Abstract
A small (96-aa) protein, virus protein R (Vpr), of human immunodeficiency virus type 1 contains one hydrophobic segment that could form a membrane-spanning helix. Recombinant Vpr, expressed in Escherichia coli and purified by affinity chromatography, formed ion channels in planar lipid bilayers when it was added to the cis chamber and when the trans chamber was held at a negative potential. The channels were more permeable to Na+ than to Cl- ions and were inhibited when the trans potential was made positive. Similar channel activity was caused by Vpr that had a truncated C terminus, but the potential dependence of channel activity was no longer seen. Antibody raised to a peptide mimicking part of the C terminus of Vpr (AbC) inhibited channel activity when added to the trans chamber but had no effect when added to the cis chamber. Antibody to the N terminus of Vpr (AbN) increased channel activity when added to the cis chamber but had no effect when added to the trans chamber. The effects of potential and antibodies on channel activity are consistent with a model in which the positive C-terminal end of dipolar Vpr is induced to traverse the bilayer membrane when the opposite (trans) side of the membrane is at a negative potential. The C terminus of Vpr would then be available for interaction with AbC in the trans chamber, and the N terminus would be available for interaction with AbN in the cis chamber. The ability of Vpr to form ion channels in vitro suggests that channel formation by Vpr in vivo is possible and may be important in the life cycle of human immunodeficiency virus type 1 and/or may cause changes in cells that contribute to AIDS-related pathologies.
Full text
PDF
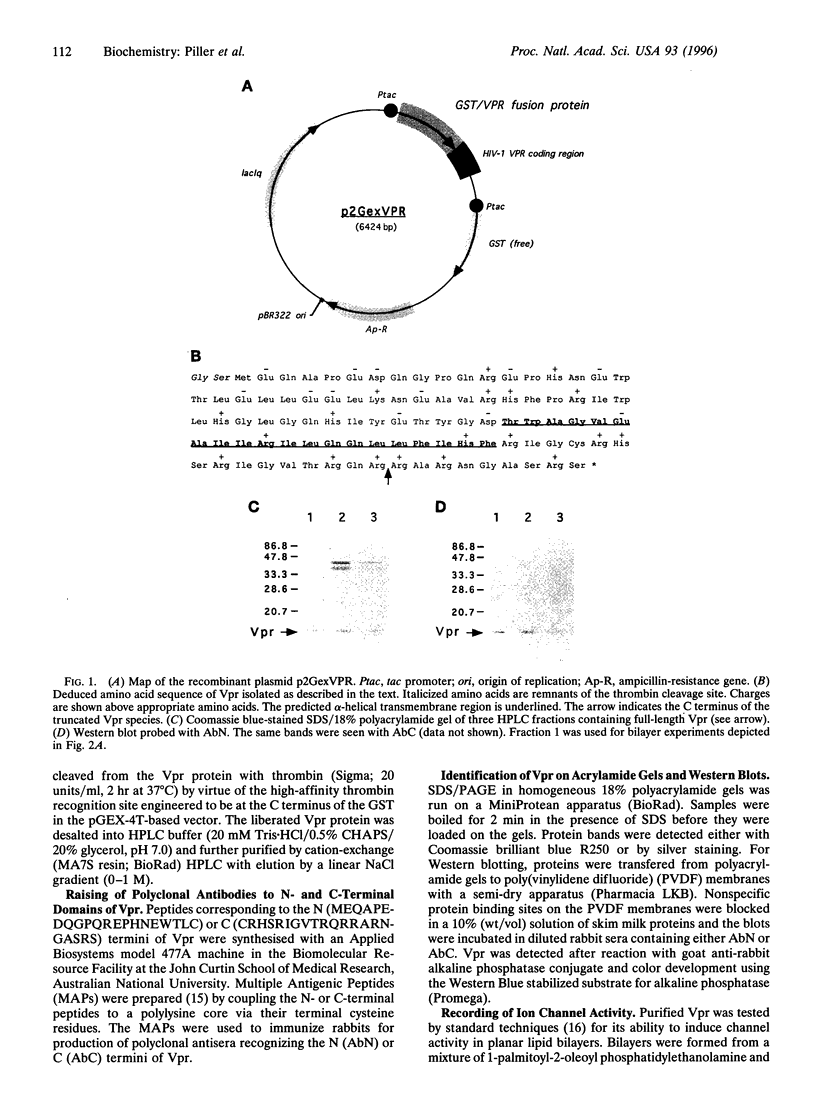
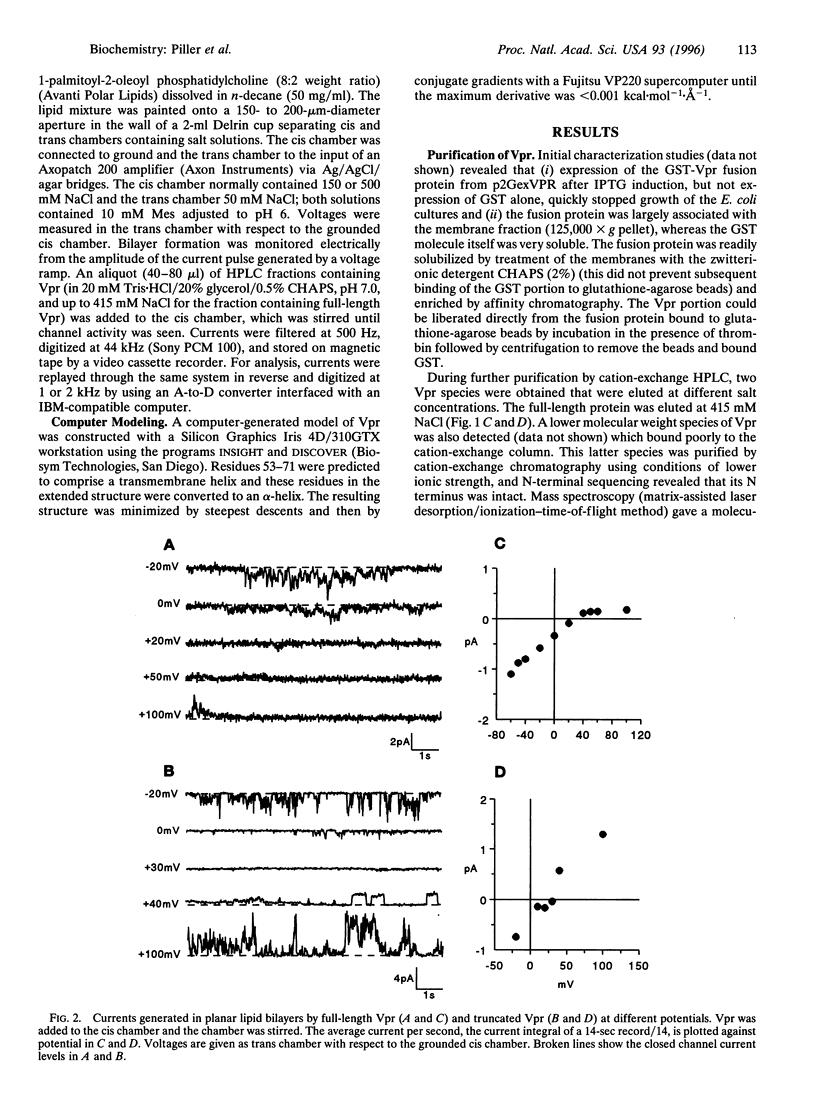

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen E. A., Dehni G., Sodroski J. G., Haseltine W. A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990 Jun;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Jalinoos Y., Proulx J., Sodroski J. G., Haseltine W. A. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3(1):11–18. [PubMed] [Google Scholar]
- Duff K. C., Ashley R. H. The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers. Virology. 1992 Sep;190(1):485–489. doi: 10.1016/0042-6822(92)91239-q. [DOI] [PubMed] [Google Scholar]
- Garry R. F. Potential mechanisms for the cytopathic properties of HIV. AIDS. 1989 Nov;3(11):683–694. doi: 10.1097/00002030-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Gras-Masse H., Ameisen J. C., Boutillon C., Gesquière J. C., Vian S., Neyrinck J. L., Drobecq H., Capron A., Tartar A. A synthetic protein corresponding to the entire vpr gene product from the human immunodeficiency virus HIV-1 is recognized by antibodies from HIV-infected patients. Int J Pept Protein Res. 1990 Sep;36(3):219–226. doi: 10.1111/j.1399-3011.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Wolstenholme A. J., Skehel J. J., Smith M. H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985 Nov;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger N. K., Bukrinsky M. I., Haggerty S. A., Ragland A. M., Kewalramani V., Lee M. A., Gendelman H. E., Ratner L., Stevenson M., Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. N., Fernandes L. S., Williams W. V., Weiner D. B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993 Feb 26;72(4):541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- Levy D. N., Refaeli Y., MacGregor R. R., Weiner D. B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. A., Clavijo P., Galantino M., Shen Z. Y., Liu W., Tam J. P. Chemically unambiguous peptide immunogen: preparation, orientation and antigenicity of purified peptide conjugated to the multiple antigen peptide system. Mol Immunol. 1991 Jun;28(6):623–630. doi: 10.1016/0161-5890(91)90131-3. [DOI] [PubMed] [Google Scholar]
- Lu Y. L., Spearman P., Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993 Nov;67(11):6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Shibata R., Kiyomasu T., Higuchi I., Kishida Y., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989 Sep;63(9):4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Sugrue R. J., Bahadur G., Zambon M. C., Hall-Smith M., Douglas A. R., Hay A. J. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO J. 1990 Nov;9(11):3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue R. J., Hay A. J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991 Feb;180(2):617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Pinto L. H., Holsinger L. J., Lamb R. A. Reconstitution of the influenza virus M2 ion channel in lipid bilayers. J Membr Biol. 1994 Oct;142(1):117–126. doi: 10.1007/BF00233389. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Henkel T., Trowbridge D. B., Orenstein J., Heuser J., Gendelman H. E., Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992 Jun;66(6):3925–3931. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Chanda P. K., Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987 Spring;3(1):33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Yuan X., Matsuda Z., Matsuda M., Essex M., Lee T. H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]