Abstract
Carbon-fiber microelectrodes (CFEs) are the primary electroanalytical tool in single-cell exocytosis and in-vivo studies. Here we report a new study on the kinetic properties of electrolyte-filled CFEs in single-cell measurements and demonstrate that the addition of outer sphere redox species, such as Fe(CN)63− and Ru(NH3)63+, in the backfill electrolyte solution can greatly enhance the kinetic response of CFEs. We show that at 750 mV, a voltage normally applied for detection of dopamine, the presence of fast outer sphere redox species in the backfilling solution significantly enhances the kinetic response of CFEs toward fast dopamine detection at single PC12 cells. Moreover, we also demonstrate that the use of Fe(CN)63− in the backfilling solution has enabled direct measurement of dopamine at applied voltages as low as 200 mV. This kinetic enhancement is believed to be due to faster electron-transfer kinetics on the coupling pole as compared to the sluggish reduction of oxygen. We anticipate that such redox-filled CFE ultramicroelectrodes will find many useful applications in single cell exocytosis and in-vivo sensing.
1 Introduction
Exocytosis is a fast cellular process through which cells secret their intracellular content. Neuronal communication is facilitated by the release of neurotransmitter molecules through exocytosis. Carbon-fiber microelectrodes (CFEs) are special ultramicroelectrodes (UMEs) suitable for studying single-cell exocytosis and brain functions.1 These electrodes have been widely used in a variety of cell types such as intact neurons,2,3 immortalized cell lines,4 blood platelets,5 chromaffin cells,6 and brain slices.7 Due to their small size and mechanical rigidity, CFEs offer superior spatial and temporal resolutions and high detection sensitivity making them especially suitable for studying fast exocytosis events. Several attempts have been taken to further improve their selectivity and sensitivity.8–15
Conventional fabrication methods for CFEs involve pulling a glass capillary to encase a small piece of carbon fiber and establishing an electrical contact by backfilling the inner electrode with a conductive epoxy or an electrolyte solution, e.g., potassium acetate.16–18 In early work with CFEs, direct electrical contact was made using mercury to fill the capillary. However, over time back-filling the electrode with an electrolyte solution, e.g., 3 M KCl, has emerged as a popular means due to the requirement to rapid change electrodes during cell analysis and the relative simplicity to comply with electrophysiology instruments. Our group has recently proposed that such electrolyte-backfilled CFEs behavior like closed bipolar electrodes.19,20 Scheme 1 illustrates the electrochemical coupling in an electrolyte-filled CFE. Here the 2-electron 2-proton oxidation of dopamine is electrically coupled to the reduction of soluble oxygen at the interior fiber. The coupling is driven by a small voltage bias, usually around < 1 V, provided by two Ag/AgCl electrodes placed on each side of the fiber.19
Scheme 1.

(a) A schematic of an electrolyte-filled CFE used in single-cell analysis. The oxidation of dopamine to dopamine-o-quinone is coupled to the reduction of a reducible species.
Although one might expect that optimal electroanalytical performance of CFEs would be obtained via a direct electrical contact to the interior fiber, a search of the literature shows no previous study characterizing the effect of the backfilling electrolyte solution. It is known that reduction of oxygen on carbon is a sluggish electrocatalytic process.21 It is thus important to understand how electrochemical coupling may affect the oxidative sensing of ultrafast neurotransmitter releasing events. Our results presented in this work indicate that significant distortions in amperometry data could arise when the electrochemistry at the interior carbon fiber is ignored.
In this work, we investigate the effect of the backfilling solution composition on the amperometric detection of dopamine from single pheochromocytoma (PC12) cells. We choose to investigate two conventional outer sphere redox species, Fe(CN)63− and Ru(NH3)63+, to compare with a traditional backfilling solutions, 3 M KCl. These redox species are selected because of their fast electron-transfer kinetics and higher solubility than oxygen. We show that at 750 mV, a voltage normally applied for detection of dopamine, the presence of fast outer sphere redox species in the backfilling solution significantly enhances the kinetic response of CFEs toward fast dopamine detection at single PC12 cells. The average amperometric peak amplitude measured with CFEs backfilled with Fe(CN)63− and Ru(NH3)63+ is ~61% higher than that measured using KCl-filled electrodes. Additionally, the average peak width measured on such CFEs is ~17% shorter than conventional KCl-filled electrodes. Moreover, our results also demonstrate that bipolar CFEs are generally slower than electrodes prepared by making a direct electrical contact in single-cell analysis. The use of Fe(CN)63− in the backfilling solution has enabled direct measurement of dopamine at applied voltages as low as 200 mV.
2 Experimental
2.1 Reagents and Chemicals
Dopamine hydrochloride (Sigma-Aldrich), potassium ferricyanide (K3Fe(CN)6, Sigma-Aldrich), hexaamineruthenium (III) chloride (Ru(NH3)6Cl3, Aldrich), and perchloric acid (HClO4, Aldrich) were of reagent grade or better and used without further purification. Other chemicals were all reagent grade or better and used without purification. Single-cell experiments were carried out in an isotonic saline solution consisting of 150 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 5 mM glucose, 10 mM HEPES, and 2 mM CaCl2 with the pH adjusted to 7.4 with 3 M NaOH. All aqueous solutions were prepared using 18 MΩ·cm water from a Barnstead NanoPure purification system (Thermo Scientific).
2.2 Electrode Fabrication
CFEs were fabricated via previously published methods.9 Briefly, a 5-μm-diameter carbon fiber was aspirated into a borosilicate glass capillary (o. d. = 1.2 mm, i. d. = 0.69 mm, Sutter Instrument Co.) and pulled with a micropipette puller (Model P-97, Sutter Instrument Co.). The pulled tip was sealed with epoxy (EPO-TEK 301-2, Epoxy Technology) and beveled (Model BV-10, Sutter Instrument Co.) to 45°. Electrical contact was obtained by backfilling the capillary with colloidal graphite in water (Energy Beam Sciences) or with an electrolyte solution and inserting a AgCl-coated silver wire into the back end of the capillary.
2.3 Cyclic Voltammetry
Cyclic voltammograms (CVs) were obtained using a Chem-Clamp voltmeter/amperometer (Dagan) and an EG&G 175 (Princeton Applied Research) function generator. The potentiostat was interfaced to a Dell computer through a PCI-6251 data acquisition board (National Instruments) via a BNC-2090 analog breakout box (National Instruments). The CV data was recorded and analyzed using an in-house written virtual instrumentation with LabView 8.5 (National Instruments).
2.4 Single-Cell Measurements
PC12 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained as previously described.9 Amperometric recordings at single cells were made on an inverted microscope (IX-71, Olympus) inside a home-built Faraday cage with culture dishes kept at 37 ± 1 °C. A CFE was positioned onto a cell by lowering the electrode tip until a slight deformation in the outline of the cell was observed. A glass micropipette was positioned roughly 50 μm away from the cell and used to inject a high concentration K+ solution (an isotonic saline solution containing 100 mM KCl) directly onto the cell surface. A 5-second, 25 psi pulse (Femtojet, Eppendorf) stimulated release of dopamine via exocytosis. For a given cell, high concentration K+ pulses were applied every 45 seconds for 3 intervals. A constant potential was applied on the CFE vs a Ag/AgCl reference electrodes.
Amperometric data from single PC12 cells was recorded using an Axopatch 200B amplifier (Molecular Devices) and observed in real-time with the Axoscope software package (Molecular Devices). Data was acquired with a 1.0 kHz low-pass filter using a Digidata 1440a (Molecular Devices) interfaced with a Dell computer. Amperometric peaks were analyzed using the MiniAnalysis software detection algorithm (Synaptosoft). A detection event was recognized when a signal with a local maximum and area under the curve exceeded a detection threshold of 5 times the root-mean-squared noise (RMS) acquired from a 2-second recording at the beginning of each individual amperometric trace. Post algorithm, the peaks were manually inspected to reject any electrical noise and to include peaks that were excluded due to their proximity to other amperometric events. Peaks which possessed a non-uniform top, double peaks, or overlapping peaks without a clear baseline were manually excluded in the analysis. All statistical values are reported ± standard error of the mean (SEM).
3 RESULTS AND DISCUSSION
3.1 Voltammetric Responses of Electrolyte-backfilled CFEs
We first examined the CV responses of CFEs filled with KCl, Fe(CN)63−, and Ru(NH3)63+. Figure 1 displays CV response for the oxidation of 100 μM dopamine on 5-μm disk CFEs backfilled with 3 M KCl (blue trace), 5 mM Fe(CN)63− in 3 M KCl (red trace), and 5 mM Ru(NH3)63+ in 3 M KCl (green trace). The black trace shows the CV response of a CFE backfilled with colloidal graphite to establish a direct electrical contact.
Figure 1.
CV response at 100 mV/s of a 5-μm disk CFE for the oxidation of 100 μM dopamine. The black trace is the CV response of a CFE back-filled with colloidal graphite, the blue, red, and green traces correspond to a CFE back-filled with 3 M KCl, 5 mM Fe(CN)63− in 3 M KCl, and 5 mM Ru(NH3)63+ in 3 M KCl, respectively.
The black trace shows a half-wave potential of ~0.17 V vs Ag/AgCl, which is around the formal potential for dopamine oxidation reported by others.22,23 Conversely, when an electrolyte solution is used to establish conductivity, the CFE becomes a bipolar electrode. As expected, the measured half-wave potential should now correspond to the difference between the formal potential for dopamine oxidation and that for the cathodic reaction. Additionally, a potential shift can be expected when the limiting current ratio of the anodic and cathodic poles is not unity. For conventional CFE the residual length of the interior fiber could be >20 mm giving an electrode area four orders of magnitude greater than that of the corresponding external disk electrode. This results in a large ratio of the limiting current on the two poles, which may account for a significant negative potential shift.19 This potential shift is clearly observed in results shown in Figure 1. The E1/2 observed from the colloidal graphite configuration is ~0.17 V vs. Ag/AgCl and the E1/2 for Fe(CN)63− reduction is 0.16 V vs. Ag/AgCl leading to an expected E1/2 around +0.01 V for the bipolar setup. However, Figure 1 shows an observed E1/2 of −0.14 V with a negative potential shift of around 0.15 V arising from the much larger reduction limiting current at the cathodic pole. Similar negative shifts are also observed for the other back-filling solutions. These results are in good agreement with previous results obtained using a CFE electrochemically coupled to a carbon fiber in a closed bipolar setup.19
The steady-state CVs of Fe(CN)63− and Ru(NH3)63+ filled electrodes show similar sigmoidal shape as the KCl-filled electrodes despite of a clear variation in the wave position. Our previous work has demonstrated that the voltammetric response of a closed bipolar electrode is primarily determined by that of the limiting pole. As mentioned above, in all bipolar settings in the present work, a large ratio between the cathodic limiting current and the anodic limiting current is obtained. Thus the shape of the steady-state CV is mainly determined by the dopamine oxidation. Although no kinetic variation is manifested in the steady-state CVs, the fact that both Fe(CN)63− and Ru(NH3)63+ have superior electron-transfer kinetics and higher solubility than oxygen could lead to better kinetic performance on single-cell measurements. Therefore, we carried out detailed measurements on single PC12 cells to explore and compare their kinetic responses in such experiments. It is also important to point out that Fe(CN)63− and Ru(NH3)63+ filling may enable stable electroanalytical performance and extended lifetime of electrolyte-filled CFEs. For a CFE with 0.5 mm inner diameter glass capillary, the volume of the electrolyte solution is roughly 5.8 μL corresponding to a total 5.8 nmole of oxygen when considering roughly ~ 1 mM oxygen concentration.24 As such, oxygen depletion after extensive electrode usage may lead to possible electrode deactivation.
3.2 Single-Cell Measurements
Figure 2 displays representative amperometric traces collected on single PC12 cells using CFEs backfilled with four different solutions, colloidal graphite, 3 M KCl, 5 mM Fe(CN)63− in 3 M KCl, and 5 mM Ru(NH3)63+ in 3 M KCl. Each amperometric recording was obtained at 750 mV from a single stimulation event shown with a red arrow. In single-cell amperometry the voltage at the electrode is usually held at ≥600 mV relative to a Ag/AgCl or SCE,25,26 such that the oxidation process at the sensing electrode is diffusion limited. For this reason a constant voltage of +750 mV was chosen for all electrode configurations. Figure 2a shows the amperometric response collected with a graphite-filled CFE indicating individually resolved exocytotic events following the stimulation. CFEs backfilled with 3 M KCl, 5 mM Fe(CN)63− in 3 M KCl, and 5 mM Ru(NH3)63+ in 3 M KCl are shown in Figs 2b, 2c, and 2d, respectively. All traces show clear detection of individual exocytotic events.
Figure 2.
Representative single-cell amperometric traces collected at 750 mV on single PC12 cells using CFEs backfilled with different solutions. Electrodes were backfilled with (a) colloidal graphite, (b) 3 M KCl, (c) 5 mM Fe(CN)63− in 3 M KCl, and (d) 5 mM Ru(NH3)63+ in 3 M KCl. The red arrow indicates the time point of a high K+ stimulant.
Figures 3a and 3b summarize the peak characteristics for each CFE configuration and the differences in detection become more pronounced. Cell data was pooled from multiple amperometric traces of each electrode configuration: colloidal graphite (n = 13 cells using 6 CFEs), Ru(NH3)63+ (n = 13 cells using 4 CFEs), Fe(CN)63− (n = 7 cells using 3 CFEs), and KCl (n = 13 cells using 3 CFEs). The data is also summarized in table 1. CFEs backfilled with graphite demonstrated the highest average peak amplitude at 16.2 ± 0.5 pA indicating the fastest kinetic response. The peak amplitudes from 5 mM Ru(NH3)63+, 5 mM Fe(CN)63−, and 3 M KCl were 14.8 ± 0.3, 13.8 ± 0.7, and 8.7 ± 0.3 pA respectively. The gradually decreasing peak amplitude is likely due to kinetic limitation from the cathodic reactions at the coupling pole. Both Ru(NH3)63+ and Fe(CN)63− are considered as fast redox species27,28 giving rise to a fast electrochemical coupling. These backfilling solutions yield average peak amplitudes only 8.6% and 14.8% lower than that collected on graphite-filled electrodes. Conversely, the reduction of oxygen is considerably slower on the cathodic pole of a carbon fiber electrode dropping the dopamine peaks nearly 50%.
Figure 3.
(a) Mean peak amplitude and (b) kinetic parameters for single exocytotic events collected on CFEs. Cell data was pooled from detection at multiple cells with multiple electrodes; black-colloidal graphite (n = 13), green-5 mM Ru(NH3)63+ in 3 M KCl (n = 13), red-5 mM Fe(CN)63− in 3 M KCl (n = 7), and blue-3 M KCl (n = 13). (c) Distribution of vesicle content detected from PC12 plotted as the cube root of the catecholamine released. Data is fit to Gaussian distributions shown in dashed lines.
Table 1.
Average kinetic parameters obtained from pooled amperometric data for the electrochemical detection of dopamine released from PC12 cells at +750 mV applied potential.
| Backfill Solution | Amplitude (pA) | Rise Time (ms) | Decay Time (ms) | Half Width (ms) | Area (fC) |
|---|---|---|---|---|---|
| Graphite | 16.2 ± 0.5 | 1.58 ± 0.04 | 1.64 ± 0.04 | 1.67 ± 0.03 | 24.7 ± 0.6 |
| 5 mM Fe(CN)63−, 3 M KCl | 13.8 ± 0.7 | 1.84 ± 0.06 | 1.85 ± 0.08 | 1.84 ± 0.07 | 22.9 ± 1.1 |
| 5 mM Ru(NH3)63+, 3 M KCl | 14.8 ± 0.4 | 1.59 ± 0.04 | 1.71 ± 0.03 | 1.76 ± 0.03 | 24.0 ± 0.5 |
| 3 M KCl | 8.70 ± 0.3 | 2.24 ± 0.05 | 2.26 ± 0.06 | 2.16 ± 0.05 | 21.2 ± 0.6 |
Figure 3b captures a large variance in the kinetic parameters observed for the different electrode configurations. The features that most highlight the difference in observed kinetics are the rise time and decay time. The rise time is directly related to the time it takes for the fusion pore to open and catecholamines to diffuse to the electrode surface, and the decay time represents the time duration needed for the fusion pore to close or all the vesicular contents to be consumed. The average rise time for CFEs filled with graphite was 1.58 ± 0.04 ms, 1.59 ± 0.04 ms for 5 mM Ru(NH3)63+, 1.84 ± 0.06 ms for 5 mM Fe(CN)63−, and 2.24 ± 0.05 ms for 3 M KCl. The rise times for graphite and Ru(NH3)63+ filled electrodes are very similar while the rise times for Fe(CN)63− and KCl filled electrodes have increased by 17% and 40%, respectively, when compared to colloidal graphite. Similar trends were observed for the decay time and half-width suggesting that oxidation of dopamine on the anodic pole is somewhat hindered by the coupling processes on the inner carbon fiber surfaces. The relative frequency of detection is unaffected by the differences in electrode configurations. To confirm this qualitative observation, a Student’s t-test was performed on the pooled data to compare the frequency of events detected after a single stimulation. At the 95% confidence level, the apparent difference in detection frequency is not statistically different between configurations. This suggests that changes of the electrode backfilling solution do not significantly impact the mean number of detection events per stimulation.
The normalized frequency histogram depicting the amount of dopamine released was plotted in Figure 3c. The data was plotted as the cube root of dopamine released as calculated from the charge for comparison between different electrode configurations.29 The distributions from each electrode configuration were fitted to Gaussian curves as shown by the dashed lines in Figure 3c. The Gaussian distributions for colloidal graphite, Ru(NH3)63+, and Fe(CN)63− are in close agreement with each other while the distribution for KCl is shifted slightly towards a smaller vesicle content. The average peak area for the colloidal graphite configuration is 24.7 ± 0.6 fC (femtoCoulomb) and is 21.2 ± 0.6 fC for 3 M KCl. The smaller peak area from the 3 M KCl data leads to a 14% decrease in total number of molecules detected compared to data obtained when colloidal graphite was used. Conversely when Ru(NH3)63+ is present in the backfill solution the average peak area is 24.0 ± 0.5 fC showing a less than 3% decrease in the number of molecules detected than colloidal graphite.
The kinetic response of a closed bipolar microelectrode can be enhanced by increasing the limiting current on the coupling pole.19 We conducted additional cell experiments where the concentration of redox molecule was increased to 100 mM for both Fe(CN)63− and Ru(NH3)63+. The results from Fe(CN)63− demonstrated a slight increase in the kinetic response from the lower concentration with an overall 3% increase in peak amplitude. The rise time remained roughly the same (it only increased from 1.85 ± 0.06 ms to 1.88 ± 0.04 ms) while both the decay time and peak half-width decreased to similar values obtained from colloidal graphite. Increasing the Ru(NH3)63+ concentration also caused a minimal increase in kinetics, but it was not determined to be statistically different than the results observed from the lower concentration. The frequency histograms did not suggest a significant shift in relative vesicle content.
A microinjection experiment was performed to further compare the electroanalytical performance of backfilled CFEs. A micropipette filled with 100 μM dopamine and a 5 μm CFE were placed in a solution of isotonic saline and positioned adjacent to one another using two micromanipulators with the tip of the electrode positioned directly next to the opening of the micropipette. Dopamine was injected directly on to the electrode by applying a 40 psi pulse for 0.1 s and the CFE was held at +750 mV for detection. In this manner the response to fast concentration changes by a controlled microinjection of dopamine at the electrode could be studied. Figure 4 shows representative amperometric peaks obtained from different bipolar configurations. Data was summarized from a minimum of 75 injections collected from 3 different electrodes at each configuration. When graphite was used, the dopamine peaks were significantly larger than when an electrolyte solution was used. The peak areas decreased to 96% for Fe(CN)63−, 84% for Ru(NH3)63+, and most notably when 3 M KCl was used the peak area was only 56% of the average value obtained with colloidal graphite. We believe this contrast arrives from the slower reduction kinetics inefficiently coupling to dopamine oxidation.
Figure 4.
Representative amperometric peaks obtained from the detection of dopamine at a 5 μm CFE via micropipette injection. A micropipette filled with 100 μM dopamine was positioned directly next to a CFE and a 0.1 s 40 psi pulse injected sample directly to the electrode surface (n = 3 electrodes). Each spike represents an individual injection. The applied potential was +750 mV. Electrodes were back-filled with different solutions as indicated.
These results are similar to those obtained from our single-cell experiments in that the observed amperometric peaks vary with the backfill solution. In contrast to the cell experiments the released chemicals are spatially confined between the cell and electrode, dopamine molecules in the injected stream move freely around the electrode. Thus, oxidation of dopamine is likely to undergo competition with fast radial-type diffusion away from the electrode. Thus the amount of dopamine a specific CFE can capture would depend strongly on its kinetic property. For a redox-filled CFE, the slow reduction process on the cathodic pole would certainly decrease its kinetic response causing a decrease in the detected dopamine amount. Interestingly, Fe(CN)63− and Ru(NH3)63+ filled electrodes show similar half peak width, ~98 ms, both of which are ~13% smaller than that of graphite-filled electrodes. KCl-filled electrodes show the smallest peak width ~89 ms. We do not understand what causes this opposite trend but believe this could be due to the free diffusion of dopamine. In summation, we believe these results in combination with our cell data confirm that the nature of the electrochemical process at the coupling pole could strongly affect the measurement of the redox processes on the limiting pole.
3.3 Dopamine detection at Lower Voltages
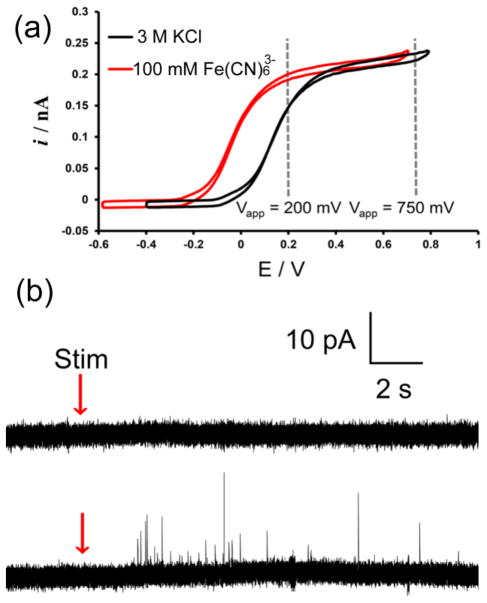
CFEs backfilled with outer sphere redox species allow for detection of exocytosis at lower applied voltages. This can be clearly seen in Figure 5a where a negative shift >200 mV is observed on the Fe(CN)63−-filled CFE. Figure 5a shows two CVs obtained from a single CFE backfilled initially with 3 M KCl (black) and then with 3 M KCl and 100 mM Fe(CN)63− (red). This lower voltage could mean smaller electrostatic distortion to the cellular membranes under the electrodes. Figure 5b is a direct comparison of amperometric traces collected at +100 mV with a CFE filled with 3 M KCl and the same CFE filled with a solution containing 3 M KCl and 100 mM Fe(CN)63−. No apparent exocytotic events were detected on the upper trace indicating that the voltage is too low to oxidize dopamine. Conversely, when the same CFE was backfilled with 100 mM Fe(CN)63−, secretory events were clearly detected upon cell stimulation at an applied voltage 600 mV lower than voltages normally applied in single-cell experiments.
Figure 5.
(a) CV response for the oxidation of 100 μM dopamine at a 5 μm CFE backfilled with 3 M KCl (black) and 100 mM Fe(CN)63− in 3 M KCl (red). The gray dashed lines in the figure represent the location at which potential was held during amperometric detection to collect data in table 2 relative to the potential of conventional cell experiments. (b) A comparison of single-cell amperometric traces collected at +100 mV with a CFE backfilled with 3 M KCl (upper panel) showing no apparent exocytotic detection after stimulation and with the same electrode backfilled with 100 mM Fe(CN)63− in 3 M KCl (bottom panel) on the same cell showing detection of individual exocytotic events.
To test the utility of amperometric detection at lower voltages, we conducted single cell experiments with electrodes backfilled with 3 M KCl and 100 mM Fe(CN)63− at +200 mV. At +200 mV the oxidative current is observable for both configurations; however in the case of 3 M KCl it is far from the mass-transfer limitation. Individual dopamine events were measured and analyzed for both 3 M KCl (n = 12 cells, 4 electrodes) and 100 mM Fe(CN)63− (n = 15, 6 electrodes) filled electrodes and are summarized in table 2. The mean peak amplitude observed for 100 mM Fe(CN)63− and 3 M KCl was 10.4 ± 0.4 and 8.4 ± 0.2 pA, respectively. The kinetic parameters are slower for both cases as compared to the values obtained with the similar electrodes at +750 mV. It is important to note that even at +200 mV, Fe(CN)63−-filled CFEs show faster detection characteristics than KCl-filled electrodes held at +750 mV. This data suggests that improved detection can be observed with an electrode backfilled with Fe(CN)63− at a lower potential than that of an electrode filled with KCl at a higher overpotential. This could have important implications for biological systems sensitive to high voltage application at the electrode surface.30 Furthermore, the potential window of detection could be catered and tuned for different electroanalytical applications by choosing redox mediators with different formal potentials.
Table 2.
Average kinetic parameters obtained from pooled amperometric data for the electrochemical detection of dopamine released from PC12 cells at +200 mV applied potential.
| Back-fill Solution | Amplitude (pA) | Rise Time (ms) | Decay Time (ms) | Half Width (ms) | Area (fC) |
|---|---|---|---|---|---|
| 5 mM Fe(CN)63−, 3 M KCl | 10.4 ± 0.4 | 1.89 ± 0.04 | 2.22 ± 0.04 | 2.16 ± 0.04 | 22.5 ± 0.7 |
| 3 M KCl | 8.4 ± 0.2 | 2.34 ± 0.05 | 3.25 ± 0.06 | 2.80 ± 0.05 | 28.4 ± 0.9 |
The total vesicular content detected with 3 M KCl at this low voltage is somewhat higher than that detected at 750 mV. We believe this is likely due to the slow kinetic response on these CFEs. Some smaller exocytotic peaks may well be detected at 750 mV could be neglected at 200 mV because their peak current are less than the 5 × RMS threshold. Therefore, some smaller peaks are precluded in the analysis effectively distorting the data towards larger events.
3.4 Electrode Stability
CFEs are known to be fouled by adsorption and polymerization of dopamine on the exterior carbon surface.31 Based on our previous discussion of small pipette volume and low oxygen concentration, it is also possible that oxygen will be depleted in the backfilling electrolyte solution. This second possible fouling mechanism may be eliminated by adding a fast electron-transfer redox mediator at much higher concentrations. To evaluate the effect of fast redox mediator on CFE stability, CFEs backfilled with graphite, 3 M KCl, and 100 mM Fe(CN)63− were exposed to repeated potential cycling over a 1.2 V voltage range at 100 mV/s in a 100 μM dopamine solution. Each electrode was continuously scanned 300 cycles in a time period of 2 hours. Our results (data not shown) show that in each case the initial CV shows the fastest response. The dopamine oxidation current decreases after repeated CV scanning along with a clear positive shift in the wave position indicating electrode fouling. This trend is largely independent of the backfilling solution. This result shows that adsorption and dopamine polymerization on exterior fiber surface is likely the primary reason for electrode fouling. The depletion of soluble oxygen in 3 M KCl is insignificant compared to faster electrode fouling by dopamine polymerization and other possible irreversible surface adsorption processes. It is possible that oxygen depletion may become important when conventional electrolyte-backfilled CFEs are used for extended time period when analyzing redox species which does not easily contaminate carbon surfaces.
4 Conclusions
We have presented the first study of the effect of electrochemical coupling on the electroanalytical performance of electrolyte-backfilled CFEs in single-cell measurements. Our results confirm that the fastest electrode response can be obtained by establishing direct electrical contact to the carbon fiber. CFEs filled with high concentration electrolyte, such as 3 M KCl, offers satisfactory detection of single dopamine secretion events. However, their kinetic properties in such measurements are slower than electrodes bearing direct electrical contact, which is likely due to kinetic limitation of cathodic reaction of oxygen. The kinetic response of these CFEs can be enhanced by adding simple outer sphere redox species, such as Fe(CN)63− and Ru(NH3)63+, in the backfill solutions. This kinetic enhancement is likely due to fast electron-transfer kinetics of these redox molecules on carbon surfaces. We anticipate that CFEs backfilled with such outer sphere redox mediators will have many useful applications in electroanalytical experiments.
Acknowledgments
We gratefully acknowledge financial support provided by the National Institutes of Health (R01GM101133). We thank Dr. Kelly L. Adams for helpful discussions and assistance. We also thank Laura Belluzzi for her assistance in cell culture.
References
- 1.Cans AS, Ewing AG. J Solid State Electrochem. 2011;15:1437–1450. [Google Scholar]
- 2.Chen GY, Gavin PF, Luo GA, Ewing AG. J Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM. Anal Chem. 2000;72:489–496. doi: 10.1021/ac991119x. [DOI] [PubMed] [Google Scholar]
- 4.Chen TK, Luo GO, Ewing AG. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 5.Ge S, Woo E, White JG, Haynes CL. Anal Chem. 2011;83:2598–2604. doi: 10.1021/ac102929y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wightman R, Jankowski J, Kennedy R, Kawaoge K, Schroeder T, Leszczyszyn D, Near J, Diliberto E, Viveros O. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JG, Moody BP, McCarty GS, Sombers LA. Langmuir. 2010;26:9116–9122. doi: 10.1021/la9048924. [DOI] [PubMed] [Google Scholar]
- 9.Adams KL, Jena BK, Percival SJ, Zhang B. Anal Chem. 2011;83:920–927. doi: 10.1021/ac102599s. [DOI] [PubMed] [Google Scholar]
- 10.Capella P, Ghasemzadeh B, Mitchell K, Adams RN. Electroanalysis. 1990;2:175–182. [Google Scholar]
- 11.Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Anal Chem. 2011;83:6658–6666. doi: 10.1021/ac2011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peairs MJ, Ross AE, Venton BJ. Anal Methods. 2011;3:2379–2386. [Google Scholar]
- 13.Xiao N, Venton BJ. Anal Chem. 2012;84:7816–7822. doi: 10.1021/ac301445w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swamy BEK, Venton BJ. Analyst. 2007;132:876–884. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- 15.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Anal Chem. 2010;82:2020–2028. doi: 10.1021/ac902753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, Sombers LA. Anal Chem. 2010;82:5205–5210. doi: 10.1021/ac100536s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge S, White JG, Haynes CL. Anal Chem. 2009;81:2935–2943. doi: 10.1021/ac8024202. [DOI] [PubMed] [Google Scholar]
- 18.Heien MLAV, Johnson MA, Wightman RM. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 19.Cox JT, Guerrette JP, Zhang B. Anal Chem. 2012;84:8797–8804. doi: 10.1021/ac302219p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrette JP, Oja SM, Zhang B. Anal Chem. 2012;84:1609–1616. doi: 10.1021/ac2028672. [DOI] [PubMed] [Google Scholar]
- 21.Song C, Zhang J. In: PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications. Zhang J, editor. Springer; New York: 2008. [Google Scholar]
- 22.Ponchon JL, Cespuglio R, Gonon F, Jouvet M, Pujol JF. Anal Chem. 1979;51:1483. doi: 10.1021/ac50045a030. [DOI] [PubMed] [Google Scholar]
- 23.Domenech A, Garcia H, Domenech-Carbo MT, Galletero MS. Anal Chem. 2002;74:562–569. doi: 10.1021/ac010657i. [DOI] [PubMed] [Google Scholar]
- 24.Gontard N, Thibault R, Cuq B, Guilbert S. J Agric Food Chem. 1996;44:1064–1069. [Google Scholar]
- 25.Ge S, White GJ, Haynes CL. Anal Chem. 2009;81:2935–2943. doi: 10.1021/ac8024202. [DOI] [PubMed] [Google Scholar]
- 26.Amatore C, Arbault S, Bouret Y, Guille M, Lemaitre F, Verchier Y. Anal Chem. 2009;81:3087–3093. doi: 10.1021/ac900059s. [DOI] [PubMed] [Google Scholar]
- 27.Chen SL, Kucernak A. J Phys Chem B. 2002;106:9396–9404. [Google Scholar]
- 28.Chen PH, McCreery RL. Anal Chem. 1996;68:3958–3965. [Google Scholar]
- 29.Zerby SE, Ewing AG. J Neurochem. 1996;66:651–657. doi: 10.1046/j.1471-4159.1996.66020651.x. [DOI] [PubMed] [Google Scholar]
- 30.Lundqvist JA, Sahlin F, Åberg MAI, Strömberg A, Eriksson PS, Orwar O. Proc Natl Acad Sci USA. 1998;95:10356–10360. doi: 10.1073/pnas.95.18.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang F, Zhou L, Mahmoud KA, Hrapovic S, Liu Y, Moynihan HA, Glennon JD, Luong JHT. Anal Chem. 2009;81:4089–4098. doi: 10.1021/ac900368m. [DOI] [PubMed] [Google Scholar]