Abstract
Signaling lymphocyte activation molecule family member 2 (SLAMF2/CD48) is a co-activator and adhesion molecule on cells with hematopoietic origin. It ligates mainly SLAMF4 on effector/memory CD8+ T cells and natural killer cells, suggesting a potential role during viral infection, with SLAMF2 acting as a ligand to activate SLAMF4-bearing cells. The ability of SLAMF2 to signal on its own after it is engaged, and the functional consequences are largely unknown. We found that cytosolic DNA-activated DCs upregulate the expression of SLAMF2 molecules. Using anti-SLAMF2 antibody and SLAMF4 recombinant protein we found that SLAMF2 engagement activates immature DCs, and more interestingly, prolongs the survival of DNA-activated DCs by inhibiting IFNβ production and IFNβ-induced apoptosis, and promotes the production of the granzyme B inhibitor protease inhibitor-9. Thus, SLAMF2 can serve as a survival molecule for DNA-activated DCs during their interaction with SLAMF4-expressing cytotoxic T cells. Based on our results we propose that SLAMF2 engagement regulates adaptive immune responses by providing longer access of putative antigen presenting cells to virus-specific effector T cells by prolonging the time frame of effective stimulation.
Keywords: dendritic cell, SLAMF2, CD48, SLAMF4, CD244, survival, cytotoxic T lymphocyte
Introduction
Antigen presentation by dendritic cells (DCs) is critical for the induction of immune responses and for the differentiation and expansion of effector and memory T cells (1). DCs are sensitive to signals originated from pathogens as well as from other immune cells. The balance of these signals ultimately determines DC activation, cytokine secretion, migration, survival and the nature and magnitude of the immune response. DCs are essential in the CD8+ T cell immune response that developed to specifically eliminate infections and to provide long-lasting protection against reinfection (2). DCs prime naïve CD8+ T cells to various pathogens (3–5) and play a significant role in the generation of an effective effector CD8+ T cell response and in the production of memory CD8+ T cells (6–8). DCs are also responsible for memory CD8+ T cell maintenance and reactivation (9–13). CD8+ T cell-dependent killing of antigen presenting DCs has been described through perforin/granzyme B secretion and it represents a negative feedback mechanism to attenuate immune responses (14). However, the phenomenon of memory CD8+ T cells protecting DCs from cytotoxic T cell (CTL) killing has also been described (2, 15–17), and granzyme B inhibitor serine protease inhibitor-6 (SPI-6) (human ortholog PI-9) has been shown to protect DCs against cytotoxicity (18, 19). The question though how DCs are able to present viral antigens to CTLs without themselves being killed through contact-mediated cytotoxicity has remained largely elusive, especially in human cells.
Myeloid DCs produce large amounts of IFNβ in response to infection signals through TLR3 and TLR4 (20) or through cytosolic receptors activated by nucleic acids (21), especially by double-stranded DNA, which can serve as a mimic of viral infection (22). Production of IFNβ is part of a defense mechanism to induce an antiviral state that prevents productive viral infection, and to modulate cell viability and function (23, 24). IFNβ has been reported to induce apoptosis in certain cell types (25, 26) (27, 28) and specifically in mouse DCs (29) where IFNβ activates the caspase-11/caspase-3 apoptotic pathway. How human DCs are able to escape from autocrine IFNβ-induced apoptosis during antigen presentation to CTLs remains an unanswered question.
Signaling lymphocytic activation molecule family 2 (SLAMF2/CD48) is a glycosyl-phosphatidyl-inositol (GPI)-anchored protein found on the surface of several hematopoietic cells and serves as adhesion and co-stimulatory protein (30). SLAMF2 is a ligand for the immunoreceptors CD2 and SLAMF4 (CD244/2B4). Despite the absence of a cytoplasmic domain SLAMF2 initiates a potent signaling cascade comparable to that of other immune-regulating molecules (31). However the SLAMF2-SLAMF4 interaction is bi-directional, most studies have focused on SLAMF2 as a ligand of SLAMF4 on natural killer (NK) cells and CD8+ effector/memory T cells reporting either activator or inhibitory effects (26, 32–35).
Here, we investigated the role of SLAMF2 in double-stranded DNA-activated human DCs (DNA-DCs), a model of viral infection. We found that SLAMF2 engagement results in the activation of immature DCs (IDCs). More interestingly, we showed for the first time that this engagement acts as a survival factor by protecting mature DNA-DCs from cell death first by inhibiting the autocrine production of apoptosis-inducing IFN-β and second by promoting the production of the granzyme B inhibitor PI-9 and protects against cytotoxicity by CTLs. Thus SLAMF2 ligation in mature DCs may empower and prolong the potent antigen presenting cell-dependent T cell activation during viral infection.
Materials and methods
Generation of monocyte-derived dendritic cells, cell culture and treatments
Human monocyte-derived DCs were generated from CD14+ blood monocytes isolated from peripheral blood mononuclear cells (PBMCs) separated from Buffy Coats by Ficoll-Paque (Fisher, Pittsburgh, PA) gradient centrifugation (36) followed by positive selection with anti-CD14-coated magnetic beads (Miltenyi Biotech, Auburn, CA). Purified CD14+ monocytes (≥95%) were plated at 2×106 cell/ml concentration and cultured in RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 2 mM glutamine, 100 U/ml penicillin and 100 g/ml streptomycin in the presence of 100 ng/ml IL-4 and 75 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ) given on days 0 and 2 and in a specific experiment on day 2 and 4 of extended culturing. Activation of IDCs were induced on day 5 by poly(dA:dT) (2.5 μg/ml) complexed with LyoVec transfection reagent (Invivogen, San Diego, CA) for 24h or for the entire period of culture. For the anti-SLAMF2 treatment 5 μg/ml BJ40 antibody (Biolegend) was coated or added soluble to IDCs or together with poly(dA:dT) on day 5 for the indicated time points. In some experiments 5 μg/ml of recombinant human SLAMF4-Fc fusion protein or control protein (Sino Biological Inc. Beijing, China) was added to the cells for 24h.
In certain experiments IDCs and DNA-DCs were be treated with the IFNβ blocking antibody cocktail anti-IFNβ and anti-IFNRA (PBL Interferon Source, Piscataway, NJ) or purified mouse immunoglobulin G1 and rabbit IgG isotype control antibodies for the indicated time points. The antibodies were left in the culture during the subsequent treatment.
The IFNs IFNα, IFNβ and IFNγ were purchased from Peprotech and used in the indicated concentrations for 24h.
Flow cytometry
The identification of DC and T cell activation were monitored by flow cytometric analysis using fluorochrome-conjugated anti-SLAMF2, anti-CD80, anti-CD83 and anti-CD86 (DCs), anti-CD3, anti-CD25 and anti-CD69 (T cells) antibodies as compared to isotype-matched control antibodies (Biolegend). To check cell viability 7-amino-actinomycin D (7AAD) staining was used and the positive cells were excluded as dead. Fluorescence intensities were measured and analyzed by FACSCalibur flow cytometer (BD Biosciences Immunocytometry Systems, Franklin Lakes, NJ). Data analysis was performed using the FlowJo Flow Cytometry Analysis software (Ashland, OR).
Real-time quantitative reverse transcriptase-polymerase chain reaction (Q-RT-PCR)
Real-time PCR was performed as described previously (37). Briefly, total RNA was isolated from DCs by RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription was performed at 37 °C for 120 min from 100 ng total RNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR for SLAMF2, CCR7, MMP-9, MMP-12, TIMP-2, TRAIL, IFNβ and PI-9 genes were performed (Light Cycler 480, Roche, Indianapolis, IN) with 40 cycles at 94 °C for 12 sec and 60 °C for 60 sec using Taqman assays (Applied Biosystems). All PCR reactions were run in triplicates with a control reaction containing no RT enzyme. The comparative Ct method was used to quantify transcripts relative to the endogenous control gene large ribosomal protein.
Enzyme-linked immunosorbent assay
IL-6, TNF-α, IFN-γ, IL-2 and TRAIL proteins were measured from cell supernatants using DuoSet human immunoassay kits (R&D Systems). IFNβ protein was measured using the VeriKine Human IFN Beta ELISA Kit (PBL Interferon Source). PI-9 protein was measured using specific ELISA kit (Antibodies-online, Atlanta, GA). The optical density of the wells was determined using a microplate reader set at 450 nm.
Migration
DCs were suspended in migration medium (0.5 % BSA in RPMI 1640) at 106 cells/ml. Transmigration inserts (diameter 6.5 mm; pore size 5 μm)were obtained from Sigma. MIP3-β chemokine (Peprotech) were diluted at 200 ng/ml in migration medium and added to the lower chambers in a final volume of 600 μl. DCs were added to the upper chamber in a final volume of 250 μl, and chemotaxis assays were conducted for 4h in 5% CO2 at 37°C. At the end of the assay, the inserts were discarded and cells migrated to the lower chamber were collected. Migrated cell numbers were counted by using polystyrene standard beads (Sigma) by flow cytometry.
Transfection of small interfering RNA
A mix of three different constructs of IFI16 siRNAs and control siRNAs (Applied Biosystems) were transfected into DCs on the third day of differentiation to a final concentration of 2.5 nM using the GenePulser X Cell electroporator and 0.4 cm cuvettes (Bio-Rad Laboratories, Hercules, CA). After 2 additional days the knockdown of the IFI16 gene were tested by Q-RT-PCR.
Mixed leukocyte reaction
DCs were differentiated from monocytes as mentioned previously and labeled with carboxyfluorescein succinimidyl ester (CFSE). IDCs and DNA-activated DCs were co-cultured with blood-purified and sorted SLAMF4−naïve or SLAMF4+ effector/memory CD8+ T cells in 1:4 ratio for 4 days and the viability of the CFSE-labeled DCs were measured by flow cytometry using 7AAD staining to exclude necrotic cells.
Statistical analysis
Paired Student one- and two-tailed t tests were used (* p≤0.05, ** p≤0.01, *** p≤0.005). The statistical calculations compared experimental samples to the IDC or non-treated samples unless noted otherwise. For all experiments, the mean and the standard deviation (SD) are reported for at least n=3 independent experiments.
Results
SLAMF2 expression is upregulated by cytosolic DNA activation in dendritic cells
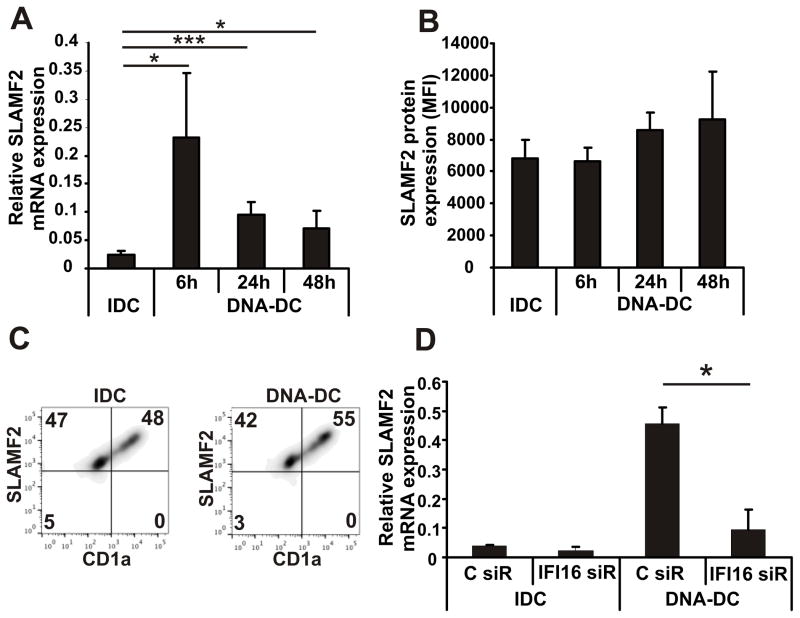
To study the role of SLAMF2 on DCs, first we investigated the expression levels of SLAMF2 on monocytes, IDCs and DNA-stimulated DCs. SLAMF2 expression was found to be high in monocytes (0h time point in Fig. S1A) but it was rapidly downregulated following incubation with IL-4 and GM-CSF (monocyte-derived IDCs) (Fig. S1A). Yet, stimulation with DNA resulted in a significant upregulation of SLAMF2 mRNA (Fig. 1A) and a moderate upregulation of the cell surface expression of SLAMF2 protein (Fig. 1B). Higher SLAMF2 expression was recorded on the surface of pro-inflammatory-prone CD1a+ subpopulation of DCs compared to the rather inhibitory CD1a− cells (Fig. 1D)(38). Since cytosolic DNA-activated DCs produce high amount of IFNβ (22) we tested whether IFNβ itself can induce SLAMF2 expression. Treatment of cells with type I (IFNα or IFNβ) but not with type II IFNγ induced SLAMF2 gene expression (Fig. S1B). Previously we had identified IFI16 as a key intracellular DNA receptor responsible for the activation of DCs by cytosolic double-stranded DNA (22). Silencing of IFI16 in DNA-DCs (IDC: 79±13%, DNA-DC: 82±9% knockdown efficiency compared to control siRNA) inhibited the upregulation of SLAMF2 (Fig. 1D) demonstrating the role of this receptor in the proper DC activation and in the regulation of SLAMF2 expression by cytoplasmic DNA, possibly, through IFNβ.
Figure 1. SLAMF2 is upregulated on mature dendritic cells.
(A) mRNA expression (n=10) and (B) cell surface expression (n=5) of SLAMF2 on IDCs and on DNA-DCs activated by transfection of poly(dA:dT) for different time points. (C) Histograms show the distribution of SLAMF2 molecules on the surface of CD1a+ and CD1a− IDC and DNA-DC populations (n=3, one representative experiment shown). (D) mRNA expression of SLAMF2 in IDCs and DNA-DCs treated with IFI16 or control siRNAs (n=3). * p≤0.05; *** p≤0.005
SLAMF2 engagement activates immature dendritic cells
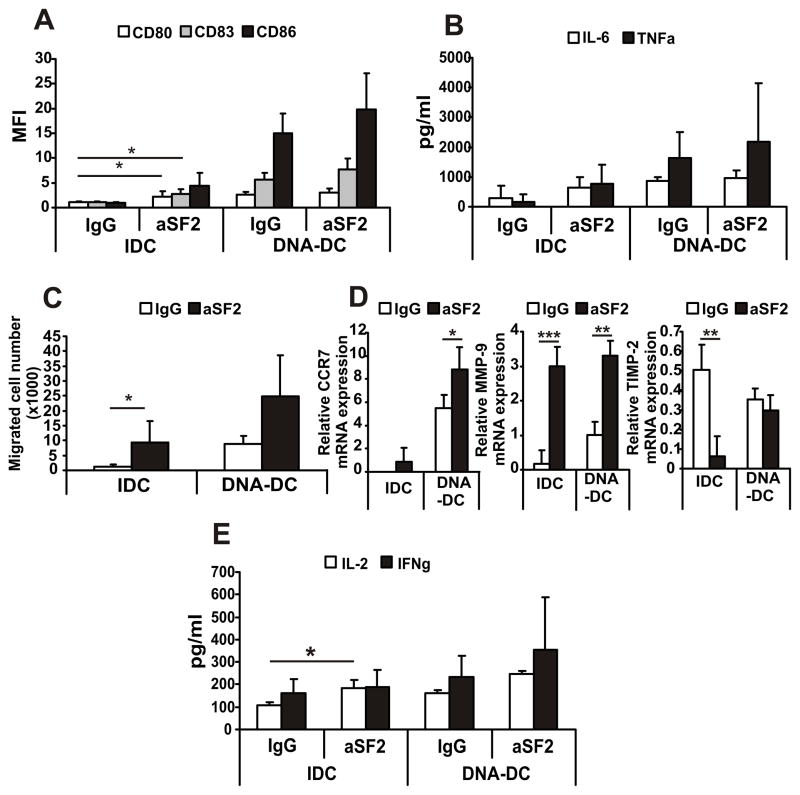
After characterizing the expression of SLAMF2 we investigated the function of SLAMF2 on DCs. To model the SLAMF2-SLAMF4 interaction we used a monoclonal antibody recognizing SLAMF2 (aSF2) and found that it activated IDCs in a dose-dependent manner (Fig. S2A) as determined by the cell surface expression of the activation molecules CD80, CD83 and CD86 (Fig. 2A), and by the production of pro-inflammatory cytokines IL-6 and TNFα (Fig. 2B). SLAMF2 engagement enhanced also the cytosolic DNA activation of DCs, however this effect was not significant (Fig. 2A and B). Cell migration towards the CCR7 ligand MIP3β is an indicator of DC maturation. aSF2-treated IDCs migrated significantly better compared to control IgG-treated cells, while antibody treatment had an enhancing effect on DNA-DC migration (Fig. 2C). Increased migration correlated with significantly increased CCR7 and matrix metalloproteinase (MMP)-9 expression. In contrast, the MMP inhibitor tissue inhibitor of MMP (TIMP)-2 levels were downregulated upon aSF2 treatment (Fig. 2D). SLAMF2 engagement enhanced the capacity of DCs to stimulate T cells as aSF2-IDCs were able to significantly upregulate IL-2 production of co-cultured T cells (Fig. 2E). To demonstrate that the DC activating effect of the aSF2 antibody is specific we used a recombinant SLAMF4 protein to treat IDCs. Significant DC activation was measured when this recombinant SLAMF4 protein was present in IDC cultures indicating that SLAMF2 ligation has an activating effect on DCs (Fig. S2B and C).
Figure 2. SLAMF2 engagement by specific antibody activates dendritic cells.
IDCs and DNA-DCs were treated with 5 μg/ml of immobilized mouse control IgG or anti-SLAMF2 antibody (aSF2) for 24h. (A) Expression of cell surface activation molecules CD80, CD83 and CD86. (B) Production of pro-inflammatory cytokines IL-6 and TNFα. (C) MIP3-β-induced cell migration of DCs. (D) Gene expression of migration-related molecules CCR7, MMP-9 and TIMP-2. (E) IgG- or aSF2-pre-treated IDCs and DNA-DCs were co-cultured with total T cells for 24h (IL-2) or for 72h (IFNγ) and the cytokine levels were measured from supernatants. n=3, * p≤0.05; ** p≤0.01; *** p≤0.005
SLAMF2 engagement selectively promotes the survival of mature dendritic cells
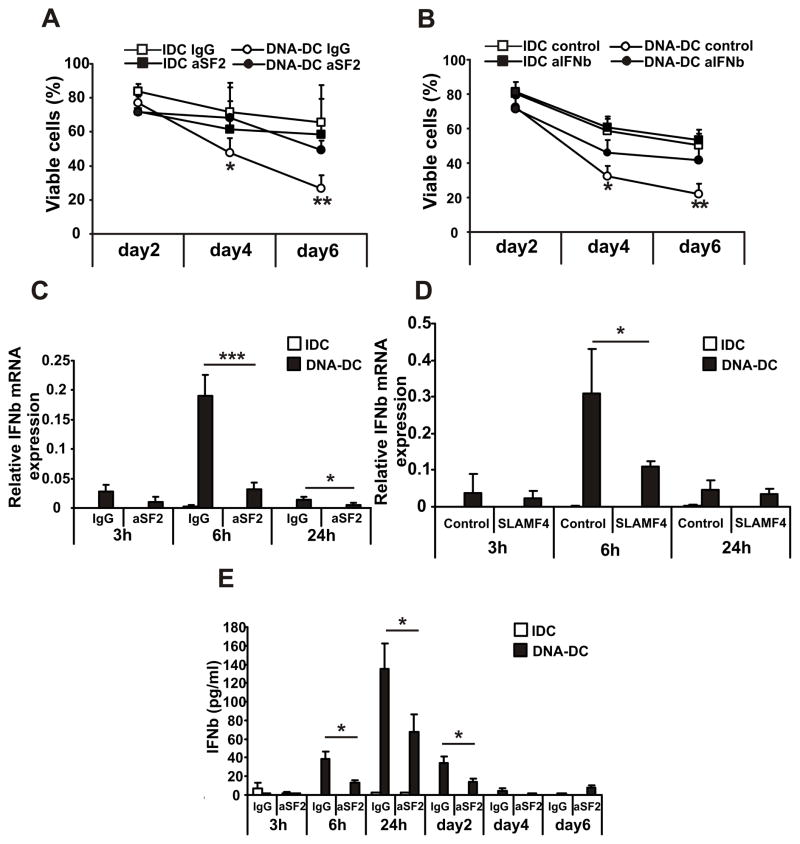
DCs are terminally differentiated cells unable to proliferate and their destiny is to mature, activate T cells and eventually die (39). Because the survival of DCs is connected to their activation status, in the next set of experiments the cell death of DCs was investigated. DCs were activated with cytosolic DNA or were left non-activated and were treated with plate-bound aSF2 or non-specific IgG. DC cell death was investigated by flow cytometry using 7AAD staining to exclude necrotic cells. The DNA-DC population showed increased cell death compared to IDCs (Fig. 3A). SLAMF2 engagement though significantly reduced the cell death of DNA-DCs at days 4 and 6 of culture (Fig. 3A). Therefore, SLAMF2 engagement rescues DNA-DCs from excessive cell death resulting in the survival of the mature DC population.
Figure 3. SLAMF2 engagement rescues mature dendritic cells from cell death by inhibiting the production of IFNβ and the expression of caspase-4.
IDCs and DNA-DCs were treated with 5 μg/ml of immobilized control IgG or aSF2 antibodies for the indicated time points. (A) Viable cell percentages of day2, day4 and day6 DC cultures measured by flow cytometry using 7AAD staining to exclude necrotic cells (n=5). (B) Viable cell percentages of day2, day4 and day6 DC cultures treated with control antibodies or with antibody cocktail against IFNβ and IFNRA receptor (aIFNb, n=3). (C) Relative IFNβ mRNA expression measured on the indicated time points of IgG or aSF2-treated IDCs and DNA-DCs by Q-PCR (n=5). (D) Relative IFNβ mRNA expression measured on the indicated time points of control or SLAMF4 protein-treated IDCs and DNA-DCs by Q-PCR (n=3). (E) IFNβ production of the aSF2-treated DCs were measured by ELISA on the indicated time points from cell supernatants (n=3). * p≤0.05; ** p≤0.01; *** p≤0.005
SLAMF2 engagement protects mature dendritic cells from cell death by inhibiting IFNβ production and caspase-4 expression
Next, we investigated the molecular events which result in increased survival of DNA-DC following engagement of SLAMF2. In our previous communication we showed that DNA-DCs are able to produce large amounts of IFNβ compared to other mature DCs and IDCs (22). Because IFNβ is able to induce apoptosis of DCs (24, 29), we investigated whether IFNβ contributed to the death of DNA-stimulated DCs. Indeed, the presence of an antibody against the IFN receptor (IFNR) A and an antibody against IFNβ the DNA-DC population became significantly more viable compared to those treated with control antibody (Fig. 3B). That the antibodies used were functional was confirmed by demonstrating decreased mRNA expression (Suppl. Fig. 3A) and protein secretion (Suppl. Fig. 3B) of the IFNβ-inducible gene TNF-related apoptosis-inducing ligand (TRAIL).
Next we tested whether SLAMF2 engagement is able to alter the production of IFNβ by DNA-DCs. Treatment of DNA-DCs with aSF2 (Fig. 3C) or SLAMF4 protein (Fig. 3D) significantly decreased IFNβ gene expression compared to controls. Treatment with aSF2 antibody also significantly decreased IFNβ protein production (Fig. 3D) compared to the non-specific IgG-treated DNA-DCs. We measured the expression and production of TRAIL to track the effect of IFNβ inhibition and found significant downregulation of gene (Suppl. Fig. 3B) and protein levels (Suppl. Fig. 3C) of TRAIL following aSF2 treatment compared to the control IgG-treated group.
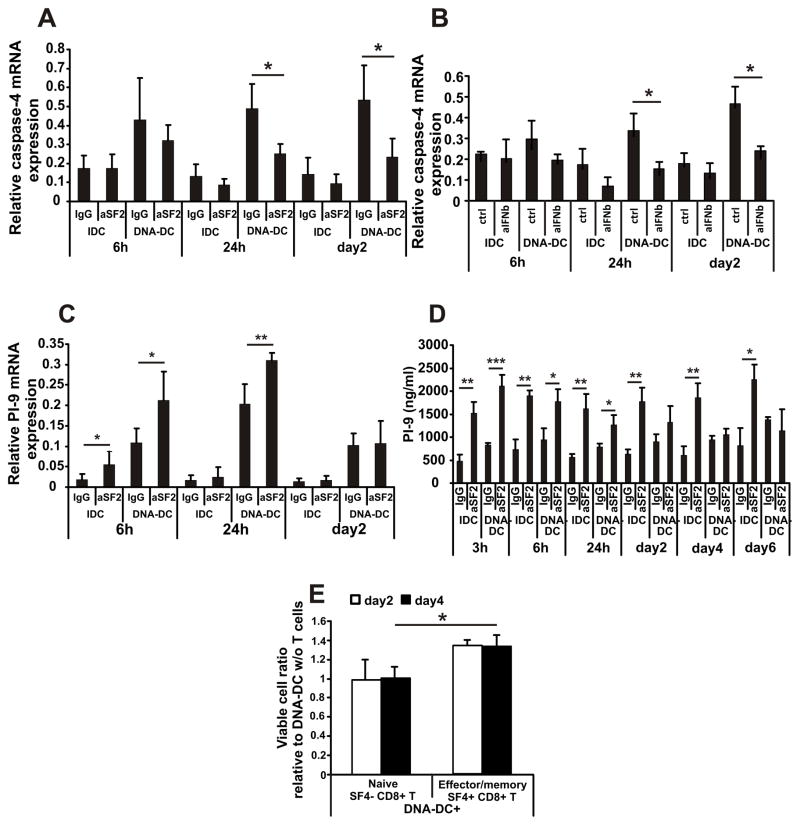
IFNβ induces mature DC apoptosis through caspase-11/caspase-3 activation (29). The human ortholog of caspase-11 is caspase-4 so in the next set of experiments we measured the expression of caspase-4 in DCs. DNA-DCs upregulated caspase-4 expression after 24–48h of activation by cytosolic DNA compared to IDCs, and aSF2 (Fig. 4A) or SLAMF4 protein (Fig. S3E) treatments were able to significantly inhibit the elevated expression of caspase-4. Similarly, using IFNβ blockade the upregulation of caspase-4 was inhibited significantly (Fig. 4B).
Figure 4. SLAMF2 engagement promotes the expression and secretion of protease inhibitor PI-9 of mature dendritic cells; SLAMF4+ CD8+ T cells promote DNA-DC survival.
(A) Relative caspase-4 mRNA expression of IDCs and DNA-DCs treated with IgG or aSF2 antibody for the indicated time points measured by Q-PCR (n=3). (B) Relative caspase-4 mRNA expression of IDCs and DNA-DCs treated with control antibodies or with antibody cocktail against IFNβ and IFNRA receptor for the indicated time points (n=3). (C) Relative PI-9 mRNA expression of IDCs and DNA-DCs treated with IgG or aSF2 antibody for the indicated time points (n=3). (D) Secreted PI-9 protein measured by ELISA (n=3). (E) CFSE-labeled DNA-DCs were co-cultured with sorted SF4− CD8+ naïve T cells or SF4+ CD8+ effector/memory T cells (1:4 DC:T cell ratio) for 2 and 4 days and the viability of DCs were measured by flow cytometry. The viable cell ratio relative to the DNA-DC viability without any T cell co-culture is plotted (n=3). * p≤0.05; ** p≤0.01; *** p≤0.005
Collectively, SLAMF2 engagement protects DCs from cell death by inhibiting the production of IFNβ and the subsequent expression of caspase-4.
SLAMF2 engagement protects mature dendritic cells from cell death by promoting the expression and secretion of protease inhibitor-9
Although we demonstrated that DNA-DCs escape the autocrine IFNβ-induced cell death by SLAMF2 engagement provided in vivo by SLAMF4+ effector/memory CD8+ T cells it remains unknown how they escape the cytotoxicity by activated killer CD8+ T cells. Murine DCs produce serine protease inhibitor-6 (SPI-6), which protects them against cytotoxicity by inhibiting granzyme B (18, 19). Accordingly, we measured the expression and secretion of the human ortholog of SPI-6, protease inhibitor-9 (PI-9), by DCs. Indeed, IDCs and DNA-DCs treated with aSF2 antibody (Fig. 4C) or with SLAMF4 protein (Fig. S3F) displayed a rapid upregulation of PI-9 gene expression compared to controls. Similarly, protein secretion of PI-9 was significantly upregulated by aSF2 treatment compared to the IgG-treated controls (Fig. 4D). Based on these data we conclude that DNA-activated DCs escape granzyme B-induced cell death by producing the inhibitor molecule PI-9.
SLAMF4-bearing CD8+ T cells can provide a survival signal to DNA-activated dendritic cells
Finally, we wished to determine the physiologic effect of SLAMF2 engagement on DCs by SLAMF4 expressed on T cells. To this end we co-cultured sorted blood-derived SLAMF4− naïve or SLAMF4+ effector/memory CD8+ T cells with DNA-activated DCs and the viability of DCs were detected 2 and 4 days later. While SLAMF4− naïve T cells had no effect on DC survival, we found that SLAMF4+ T cells were able to significantly prolong DC survival (Fig. 4C). Collectively, these data support that DNA-DC/CD8+ T cell interaction though SLAMF4/SLAMF2 results in prolonged DC survival.
Discussion
In this communication we present evidence that SLAMF2 on human DCs serves not only as stimulatory molecule for immature DCs, but more importantly as a survival molecule protecting mature DCs from cell death during anti-viral immune responses.
Virus invasion requires the rapid response of the immune system to inhibit the spreading of the infection. Cell death is an effective strategy to limit intracellular infections. The killing of infected cells by CD8+ T cells therefore is critical for immunity (19). DCs are the most potent antigen presenting cells that stimulate both naïve CD8+ T cells and memory CD8+ T cells to differentiate into CTLs (3, 11). By presenting the viral antigen to CTLs DCs flag themselves as ‘infected’ and serve as potential targets of cytotoxicity. Moreover, during the encounter with the pathogen, DCs become activated and produce large amounts of type I IFNs (predominantly IFNβ) to protect the neighboring cells from the infection but meanwhile they activate the IFNβ-induced apoptotic program. Thus to fulfill their role as antigen presenting cells, DCs need to develop effective protection against cell death. In the series of experiments presented above we show for the first time that SLAMF2 molecules serve as survival factors during contact with SLAMF4+ CD8+ cytotoxic T cells.
Using transfected double-stranded DNA to mimic viral infections in human DCs (DNA-DCs) we previously observed massive amount of IFNβ production and effective CD8+ T cell activation by DNA-DCs (22). Simultaneously with the IFNβ production, DNA-DCs upregulate the expression of SLAMF2 molecules to interact with the SLAMF4 molecules on the cell surface of effector/memory CD8+ T cells. This interaction results in rescuing DNA-DCs from excessive cell death through two distinct pathways: (a) though the inhibition of IFNβ production and IFNβ-induced apoptosis, and (b) by triggering the production of the granzyme B inhibitor PI-9.
SLAM family molecule interactions are difficult to explore because of the complex expression patterns of the members on different cell populations. Moreover, SLAMF2 expression is dynamically regulated, thus time- and localization-dependent fine-tuning is crucial. The gene expression and protein levels of SLAMF2 seem to be regulated at different magnitudes which could be due to the complex modulation of molecule which include surface expression, internalization and the production of a soluble form of SLAMF2. We showed higher SLAMF2 expression on double-stranded DNA-activated DCs compared to IDCs. These data are in agreement with the original observation of elevated SLAMF2 expression on EBV-infected B cells (40) as well as with our previous work showing that DNA-activated DCs are very potent antigen presenting cells (22). Moreover, expression differences of SLAMF2 CD1a+ and CD1a− DCs seem to follow the reported differences in their functional properties (38).
DC maturation stimuli usually serve as survival signals. For example, CpG DNA upregulates the expression of the cellular inhibitor of apoptosis (c-IAP) 1 and 2, while it downregulates the levels of active caspase-3 in a PI3K-dependent manner (41); HIV-infected DCs become resistant to NK-induced TRAIL-mediated apoptosis due to the upregulation of the cellular-Flice-like inhibitory protein and c-IAP2 (42); LPS activates ERK, which regulates DC survival (43). Interestingly, we found elevated rates of cell death in DNA-DCs compared to IDCs, which was the consequence of the autocrine production of IFNβ. Mouse caspase-11, an ortholog of human caspase-4, is induced in hematopoietic cells by LPS and IFNs, and activates caspase-1 and caspase-3 (44, 45). We found increased gene expression of caspase-4 in DNA-DCs compared to IDCs, explaining the excessive cell death of these mature DCs. Elevated caspase-4 expression was found to be inhibited by aSF2 treatment and by blocking the IFNβ pathway indicating that the autocrine production of IFNβ activates the pyroptotic pathway of cell death possibly through the AIM2 and/or IFI16 cytosolic DNA receptors (46). The effects of IFNβ on mature DC apoptosis could be controlled temporarily by SLAMF2 engagement during the intercourse of DCs and CD8+ T cells. To our knowledge this is the first study to show the role of caspase-4 in IFNβ-induced cell death of human cells. The virus-infected DCs need to fulfill two requirements to be eligible for SLAMF2-mediated survival: (a) they have to be matured enough to professionally present antigen to T cells and (b) they should be present at the right place to be able to make interactions with CD8+ CTLs and possibly NK cells which provide SLAMF4 to engage SLAMF2. In this setting our model which depicts SLAMF2 as a survival factor (Figure 5) can explain how effector/memory CD8+ SALMF4+ T cells are able to activate and rescue the antigen-carrying DCs from killing by the same CTLs that they induce. This in turn will prolong the time frame of effective stimulation of the expanding population of antigen-specific CD8+ T cells (2, 15–17).
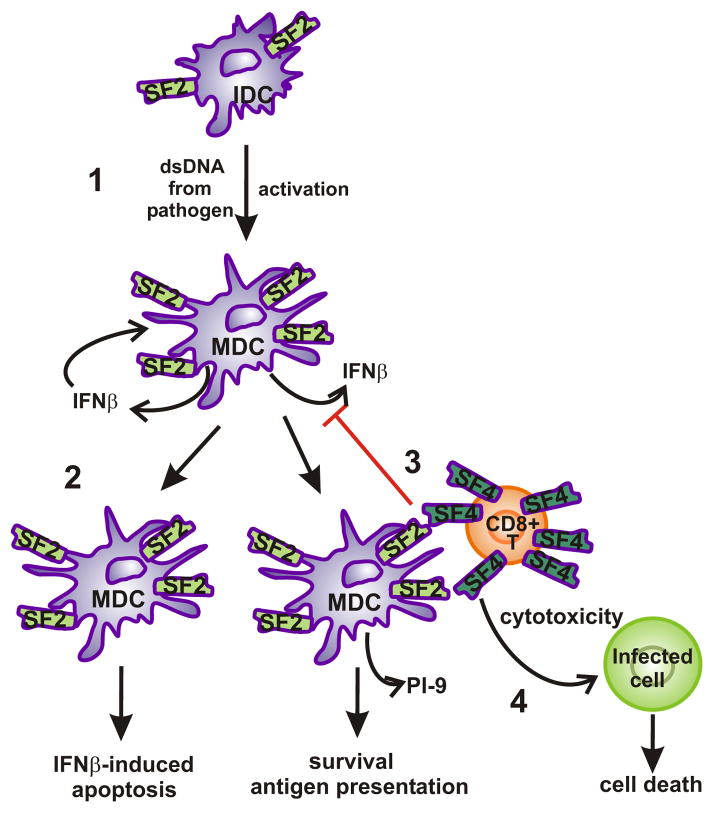
Figure 5. Hypothesis of SLAMF2-SLAMF4 interaction on dendritic cells.
(1) Upon DNA activation from pathogen or other sources (e.g. self-DNA from dead cells) immature DCs become activated, produce IFNβ and upregulate the expression of SLAMF2 molecules on the cell surface. (2) Without SLAMF2 engagement by SLAMF4, mature dendritic cells die by IFNβ-induced apoptosis. (3) SLAMF2 engagement by SLAMF4 on the surface of effector/memory CD8+ T cells inhibits the IFNβ production and promotes the production of protein inhibitor-9 by mature DCs, thus protects DCs from either the IFNβ-induced apoptosis and from granzyme B-induced cytotoxicity of T cells. (4) Meanwhile, SLAMF2-SLAMF4 interaction can promote the cytotoxic effect of SLAMF4+ CD8+ T cells against infected cells.
The IFNβ-induced cell death and caspase-11 involvement as well as the role of SPI-6 in DCs have been investigated almost exclusively in mouse cells and cell lines. Our work expands these investigations to human DCs and more importantly, it identifies the involved molecular events.
Given the critical role in antigen presentation and T cell activation, DCs have attracted major attention for the improvement of vaccine efficacy, including the use of adjuvants to optimally activate DCs and consequently boost immunity. We hope that with the data presented in this communication new strategies can be developed to maximize the antigen presenting and T cell activating capacity of DCs, thus to enhance the effectiveness of DC-based vaccine therapies.
Supplementary Material
Abbreviations
- IDC
immature dendritic cell
- MDC
mature dendritic cell
- SLAM
Signaling Lymphocyte Activation Molecule
- aSF2
anti-human SLAMF2 antibody
Footnotes
This work was supported by the NIH grant 2P01AI065687-06A1.
References
- 1.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual review of immunology. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 2.Gurunathan S, Stobie L, Prussin C, Sacks DL, Glaichenhaus N, Iwasaki A, Fowell DJ, Locksley RM, Chang JT, Wu CY, Seder RA. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. Journal of immunology. 2000;165:915–924. doi: 10.4049/jimmunol.165.2.915. [DOI] [PubMed] [Google Scholar]
- 3.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clinical and experimental immunology. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. Journal of immunology. 2005;175:3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- 6.Brzoza KL, Rockel AB, Hiltbold EM. Cytoplasmic entry of Listeria monocytogenes enhances dendritic cell maturation and T cell differentiation and function. Journal of immunology. 2004;173:2641–2651. doi: 10.4049/jimmunol.173.4.2641. [DOI] [PubMed] [Google Scholar]
- 7.Feng H, Zhang D, Palliser D, Zhu P, Cai S, Schlesinger A, Maliszewski L, Lieberman J. Listeria-infected myeloid dendritic cells produce IFN-beta, priming T cell activation. Journal of immunology. 2005;175:421–432. doi: 10.4049/jimmunol.175.1.421. [DOI] [PubMed] [Google Scholar]
- 8.Muraille E, Giannino R, Guirnalda P, Leiner I, Jung S, Pamer EG, Lauvau G. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. European journal of immunology. 2005;35:1463–1471. doi: 10.1002/eji.200526024. [DOI] [PubMed] [Google Scholar]
- 9.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. Journal of immunology. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 10.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. Journal of immunology. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 11.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AW, Cariappa A, Chase C, Russell P, Starnbach MN, Koni PA, Pillai S, Weninger W, von Andrian UH. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nature immunology. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit DJ, Lefrancois L. Dendritic cell-T cell interactions in the generation and maintenance of CD8 T cell memory. Microbes and infection/Institut Pasteur. 2006;8:1108–1115. doi: 10.1016/j.micinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. The Journal of experimental medicine. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Watchmaker P, Urban J, Sheridan B, Giermasz A, Nishimura F, Sasaki K, Cumberland R, Muthuswamy R, Mailliard RB, Larregina AT, Falo LD, Gooding W, Storkus WJ, Okada H, Hendricks RL, Kalinski P. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer research. 2007;67:10012–10018. doi: 10.1158/0008-5472.CAN-07-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watchmaker PB, Urban JA, Berk E, Nakamura Y, Mailliard RB, Watkins SC, van Ham SM, Kalinski P. Memory CD8+ T cells protect dendritic cells from CTL killing. Journal of immunology. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrew KA, Simkins HM, Witzel S, Perret R, Hudson J, Hermans IF, Ritchie DS, Yang J, Ronchese F. Dendritic cells treated with lipopolysaccharide up-regulate serine protease inhibitor 6 and remain sensitive to killing by cytotoxic T lymphocytes in vivo. Journal of immunology. 2008;181:8356–8362. doi: 10.4049/jimmunol.181.12.8356. [DOI] [PubMed] [Google Scholar]
- 19.Lovo E, Zhang M, Wang L, Ashton-Rickardt PG. Serine protease inhibitor 6 is required to protect dendritic cells from the kiss of death. Journal of immunology. 2012;188:1057–1063. doi: 10.4049/jimmunol.1102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coccia EM. IFN regulation and functions in myeloid dendritic cells. Cytokine & growth factor reviews. 2008;19:21–32. doi: 10.1016/j.cytogfr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Unterholzner L. The interferon response to intracellular DNA: Why so many receptors? Immunobiology. 2013;218:1312–1321. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Kis-Toth K, Szanto A, Thai TH, Tsokos GC. Cytosolic DNA-activated human dendritic cells are potent activators of the adaptive immune response. Journal of immunology. 2011;187:1222–1234. doi: 10.4049/jimmunol.1100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Current medicinal chemistry. 2007;14:1279–1289. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- 24.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O’Reilly LA, Schneider P, Fasel N, Shortman K, Strasser A, Acha-Orbea H. Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo. PloS one. 2011;6:e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamero AM, Potla R, Wegrzyn J, Szelag M, Edling AE, Shimoda K, Link DC, Dulak J, Baker DP, Tanabe Y, Grayson JM, Larner AC. Activation of Tyk2 and Stat3 is required for the apoptotic actions of interferon-beta in primary pro-B cells. The Journal of biological chemistry. 2006;281:16238–16244. doi: 10.1074/jbc.M509516200. [DOI] [PubMed] [Google Scholar]
- 26.Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren HG. 2B4/CD48-mediated regulation of lymphocyte activation and function. Journal of immunology. 2005;175:2045–2049. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]
- 27.Khvalevsky E, Rivkin L, Rachmilewitz J, Galun E, Giladi H. TLR3 signaling in a hepatoma cell line is skewed towards apoptosis. Journal of cellular biochemistry. 2007;100:1301–1312. doi: 10.1002/jcb.21119. [DOI] [PubMed] [Google Scholar]
- 28.van Koetsveld PM, Vitale G, de Herder WW, Feelders RA, van der Wansem K, Waaijers M, van Eijck CH, Speel EJ, Croze E, van der Lely AJ, Lamberts SW, Hofland LJ. Potent inhibitory effects of type I interferons on human adrenocortical carcinoma cell growth. The Journal of clinical endocrinology and metabolism. 2006;91:4537–4543. doi: 10.1210/jc.2006-0620. [DOI] [PubMed] [Google Scholar]
- 29.Yen JH, Ganea D. Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood. 2009;114:1344–1354. doi: 10.1182/blood-2008-12-196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunological reviews. 2001;181:234–249. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 31.Shin JS, Abraham SN. Glycosylphosphatidylinositol-anchored receptor-mediated bacterial endocytosis. FEMS microbiology letters. 2001;197:131–138. doi: 10.1111/j.1574-6968.2001.tb10594.x. [DOI] [PubMed] [Google Scholar]
- 32.Messmer B, Eissmann P, Stark S, Watzl C. CD48 stimulation by 2B4 (CD244)-expressing targets activates human NK cells. Journal of immunology. 2006;176:4646–4650. doi: 10.4049/jimmunol.176.8.4646. [DOI] [PubMed] [Google Scholar]
- 33.Veillette A. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunological reviews. 2006;214:22–34. doi: 10.1111/j.1600-065X.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 34.Vaidya SV, Mathew PA. Of mice and men: different functions of the murine and human 2B4 (CD244) receptor on NK cells. Immunology letters. 2006;105:180–184. doi: 10.1016/j.imlet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) Journal of immunology. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 36.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. Journal of immunological methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 37.Szatmari I, Gogolak P, Im JS, Dezso B, Rajnavolgyi E, Nagy L. Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity. 2004;21:95–106. doi: 10.1016/j.immuni.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Gogolak P, Rethi B, Szatmari I, Lanyi A, Dezso B, Nagy L, Rajnavolgyi E. Differentiation of CD1a− and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPARgamma. Blood. 2007;109:643–652. doi: 10.1182/blood-2006-04-016840. [DOI] [PubMed] [Google Scholar]
- 39.Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nature immunology. 2004;5:583–589. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama S, Staunton D, Fisher R, Amiot M, Fortin JJ, Thorley-Lawson DA. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. Journal of immunology. 1991;146:2192–2200. [PubMed] [Google Scholar]
- 41.Park Y, Lee SW, Sung YC. Cutting Edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. Journal of immunology. 2002;168:5–8. doi: 10.4049/jimmunol.168.1.5. [DOI] [PubMed] [Google Scholar]
- 42.Melki MT, Saidi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS pathogens. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. The Journal of experimental medicine. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell death and differentiation. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 45.Nadiri A, Wolinski MK, Saleh M. The inflammatory caspases: key players in the host response to pathogenic invasion and sepsis. Journal of immunology. 2006;177:4239–4245. doi: 10.4049/jimmunol.177.7.4239. [DOI] [PubMed] [Google Scholar]
- 46.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.