Abstract
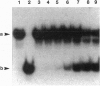
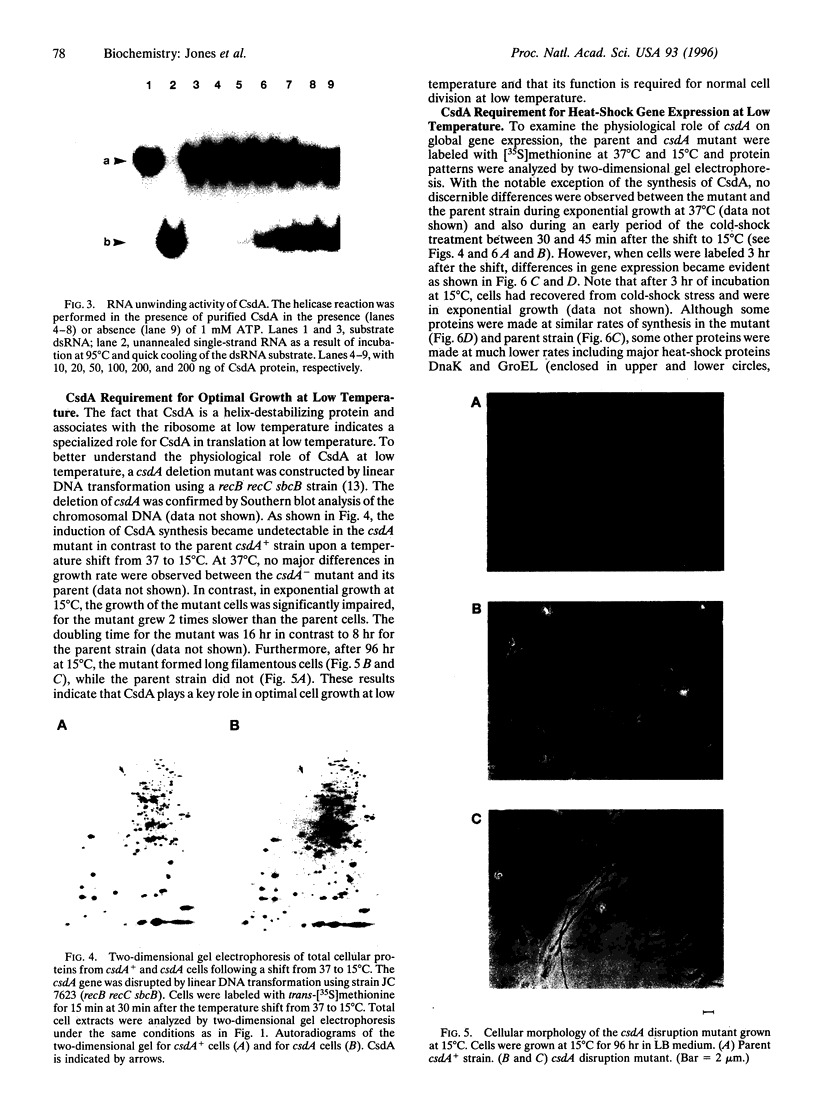
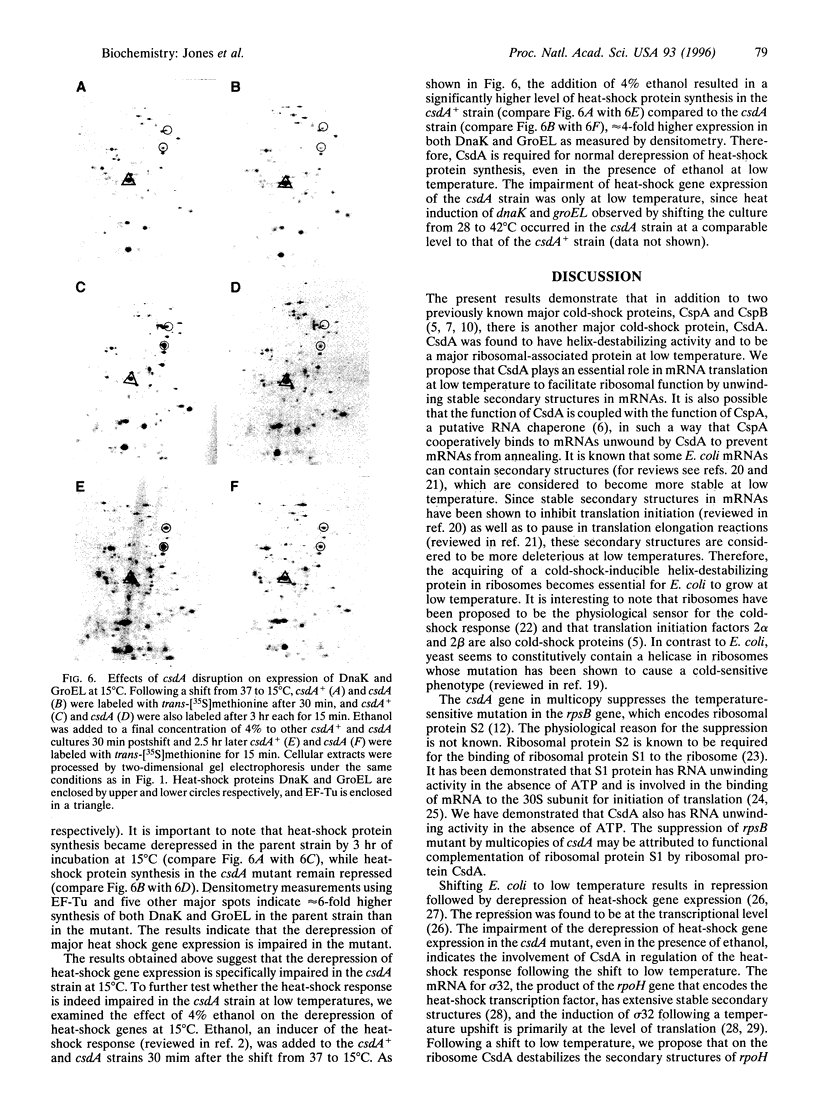
A 70-kDa protein was specifically induced in Escherichia coli when the culture temperature was shifted from 37 to 15 degrees C. The protein was identified to be the product of the deaD gene (reassigned csdA) encoding a DEAD-box protein. Furthermore, after the shift from 37 to 15 degrees C, CsdA was exclusively localized in the ribosomal fraction and became a major ribosomal-associated protein in cells grown at 15 degrees C. The csdA deletion significantly impaired cell growth and the synthesis of a number of proteins, specifically the derepression of heat-shock proteins, at low temperature. Purified CsdA was found to unwind double-stranded RNA in the absence of ATP. Therefore, the requirement for CsdA in derepression of heat-shock protein synthesis is a cold shock-induced function possibly mediated by destabilization of secondary structures previously identified in the rpoH mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coetzee T., Herschlag D., Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994 Jul 1;8(13):1575–1588. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs E. R., Wittman H. G. A strain of Escherichia coli which gives rise to mutations in a large number of ribosomal proteins. Mol Gen Genet. 1976 Dec 22;149(3):303–309. doi: 10.1007/BF00268532. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H., Hurwitz J. Characterization of a new RNA helicase from nuclear extracts of HeLa cells which translocates in the 5' to 3' direction. J Biol Chem. 1993 Oct 5;268(28):21372–21383. [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Iost I., Dreyfus M. mRNAs can be stabilized by DEAD-box proteins. Nature. 1994 Nov 10;372(6502):193–196. doi: 10.1038/372193a0. [DOI] [PubMed] [Google Scholar]
- Jones P. G., Cashel M., Glaser G., Neidhardt F. C. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J Bacteriol. 1992 Jun;174(12):3903–3914. doi: 10.1128/jb.174.12.3903-3914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., Inouye M. The cold-shock response--a hot topic. Mol Microbiol. 1994 Mar;11(5):811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpel R. L., Miller N. S., Fresco J. R. Mechanistic studies of ribonucleic acid renaturation by a helix-destabilizing protein. Biochemistry. 1982 Apr 27;21(9):2102–2108. doi: 10.1021/bi00538a019. [DOI] [PubMed] [Google Scholar]
- Karpel R. L., Swistel D. G., Miller N. S., Geroch M. E., Lu C., Fresco J. R. Acceleration of RNA renaturation by nucleic acid unwinding proteins. Brookhaven Symp Biol. 1975 Jul;(26):165–174. [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. On the relationship between the binding of ribosomal protein S1 to the 30 S subunit of Escherichia coli and 3' terminus of 16 S RNA. J Mol Biol. 1978 Jun 5;121(4):411–430. doi: 10.1016/0022-2836(78)90391-1. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Xie A., Jiang W., Etchegaray J. P., Jones P. G., Inouye M. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol Microbiol. 1994 Mar;11(5):833–839. doi: 10.1111/j.1365-2958.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Nagai H., Yuzawa H., Yura T. Interplay of two cis-acting mRNA regions in translational control of sigma 32 synthesis during the heat shock response of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk K., Feng W., Jiang W., Tejero R., Emerson S. D., Inouye M., Montelione G. T. Solution NMR structure of the major cold shock protein (CspA) from Escherichia coli: identification of a binding epitope for DNA. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5114–5118. doi: 10.1073/pnas.91.11.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin H., Jiang W., Inouye M., Heinemann U. Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5119–5123. doi: 10.1073/pnas.91.11.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. R., Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992 Feb;6(3):283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Toone W. M., Rudd K. E., Friesen J. D. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol. 1991 Jun;173(11):3291–3302. doi: 10.1128/jb.173.11.3291-3302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Hutton M. E., Neidhardt F. C. Gene-protein database of Escherichia coli K-12: edition 3. Electrophoresis. 1990 Dec;11(12):1131–1166. doi: 10.1002/elps.1150111205. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Mitani T., Ogura T., Niki H., Hiraga S. Cloning, sequencing, and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol Microbiol. 1994 Jul;13(2):301–312. doi: 10.1111/j.1365-2958.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]