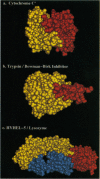
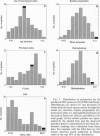
Abstract
This review examines protein complexes in the Brookhaven Protein Databank to gain a better understanding of the principles governing the interactions involved in protein-protein recognition. The factors that influence the formation of protein-protein complexes are explored in four different types of protein-protein complexes--homodimeric proteins, heterodimeric proteins, enzyme-inhibitor complexes, and antibody-protein complexes. The comparison between the complexes highlights differences that reflect their biological roles.
Full text
PDF
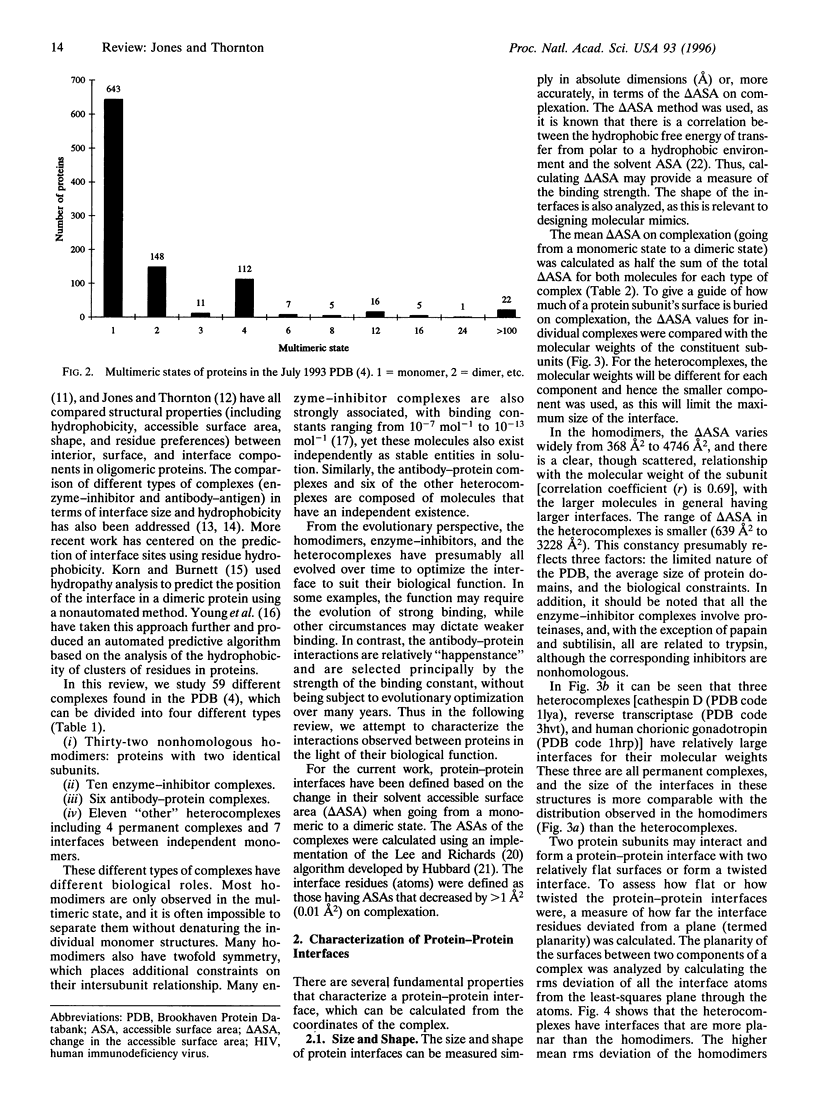

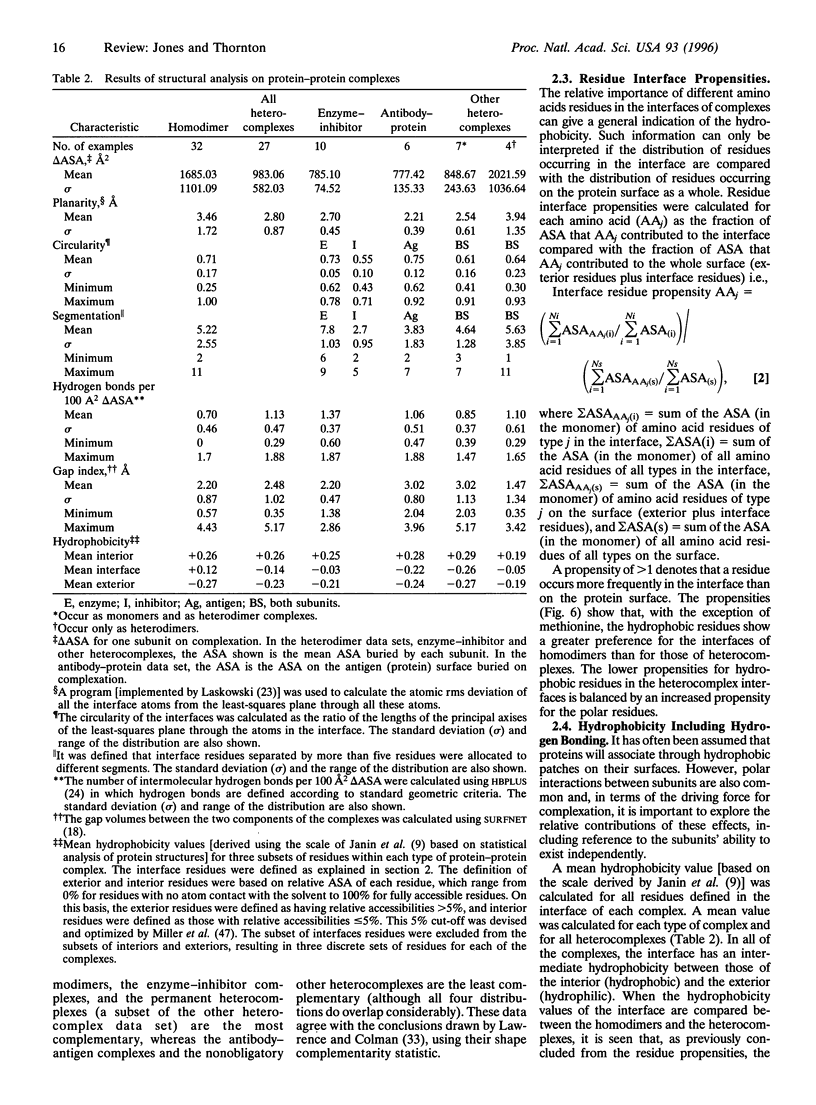
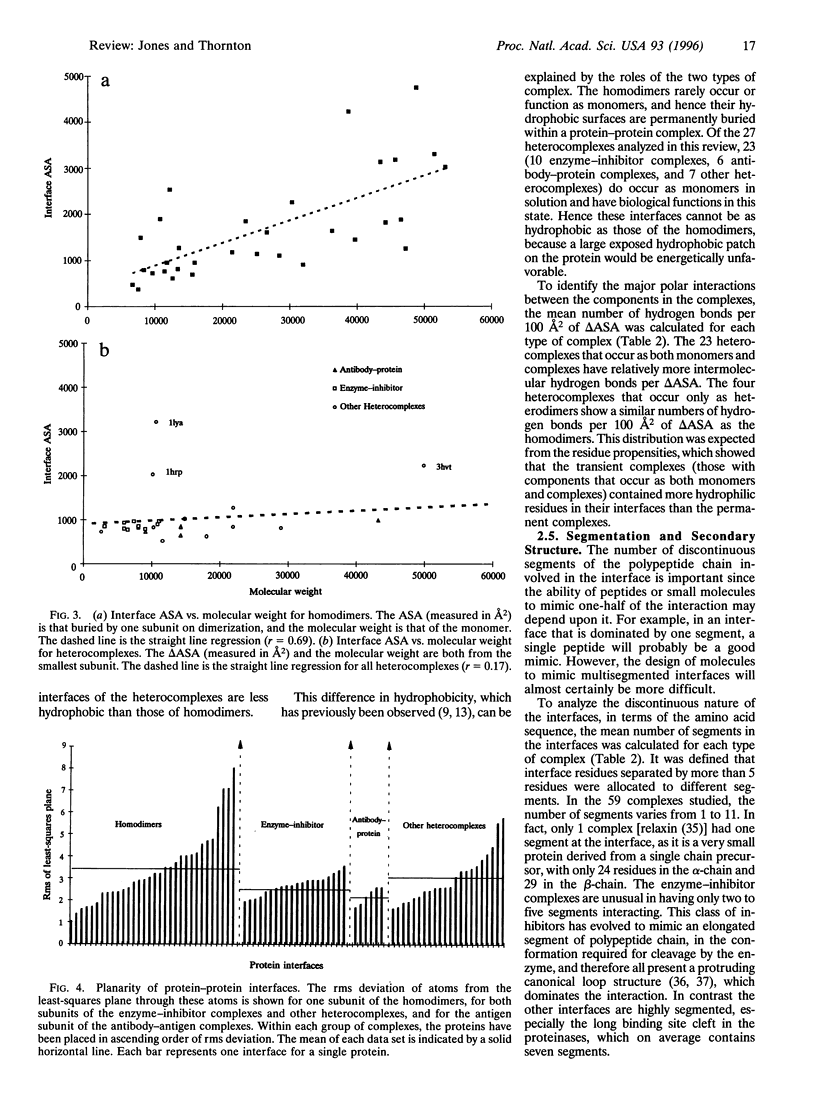

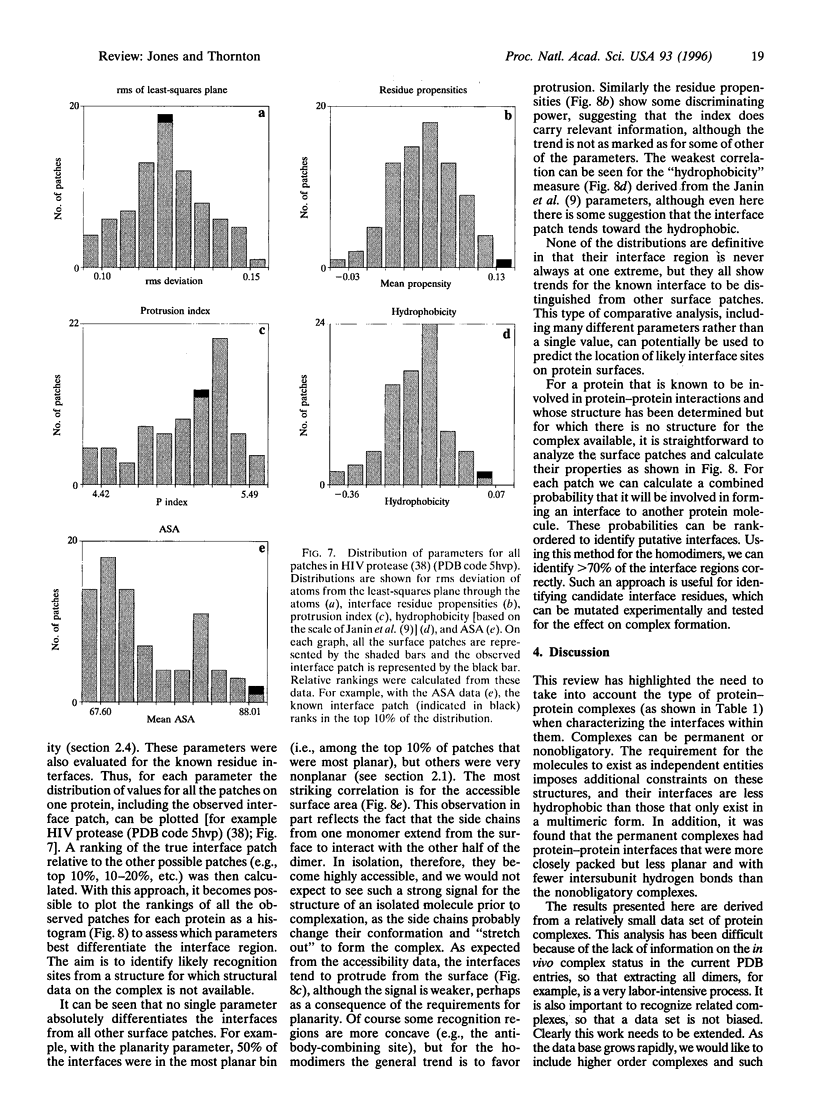

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. An investigation of protein subunit and domain interfaces. Protein Eng. 1988 Jul;2(2):101–113. doi: 10.1093/protein/2.2.101. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Chothia C. Hydrophobic bonding and accessible surface area in proteins. Nature. 1974 Mar 22;248(446):338–339. doi: 10.1038/248338a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Janin J. Principles of protein-protein recognition. Nature. 1975 Aug 28;256(5520):705–708. doi: 10.1038/256705a0. [DOI] [PubMed] [Google Scholar]
- Connolly M. L. Shape complementarity at the hemoglobin alpha 1 beta 1 subunit interface. Biopolymers. 1986 Jul;25(7):1229–1247. doi: 10.1002/bip.360250705. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Cohen G. H. Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquerroy S., Cherfils J., Janin J. Protein-protein interaction: an analysis by computer simulation. Ciba Found Symp. 1991;161:237–252. doi: 10.1002/9780470514146.ch15. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C., Randal M., Quan C., Burnier J., O'Connell L., Rinderknecht E., Kossiakoff A. A. X-ray structure of human relaxin at 1.5 A. Comparison to insulin and implications for receptor binding determinants. J Mol Biol. 1991 Sep 5;221(1):15–21. [PubMed] [Google Scholar]
- Finzel B. C., Weber P. C., Hardman K. D., Salemme F. R. Structure of ferricytochrome c' from Rhodospirillum molischianum at 1.67 A resolution. J Mol Biol. 1985 Dec 5;186(3):627–643. doi: 10.1016/0022-2836(85)90135-4. [DOI] [PubMed] [Google Scholar]
- Freymann D., Down J., Carrington M., Roditi I., Turner M., Wiley D. 2.9 A resolution structure of the N-terminal domain of a variant surface glycoprotein from Trypanosoma brucei. J Mol Biol. 1990 Nov 5;216(1):141–160. doi: 10.1016/S0022-2836(05)80066-X. [DOI] [PubMed] [Google Scholar]
- Gerstein M., Anderson B. F., Norris G. E., Baker E. N., Lesk A. M., Chothia C. Domain closure in lactoferrin. Two hinges produce a see-saw motion between alternative close-packed interfaces. J Mol Biol. 1993 Nov 20;234(2):357–372. doi: 10.1006/jmbi.1993.1592. [DOI] [PubMed] [Google Scholar]
- Gerstein M., Schulz G., Chothia C. Domain closure in adenylate kinase. Joints on either side of two helices close like neighboring fingers. J Mol Biol. 1993 Jan 20;229(2):494–501. doi: 10.1006/jmbi.1993.1048. [DOI] [PubMed] [Google Scholar]
- Helmer-Citterich M., Tramontano A. PUZZLE: a new method for automated protein docking based on surface shape complementarity. J Mol Biol. 1994 Jan 21;235(3):1021–1031. doi: 10.1006/jmbi.1994.1054. [DOI] [PubMed] [Google Scholar]
- Hubbard S. J., Thornton J. M., Campbell S. F. Substrate recognition by proteinases. Faraday Discuss. 1992;(93):13–23. doi: 10.1039/fd9929300013. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Thorsness P. E., Ramalingam V., Helmers N. H., Koshland D. E., Jr, Stroud R. M. Structure of a bacterial enzyme regulated by phosphorylation, isocitrate dehydrogenase. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8635–8639. doi: 10.1073/pnas.86.22.8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Chothia C. The structure of protein-protein recognition sites. J Biol Chem. 1990 Sep 25;265(27):16027–16030. [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Johnson J. E. Functional implications of protein-protein interactions in icosahedral viruses. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):27–33. doi: 10.1073/pnas.93.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Thornton J. M. Protein-protein interactions: a review of protein dimer structures. Prog Biophys Mol Biol. 1995;63(1):31–65. doi: 10.1016/0079-6107(94)00008-w. [DOI] [PubMed] [Google Scholar]
- Korn A. P., Burnett R. M. Distribution and complementarity of hydropathy in multisubunit proteins. Proteins. 1991;9(1):37–55. doi: 10.1002/prot.340090106. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lawrence M. C., Colman P. M. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993 Dec 20;234(4):946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- McDonald I. K., Thornton J. M. Satisfying hydrogen bonding potential in proteins. J Mol Biol. 1994 May 20;238(5):777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- Miller S., Lesk A. M., Janin J., Chothia C. The accessible surface area and stability of oligomeric proteins. 1987 Aug 27-Sep 2Nature. 328(6133):834–836. doi: 10.1038/328834a0. [DOI] [PubMed] [Google Scholar]
- Miller S. The structure of interfaces between subunits of dimeric and tetrameric proteins. Protein Eng. 1989 Nov;3(2):77–83. doi: 10.1093/protein/3.2.77. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Satow Y., Watanabe Y., Hirono S., Iitaka Y. Crystal structures of Streptomyces subtilisin inhibitor and its complex with subtilisin BPN'. Nature. 1979 Feb 8;277(5696):447–452. doi: 10.1038/277447a0. [DOI] [PubMed] [Google Scholar]
- Morgan R. S., Miller S. L., McAdon J. M. The symmetry of self-complementary surfaces. J Mol Biol. 1979 Jan 5;127(1):31–38. doi: 10.1016/0022-2836(79)90456-x. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Almo S. C., Toney M. D., Ringe D. 2.8-A-resolution crystal structure of an active-site mutant of aspartate aminotransferase from Escherichia coli. Biochemistry. 1989 Oct 3;28(20):8161–8167. doi: 10.1021/bi00446a030. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Takimoto-Kamimura M., Rini J. M., Profy A. T., Wilson I. A. Major antigen-induced domain rearrangements in an antibody. Structure. 1993 Oct 15;1(2):83–93. doi: 10.1016/0969-2126(93)90024-b. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Orengo C. A. Protein structure alignment. J Mol Biol. 1989 Jul 5;208(1):1–22. doi: 10.1016/0022-2836(89)90084-3. [DOI] [PubMed] [Google Scholar]
- Thornton J. M., Edwards M. S., Taylor W. R., Barlow D. J. Location of 'continuous' antigenic determinants in the protruding regions of proteins. EMBO J. 1986 Feb;5(2):409–413. doi: 10.1002/j.1460-2075.1986.tb04226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunogae Y., Tanaka I., Yamane T., Kikkawa J., Ashida T., Ishikawa C., Watanabe K., Nakamura S., Takahashi K. Structure of the trypsin-binding domain of Bowman-Birk type protease inhibitor and its interaction with trypsin. J Biochem. 1986 Dec;100(6):1637–1646. doi: 10.1093/oxfordjournals.jbchem.a121872. [DOI] [PubMed] [Google Scholar]
- Vakser I. A., Aflalo C. Hydrophobic docking: a proposed enhancement to molecular recognition techniques. Proteins. 1994 Dec;20(4):320–329. doi: 10.1002/prot.340200405. [DOI] [PubMed] [Google Scholar]
- Walls P. H., Sternberg M. J. New algorithm to model protein-protein recognition based on surface complementarity. Applications to antibody-antigen docking. J Mol Biol. 1992 Nov 5;228(1):277–297. doi: 10.1016/0022-2836(92)90506-f. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991 Dec 13;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- Wells J. A. Binding in the growth hormone receptor complex. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):1–6. doi: 10.1073/pnas.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Stanfield R. L. Antibody-antigen interactions: new structures and new conformational changes. Curr Opin Struct Biol. 1994 Dec;4(6):857–867. doi: 10.1016/0959-440x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Young L., Jernigan R. L., Covell D. G. A role for surface hydrophobicity in protein-protein recognition. Protein Sci. 1994 May;3(5):717–729. doi: 10.1002/pro.5560030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielenkiewicz P., Rabczenko A. Methods of molecular modelling of protein-protein interactions. Biophys Chem. 1988 Apr;29(3):219–224. doi: 10.1016/0301-4622(88)85042-7. [DOI] [PubMed] [Google Scholar]