reinforcing effects of ethanol (Becker, 2013; Meisch, 1975). Because the SIP procedure employed by the Grant laboratory in macaques uses fixed induction doses and typically transitions subjects from a variable-length induction session (~1–4 h) to a 22-h open-access session, it is not possible to directly compare ethanol intakes between experimental phases or to compare the influence of schedule removal between macaque and rodent procedures.
Although the reduction of ethanol consumption upon removal of the generator schedule is a major limitation for ethanol SIP in rodents, this feature is shared by other excessive intake models that depend upon a form of intermittency to support escalation in intake over baseline conditions. For example, chronic intermittent (every other day) access to ethanol in B6 mice results in a 2-fold increase in consumption of a 15% ethanol solution over seven consecutive sessions when compared to a control group receiving continuous ethanol access (Melendez, 2011). When the intermittent ethanol group was subsequently exposed to a continuous-access condition the elevated intakes returned to baseline levels over a subsequent seven sessions.
A second limitation for ethanol SIP is the necessity for food restriction of animal subjects (see section I.a above). This feature also distinguishes SIP from other excessive intake models (DID, ADE, and chronic intermittent access) which do not require this manipulation. The biggest concern with food restriction is the uncertainty as to whether animals are excessively drinking ethanol for its caloric content versus its positive reinforcing and motivational properties. This concern mimics a broader debate in the alcohol literature over the role that its caloric value plays in its self-administration. One viewpoint is epitomized by observations that ethanol-preferring B6 mice consume intoxicating levels of ethanol only in concert with daily patterns of feeding behavior and reduce their intakes when food diets are enriched with sugar (Dole & Gentry, 1984; Dole, Ho, & Gentry, 1985). This led to the conclusion that mice are consuming ethanol for its nutritional and not its pharmacological properties. The opposing viewpoint is typified by findings in Long-Evans rats that total ethanol and total food intake are not significantly correlated, food bouts are of similar size regardless of whether accompanied by ethanol or water drinking, and ethanol is often consumed in larger bouts than water (Gill, Amit, & Smith, 1996). In contrast, these findings led to the conclusion that the ethanol consumption is not regulated as a calorie source, but is consumed in discrete bouts that exert pharmacologically relevant effects. Regardless of how each set of findings is interpreted, food restriction places an animal in a calorie deficit that magnifies the issue of ethanol as calories. Perhaps the strongest argument against ethanol SIP as being calorically driven is provided by numerous reports that rodents readily participate in adjunctive drinking of water and non-caloric drugs (Falk et al., 1994; McMillan & Leander, 1976; Singer & Wallace, 1984). Further, concurrent presentation of either a 0.25% w/v saccharin or a 0.9% sodium chloride solution alongside a 5% ethanol solution resulted in rats shifting their preference away from ethanol, even after an extensive history of ethanol SIP (Falk & Tang, 1988; Samson & Falk, 1974a; Tang & Falk, 1986). This suggests that caloric content of the excessively consumed fluid is not the most salient feature, and that taste is of equal or greater importance. This realization also has implications for the finding by Dole and colleagues (1985) that sugar-supplemented food was able to supplant preference for ethanol. Lastly, SIP is considered a displacement behavior that is non-regulatory. In contrast, calorie balance is tightly regulated, and so it is not expected that a non-regulatory behavior would be engaged in as a means to restore caloric intake. While the caloric content of ethanol cannot be discounted, it is not the only factor that contributes to its excessive intake under scheduled conditions.
Oral self-administration of ethanol can present some methodological issues, and ethanol SIP is not immune to these procedural challenges. In an earlier review, Mello (1975) outlined some common problems in evaluating excessive ethanol intake in experimental animals that included ensuring that the fluid is actually ingested and measuring blood ethanol concentrations to demonstrate efficacy of the oral route. One way to address these concerns in rodent studies is to incorporate lickometer circuits for the detection of licks (as shown in Fig. 1). This modification to the apparatus accomplishes two goals in that correlations between volume displaced and sipper contacts can be assessed and time-stamped lick patterns can be monitored for evidence of sipper tampering (i.e., rates resulting from licking versus clawing at sipper can be discerned). In our experience with ethanol SIP in C57BL/6 and DBA/2 male mice, correlations between g/kg dose and licks were r = 0.85–0.89, and post-session blood samples revealed that g/kg dose and BECs were also strongly correlated (r = 0.77–0.87; p < 0.001 in each case [Ford et al., 2013]). These correlative measures can help address some of the interpretative challenges associated with oral ethanol intake by rodents within a SIP procedure.
IV. Steps toward enhancing the utility of SIP in the study of excessive drinking in rodents
Ethanol possesses several qualities (i.e., rewarding, anxiolytic, intoxicating, caloric) that distinguish it from water, and when it is consumed in excessive quantities there are likely to be significant differences in the polydipsic response with ethanol versus water. Despite this, parametric analyses of the experimental variables for ethanol SIP in rodents has yet to be thoroughly examined, and most variables are chosen without species consideration (i.e., 5% ethanol for rats is also commonly used for mice) and are based on the historical parameters used for water SIP. Customization of the procedure for ethanol as the available fluid and for the species/strain under examination would be advisable.
Another related issue surrounds the loosely defined volume threshold for the demonstration of SIP versus spontaneous (or regulatory) drinking. Are all studies that claim to be studying SIP actually investigating behavior that is excessive beyond what is spontaneously observed in the animal species/strain under examination? Is the volume of ethanol consumed the most appropriate endpoint to make this determination, especially considering that the concentration of ethanol chosen will likely influence the outcome? In an earlier review on adjunctive behavior, Falk described the search for the shortest interval schedule that culminated in water polydipsia when compared to baseline intake levels (when the same total number of pellets were provided in aggregate at session onset for the same session duration), and a factor of 2 (or doubling) in a session as short as 15 min was considered ‘unequivocal polydipsia’ (Falk, 1971). To our knowledge a similar threshold for ethanol SIP and for mice in general has not been clearly defined. Further, when considering different rodent strains and their exposure to ethanol SIP, the baseline intakes (that encompass extremes of innate preference or avoidance) may dictate whether or not a 2-fold or similar threshold is reached under schedule conditions. For example, B6 mice will drink considerable amounts of ethanol under baseline conditions, but even when these mice are exposed to an interval schedule that generates the greatest volume of intake (i.e., FT-3 or FT-5), this often does not approach a doubling of volume intake for most subjects when compared to within-subject baseline measures when the entire pellet total was presented at session onset (Ford et al., 2013). Observations of a similar nature were found in several selected lines of rats bred for high and low ethanol preference (Gilpin et al., 2008), with all lines demonstrating a significant decline in volume intake of a 4% w/v ethanol solution (which closely approximates the standard rat ethanol concentration of 5% v/v) when compared to within-subject water intakes under identical schedule conditions. At least in the case of our earlier work with mice, if the occurrence of excessive intake was evaluated based upon the metric of BECs then the 4-fold increase (0.5 mg/mL to 2.0 mg/mL) over baseline values observed after 1-h of schedule exposure (Ford et al., 2013) would certainly qualify as excessive according to Falk’s criterion. Thus, BECs may be the most appropriate measure to confirm the physiological relevance of ethanol intake that occurs, and this index also allows for consideration of various ethanol concentrations and establishes confidence that dissipated volumes are actually consumed (see Mello, 1975). A combination of BECs and behavioral measures of schedule control that confirm SIP independent of intake volume would be ideal for interpreting experimental findings.
Overall, a greater mechanistic understanding of the hormonal signaling involved in the regulation of adjunctive ethanol drinking and how these neuromodulators influence neurochemical systems is needed. As documented above, the primary focus to date in rodents has been on the glucocorticoid CORT as a means to explain schedule-induced drinking as a coping response in face of stressful environmental contingencies. Preliminary findings from a quantitative trait locus (QTL) analysis of recombinant BXD inbred lines for ethanol consumption during SIP identified numerous candidate genes whose expression was highly correlated with this trait (Goldowitz et al., 2006). Amongst a group of stress-related genes located in the QTL region on mouse chromosome 8 was mevalonate decarboxylase (Mvd), which is involved in regulating the biosynthesis of the steroid precursor cholesterol. In addition to CORT, several other adrenal-derived stress axis mediators may influence excessive ethanol drinking, such as deoxycorticosterone (DOC) and its metabolites, which are well known for their modulation of central GABAergic function (Reddy & Rogawski, 2002). Interestingly, DOC also serves as a steroid precursor to aldosterone, which is crucial in maintaining sodium and potassium homeostasis (Müller, 1995). Aldosterone function may be particularly relevant when the body’s fluid homeostasis mechanisms are challenged during excessive ethanol drinking. Investigation of polydipsia stemming from diabetes insipidus in humans indicates that dysregulation or mutation of the type-2 vasopressin receptor is a common cause (Moeller et al., 2013). Consistent with this notion, the STR/N inbred mouse strain, which exhibits a spontaneous water polydipsia, had 7- to 10-fold greater arginine vasopressin (AVP) binding in the thalamic paraventricular nucleus, and binding was absent in the hypothalamic paraventricular nucleus when compared to non-polydipsic control mice (Tribollet et al., 2002). Taken in conjunction with recent work demonstrating the role of AVP and type 1B receptors in the modulation of HPA axis function and the transition from excessive ethanol intake to dependence in rats (Edwards, Guerrero, Ghomeim, Roberts, & Koob, 2012), the potential role of AVP signaling in ethanol SIP deserves further attention. Lastly, sex differences in the acquisition and maintenance of SIP (see above) suggest that perhaps estrogen and progesterone-derived steroids may play a role in governing adjunctive drinking. This notion would be consistent with previous observations from our laboratory group demonstrating crucial roles for estradiol (Ford, Eldridge, & Samson, 2004) and sex-specific effects of progesterone derivatives (Finn, Beckley, Kaufman, & Ford, 2010) in modulating ethanol self-administration in rodents.
The potential use of SIP for modeling compulsive behavioral patterns associated with chronic ethanol use remains under-explored. Compulsive alcohol drinking in alcoholics represents a loss of control over highly repetitive drug seeking routines following chronic exposure, and this dysfunction is attributable in part to changes in prefrontal cortex circuits that process inhibitory control of behavior (Vengeliene, Celerier, Chaskiel, Penzo, & Spanagel, 2009). These same brain regions are functionally deficient in patients suffering from obsessive-compulsive disorder (OCD). The value of a SIP procedure to model behavioral compulsions that accompany OCD was recently reviewed, and it was concluded that drug treatments found to be clinically effective in treating OCD (in particular, selective serotonin reuptake inhibitors [SSRIs]) were also effective in reducing water polydipsia in rodents (Moreno & Flores, 2012; Platt et al., 2008). This pharmacological validation of SIP for modeling compulsive-like behavior opens up the possibility that similar pharmacotherapy strategies could be investigated for their efficacy in curtailing excessive ethanol intake. A logical next step would be to determine whether SSRI treatment exhibits efficacy in reducing ethanol SIP in the absence of effects on regulatory water drinking.
Schedule-induced polydipsia, like other excessive intake models, is best viewed as a partial model to study alcoholism, and likely reflects only a subtype of alcoholics or a single aspect of the disease. It may not be surprising then that conventional therapeutics are largely non-selective in their reduction of excessive ethanol intake generated under scheduled conditions (see Escher, Call, et al., 2006; Escher & Mittleman, 2006). These findings do not necessarily reflect a weakness of the ethanol SIP model, but may echo the low efficacy of current medications in reducing excessive intake patterns originating from compulsive/displacement behavior or as a coping/escape strategy. This would be consistent with findings from a clinical laboratory setting that suggested naltrexone effectiveness may be dependent on a certain drinking repertoire (Anton, Drobes, Voronin, Durazo-Avizu, & Moak, 2004). Further, rats with a 12-day history of six 1-h schedule exposures per day and access to a 5% ethanol solution showed a 96% preference for ethanol when water was made concurrently available over a subsequent 3-day period (Samson & Falk, 1974a). This work indicated that in ethanol-dependent rats, ethanol SIP possessed unique properties (or led to neuroplastic changes) that distinguished it from adjunctive water drinking. Although pharmacological interventions have yet to demonstrate selectivity for ethanol, there is necessarily a neurobiological basis for this ethanol preference during schedule exposure. Additional investigation could identify the neural system(s) underlying this preference, and perhaps unveil novel therapeutic avenues for selectively reducing excessive ethanol intake.
Acknowledgments
This research was supported by the NIH, including a pilot project grant from the Integrative Neuroscience Initiative on Alcoholism: Stress and Anxiety of Alcohol Abuse (AA013641), a Mentored Research Scientist Development Award (AA016849), and the Oregon National Primate Research Center (OD 011092).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbey A, Smith MJ, Scott RO. The relationship between reasons for drinking alcohol and alcohol consumption: an interactional approach. Addictive Behaviors. 1993;18:659–670. doi: 10.1016/0306-4603(93)90019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Kenshalo DR. Schedule-induced drinking as a function of interreinforcement interval in the rhesus monkey. Journal of the Experimental Analysis of Behavior. 1976;26:257–267. doi: 10.1901/jeab.1976.26-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Kenshalo DR. Schedule-induced drinking as functions of interpellet interval and draught size in the Java macaque. Journal of the Experimental Analysis of Behavior. 1978;30:139–151. doi: 10.1901/jeab.1978.30-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. The Journal of Clinical Investigation. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Armstrong EA. The nature and function of displacement activities. Symposia of the Society for Experimental Biology. 1950;4:361–384. [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Current Topics in Behavioral Neurosciences. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Richards SP, O’Toole LA, Helms ML, Phillips TJ. Short-term selective breeding as a tool for QTL mapping: ethanol preference drinking in mice. Behavior Genetics. 1997;27:55–66. doi: 10.1023/a:1025615409383. [DOI] [PubMed] [Google Scholar]
- Brett LP, Levine S. Schedule-induced polydipsia suppresses pituitary-adrenal activity in rats. Journal of Comparative and Physiological Psychology. 1979;93:946–956. doi: 10.1037/h0077619. [DOI] [PubMed] [Google Scholar]
- Brett LP, Levine S. The pituitary-adrenal response to “minimized” schedule-induced drinking. Physiology & Behavior. 1981;26:153–158. doi: 10.1016/0031-9384(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Mormède P. Pituitary-adrenal consequences of adjunctive activities in pigs. Hormones and Behavior. 1981;15:386–395. doi: 10.1016/0018-506x(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Terlouw C, Mormède P, Le Moal M. Schedule-induced polydipsia experience decreases plasma corticosterone levels but increases plasma prolactin levels. Physiology & Behavior. 1988a;43:275–279. doi: 10.1016/0031-9384(88)90187-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Terlouw C, Tazi A, Koolhaas JM, Bohus B, Koob GF, et al. The propensity for schedule-induced polydipsia is related to differences in conditioned avoidance behaviour and in defense reactions in a defeat test. Physiology & Behavior. 1988b;43:269–273. doi: 10.1016/0031-9384(88)90186-2. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Ho A, Gentry RT. Toward an analogue of alcoholism in mice: criteria for recognition of pharmacologically motivated drinking. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3469–3471. doi: 10.1073/pnas.82.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TF, Samson HH. Adjunctive alcohol drinking in humans. Physiology & Behavior. 1988;44:775–779. doi: 10.1016/0031-9384(88)90061-3. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction Biology. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher T, Call SB, Blaha CD, Mittleman G. Behavioral effects of aminoadamantane class NMDA receptor antagonists on schedule-induced alcohol and self-administration of water in mice. Psychopharmacology. 2006;187:424–434. doi: 10.1007/s00213-006-0465-5. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Schedule-induced alcohol drinking: non-selective effects of acamprosate and naltrexone. Addiction Biology. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Falk JL. Production of polydipsia in normal rats by an intermittent food schedule. Science. 1961;133:195–196. doi: 10.1126/science.133.3447.195. [DOI] [PubMed] [Google Scholar]
- Falk JL. Schedule-induced polydipsia as a function of fixed interval length. Journal of the Experimental Analysis of Behavior. 1966a;9:37–39. doi: 10.1901/jeab.1966.9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Analysis of water and NaCl solution acceptance by schedule-induced polydipsia. Journal of the Experimental Analysis of Behavior. 1966b;9:111–118. doi: 10.1901/jeab.1966.9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Control of schedule-induced polydipsia: type, size, and spacing of meals. Journal of the Experimental Analysis of Behavior. 1967;9:199–206. doi: 10.1901/jeab.1967.10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Conditions producing psychogenic polydipsia in animals. Annals of the New York Academy of Sciences. 1969;157:569–593. doi: 10.1111/j.1749-6632.1969.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Falk JL. The nature and determinants of adjunctive behavior. Physiology & Behavior. 1971;6:577–588. doi: 10.1016/0031-9384(71)90209-5. [DOI] [PubMed] [Google Scholar]
- Falk JL. Excessive Behavior and Drug-Taking: Environmental Generation and Self-Control. In: Levison PK, editor. Substance Abuse, Habitual Behavior, and Self-Control. Boulder: Westview Press; 1984. pp. 81–122. [Google Scholar]
- Falk JL. Solvay Award address. Drug abuse as an adjunctive behavior. Drug and Alcohol Dependence. 1998;52:91–98. doi: 10.1016/s0376-8716(98)00084-2. [DOI] [PubMed] [Google Scholar]
- Falk JL, Samson HH. Schedule-induced physical dependence on ethanol. Pharmacological Reviews. 1975;27:449–464. [PubMed] [Google Scholar]
- Falk JL, Samson HH, Winger G. Behavioral maintenance of high concentrations of blood ethanol and physical dependence in the rat. Science. 1972;177:811–813. doi: 10.1126/science.177.4051.811. [DOI] [PubMed] [Google Scholar]
- Falk JL, Tang M. What schedule-induced polydipsia can tell us about alcoholism. Alcoholism: Clinical and Experimental Research. 1988;12:577–585. doi: 10.1111/j.1530-0277.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Falk JL, Zhang J, Chen R, Lau CE. A schedule induction probe technique for evaluating abuse potential: comparison of ethanol, nicotine and caffeine, and caffeine-midazolam interaction. Behavioural Pharmacology. 1994;5:513–520. doi: 10.1097/00008877-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Farber PD, Khavari KA, Douglass FM., 4th A factor analytic study of reasons for drinking: empirical validation of positive and negative reinforcement dimensions. Journal of Consulting and Clinical Psychology. 1980;48:780–781. doi: 10.1037//0022-006x.48.6.780. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Hormones and Behavior. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcoholism: Clinical and Experimental Research. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Flory RK. The control of schedule-induced polydipsia: Frequency and magnitude of reinforcement. Learning and Motivation. 1971;2:215–227. [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcoholism: Clinical and Experimental Research. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Ford MM, Steele AM, McCracken AD, Finn DA, Grant KA. The relationship between adjunctive drinking, blood ethanol concentration and plasma corticosterone across fixed-time intervals of food delivery in two inbred mouse strains. Psychoneuroendocrinology. 2013;38:2598–2610. doi: 10.1016/j.psyneuen.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RM. Schedule induction and sweetness as factors in ethanol consumption and preference by rats. Pharmacology, Biochemistry, and Behavior. 1978;8:739–741. doi: 10.1016/0091-3057(78)90275-7. [DOI] [PubMed] [Google Scholar]
- Gill K, Amit Z, Smith BR. Alcohol as a food: a commentary on Richter. Physiology & Behavior. 1996;60:1485–1490. doi: 10.1016/s0031-9384(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Badia-Elder NE, Elder RL, Stewart RB. Schedule-induced polydipsia in lines of rats selectively bred for high and low ethanol preference. Behavior Genetics. 2008;38:515–524. doi: 10.1007/s10519-008-9224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi O, Orlandi M, Escorihuela RM, Driscoll P, Lecca D, Corda MG. GABAergic and dopaminergic transmission in the brain of Roman high-avoidance and Roman low-avoidance rats. Brain Research. 1994;638:133–138. doi: 10.1016/0006-8993(94)90642-4. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Piras G, Corda MG. The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neuroscience and Biobehavioral Reviews. 2007;31:148–163. doi: 10.1016/j.neubiorev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clinical Schizophrenia & Related Psychoses. 2010;4:115–123. doi: 10.3371/CSRP.4.2.3. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Matthews DB, Hamre KM, Mittleman G, Chesler EJ, Becker HC, et al. Progress in using mouse inbred strains, consomics, and mutants to identify genes related to stress, anxiety, and alcohol phenotypes. Alcoholism: Clinical and Experimental Research. 2006;30:1066–1078. doi: 10.1111/j.1530-0277.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Johanson CE. The nature of the scheduled reinforcer and adjunctive drinking in nondeprived rhesus monkeys. Pharmacology, Biochemistry, and Behavior. 1988;29:295–301. doi: 10.1016/0091-3057(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcoholism: Clinical and Experimental Research. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology. 2003;28:620–628. doi: 10.1038/sj.npp.1300070. [DOI] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Rogers LS, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 2013;228:541–549. doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S, Dalvi A. Acceleration of onset of action in schedule-induced polydipsia: combinations of SSRI and 5-HT1A and 5-HT1B receptor antagonists. Pharmacology, Biochemistry, and Behavior. 2004;77:69–75. doi: 10.1016/j.pbb.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism: Clinical and Experimental Research. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katovic NM, Gresack JE, Spear LP. Schedule-induced polydipsia: gender-specific effects and consequences of prenatal cocaine and postnatal handling. Pharmacology, Biochemistry, and Behavior. 1999;64:695–704. doi: 10.1016/s0091-3057(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ. Effect of addition of ethanol and NaCl on saccharin + glucose polydipsia. Pharmacology, Biochemistry, and Behavior. 1979;10:277–283. doi: 10.1016/0091-3057(79)90101-1. [DOI] [PubMed] [Google Scholar]
- Lester D. Self-maintenance of intoxication in the rat. Quarterly Journal of Studies on Alcohol. 1961;22:223–231. [PubMed] [Google Scholar]
- Levine R, Levine S. Role of the pituitary-adrenal hormones in the acquisition of schedule-induced polydipsia. Behavioral Neuroscience. 1989;103:621–637. doi: 10.1037//0735-7044.103.3.621. [DOI] [PubMed] [Google Scholar]
- Levitsky D, Collier G. Schedule-induced wheel running. Physiology & Behavior. 1968;3:571–573. [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- López-Crespo G, Rodríguez M, Pellón R, Flores P. Acquisition of schedule-induced polydipsia by rats in proximity to upcoming food delivery. Learning & Behavior. 2004;32:491–499. doi: 10.3758/bf03196044. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, López-Crespo G, Venero C, Cañadas F, Sánchez-Santed F, Sandi C, et al. Differences in corticosterone level due to inter-food interval length: implications for schedule-induced polydipsia. Hormones and Behavior. 2006;49:166–172. doi: 10.1016/j.yhbeh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, Lopez-Crespo G, Sanchez-Amate MC, Flores P. Individual differences in schedule-induced polydipsia and the role of gabaergic and dopaminergic systems. Psychopharmacology. 2008;197:487–498. doi: 10.1007/s00213-007-1059-6. [DOI] [PubMed] [Google Scholar]
- Martin JR, Baettig K. Schedule induced ethanol polydipsia in psychogenetically selected lines of rats. Pharmacology, Biochemistry, and Behavior. 1981;14:857–862. doi: 10.1016/0091-3057(81)90374-9. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Developments in Alcoholism. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- McCaffrey RJ, Pavlik MK, Allen JD. Schedule-induced polydipsia in aged rats. Physiology & Behavior. 1981;26:337–339. doi: 10.1016/0031-9384(81)90034-2. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Leander JD. Schedule-induced oral self administration of etonitazene. Pharmacology, Biochemistry, and Behavior. 1976;4:137–141. doi: 10.1016/0091-3057(76)90005-8. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Leander JD, Ellis FW, Lucot JB, Frye GD. Characteristics of ethanol drinking patterns under schedule-induced polydipsia. Psychopharmacology. 1976;49:49–55. doi: 10.1007/BF00427470. [DOI] [PubMed] [Google Scholar]
- Meisch RA. The function of schedule-induced polydipsia in establishing ethanol as a positive reinforcer. Pharmacological Reviews. 1975;27:465–473. [PubMed] [Google Scholar]
- Meisch RA, Thompson T. Ethanol intake during schedule-induced polydipsia. Physiology & Behavior. 1972;8:471–475. doi: 10.1016/0031-9384(72)90331-9. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. Schedule-induced polydipsia and oral intake of drugs. Pharmacological Reviews. 1975;27:489–498. [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Blaha CD, Phillips AG. Pituitary-adrenal and dopaminergic modulation of schedule-induced polydipsia: behavioral and neurochemical evidence. Behavioral Neuroscience. 1992;106:408–420. doi: 10.1037//0735-7044.106.2.408. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Call SB, Cockroft JL, Goldowitz D, Matthews DB, Blaha CD. Dopamine dynamics associated with, and resulting from, schedule-induced alcohol self-administration: analyses in dopamine transporter knockout mice. Alcohol. 2011;45:325–339. doi: 10.1016/j.alcohol.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Castañeda E, Robinson TE, Valenstein ES. The propensity for nonregulatory ingestive behavior is related to differences in dopamine systems: behavioral and biochemical evidence. Behavioral Neuroscience. 1986;100:213–220. doi: 10.1037//0735-7044.100.2.213. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Jones GH, Robbins TW. The relationship between schedule-induced polydipsia and pituitary-adrenal activity: pharmacological and behavioral manipulations. Behavioural Brain Research. 1988;28:315–324. doi: 10.1016/0166-4328(88)90134-9. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Rosner AL, Schaub CL. Polydipsia and dopamine: behavioral effects of dopamine D1 and D2 receptor agonists and antagonists. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:638–650. [PubMed] [Google Scholar]
- Mittleman G, Valenstein ES. Ingestive behavior evoked by hypothalamic stimulation and schedule-induced polydipsia are related. Science. 1984;224:415–417. doi: 10.1126/science.6710151. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocrine Reviews. 2013;34:278–301. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Cardona D, Gómez MJ, Sánchez-Santed F, Tobeña A, Fernández-Teruel A, et al. Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology. 2010;35:1198–1208. doi: 10.1038/npp.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Flores P. Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases. Psychopharmacology. 2012;219:647–659. doi: 10.1007/s00213-011-2570-3. [DOI] [PubMed] [Google Scholar]
- Müller J. Aldosterone: the minority hormone of the adrenal cortex. Steroids. 1995;60:2–9. doi: 10.1016/0039-128x(94)00021-4. [DOI] [PubMed] [Google Scholar]
- Mumby D, Beck CH. Schedule-induced polydipsia: attenuating effects of decreased size of food granulations. Physiology & Behavior. 1988;43:375–381. doi: 10.1016/0031-9384(88)90202-8. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ogata H, Ogato F, Mendelson JH, Mello NK. A comparison of techniques to induce alcohol dependence and tolerance in the mouse. The Journal of Pharmacology and Experimental Therapeutics. 1972;180:216–230. [PubMed] [Google Scholar]
- Palfai T, Kutscher CL, Symons JP. Schedule-induced polydipsia in the mouse. Physiology & Behavior. 1971;6:461–462. doi: 10.1016/0031-9384(71)90184-3. [DOI] [PubMed] [Google Scholar]
- Platt B, Beyer CE, Schechter LE, Rosenzweig-Lipson S. Schedule-induced polydipsia: a rat model of obsessive-compulsive disorder. Current Protocols in Neuroscience. 2008;Chapter 9(Unit 9.27) doi: 10.1002/0471142301.ns0927s43. [DOI] [PubMed] [Google Scholar]
- Polich JM, Orvis BR. Alcohol problems: Patterns and prevalence in the US Air Force, Report No R-2308-AF. Santa Monica, CA: RAND; 1979. [Google Scholar]
- Porter JH. Schedule-induced polydipsia as a function of percent of body weight in the Mongolian gerbil. Physiology & Behavior. 1983;31:137–139. doi: 10.1016/0031-9384(83)90108-7. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. The Journal of Neuroscience. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds TJ, Kenny JT, Wright JW. A developmental study of schedule-induced polydipsia in the rat. Learning and Motivation. 1977;8:284–288. [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcoholism: Clinical and Experimental Research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Koob GF. Selective disruption of displacement behaviour by lesions of the mesolimbic dopamine system. Nature. 1980;285:409–412. doi: 10.1038/285409a0. [DOI] [PubMed] [Google Scholar]
- Roehrs TA, Samson HH. Schedule-induced ethanol polydipsia: function of ethanol concentration. Pharmacology, Biochemistry, and Behavior. 1980;13:291–294. doi: 10.1016/0091-3057(80)90086-6. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology. 2007;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- Samson HH, Falk JL. Alteration of fluid preference in ethanol-dependent animals. The Journal of Pharmacology and Experimental Therapeutics. 1974a;190:365–376. [PubMed] [Google Scholar]
- Samson HH, Falk JL. Schedule-induced ethanol polydipsia: enhancement by saccharin. Pharmacology, Biochemistry, and Behavior. 1974b;2:835–838. doi: 10.1016/0091-3057(74)90118-x. [DOI] [PubMed] [Google Scholar]
- Samson H, Falk JL. Pattern of daily blood ethanol elevation and the development of physical dependence. Pharmacology, Biochemistry, and Behavior. 1975;3:1119–1123. doi: 10.1016/0091-3057(75)90026-x. [DOI] [PubMed] [Google Scholar]
- Segal EF, Oden DL, Deadwyler SA. Determinants of polydipsia: IV. Free-reinforcement schedules. Psychonomic Science. 1965;3:11–12. [Google Scholar]
- Singer G, Wallace M. Schedule-induced self injection of drugs. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1984;8:171–178. doi: 10.1016/0278-5846(84)90147-7. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescence and the trajectory of alcohol use: introduction to part VI. Annals of the New York Academy of Sciences. 2004;1021:202–205. doi: 10.1196/annals.1308.025. [DOI] [PubMed] [Google Scholar]
- Stöhr T, Szuran T, Welzl H, Pliska V, Feldon J, Pryce CR. Lewis/Fischer rat strain differences in endocrine and behavioural responses to environmental challenge. Pharmacology, Biochemistry, and Behavior. 2000;67:809–819. doi: 10.1016/s0091-3057(00)00426-3. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcoholism: Clinical and Experimental Research. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tang M, Brown C, Falk JL. Complete reversal of chronic ethanol polydipsia by schedule withdrawal. Pharmacology, Biochemistry, and Behavior. 1982;16:155–158. doi: 10.1016/0091-3057(82)90028-4. [DOI] [PubMed] [Google Scholar]
- Tang M, Falk JL. Ethanol polydipsic choice: effects of alternative fluid polydipsic history. Alcohol. 1986;3:361–365. doi: 10.1016/0741-8329(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Mormede P, Le Moal M. Pituitary-adrenal correlates of schedule-induced polydipsia and wheel running in rats. Behavioural Brain Research. 1986;19:249–256. doi: 10.1016/0166-4328(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Timmer SG, Veroff J, Colten ME. Life stress, helplessness, and the use of alcohol and drugs to cope: An analysis of national survey data. In: Shiffman S, Wills TA, editors. Coping and Substance Use. London: Academic Press; 1985. pp. 171–198. [Google Scholar]
- Todd KG, Beck CH, Martin-Iverson MT. Effects of D1 and D2 dopamine antagonists on behavior of polydipsic rats. Pharmacology, Biochemistry, and Behavior. 1992;42:381–388. doi: 10.1016/0091-3057(92)90130-8. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Ueta Y, Heitz F, Marguerat A, Koizumi K, Yamashita H. Up-regulation of vasopressin and angiotensin II receptors in the thalamus and brainstem of inbred polydipsic mice. Neuroendocrinology. 2002;75:113–123. doi: 10.1159/000048227. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addiction Biology. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Wallace M, Singer G. Schedule induced behavior: a review of its generality, determinants and pharmacological data. Pharmacology, Biochemistry, and Behavior. 1976;5:483–490. doi: 10.1016/0091-3057(76)90114-3. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Blaha CD, Winn P, Phillips AG. Schedule-induced polydipsia and the nucleus accumbens: electrochemical measurements of dopamine efflux and effects of excitotoxic lesions in the core. Behavioural Brain Research. 1996;75:147–158. doi: 10.1016/0166-4328(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Williams A, Clark D. Alcohol consumption in university students: the role of reasons for drinking, coping strategies, expectancies, and personality traits. Addictive Behaviors. 1998;23:371–378. doi: 10.1016/s0306-4603(97)80066-4. [DOI] [PubMed] [Google Scholar]
- Woods A, Smith C, Szewczak M, Dunn RW, Cornfeldt M, Corbett R. Selective serotonin re-uptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive compulsive disorder. Psychopharmacology. 1993;112:195–198. doi: 10.1007/BF02244910. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Flory RK. Schedule-induced polydipsia and reinforcement magnitude. Physiology & Behavior. 1977;18:787–791. doi: 10.1016/0031-9384(77)90184-6. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]