Abstract
Invasive Salmonella infections for which improved or new vaccines are being developed include enteric fever caused by Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B and sepsis and meningitis in young children in sub-Saharan Africa caused by nontyphoidal Salmonella (NTS) serovars, particularly S. enterica serovars Typhimurium and Enteritidis. Assays are needed to measure functional antibodies elicited by the new vaccines to assess their immunogenicities and potential protective capacities. We developed in vitro assays to quantify serum bactericidal antibody (SBA) activity induced by S. Typhi, S. Paratyphi A, S. Typhimurium, and S. Enteritidis vaccines in preclinical studies. Complement from various sources was tested in assays designed to measure antibody-dependent complement-mediated killing. Serum from rabbits 3 to 4 weeks of age provided the best complement source compared to serum from pigs, goats, horses, bovine calves, or rabbits 8 to 12 weeks of age. For S. Enteritidis, S. Typhimurium, and S. Typhi SBA assays to be effective, bacteria had to be harvested at log phase. In contrast, S. Paratyphi A was equally susceptible to killing whether it was grown to the stationary or log phase. The typhoidal serovars were more susceptible to complement-mediated killing than were the nontyphoidal serovars. Lastly, the SBA endpoint titers correlated with serum IgG anti-lipopolysaccharide (LPS) titers in mice immunized with mucosally administered S. Typhimurium, S. Enteritidis, and S. Paratyphi A but not S. Typhi live attenuated vaccines. The SBA assay described here is a useful tool for measuring functional antibodies elicited by Salmonella vaccine candidates.
INTRODUCTION
Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B, which cause enteric fever, remain a public health concern in many developing countries (1–3), and in sub-Saharan Africa, nontyphoidal Salmonella (NTS) serovars Typhimurium and Enteritidis (including some emerging genetically distinct strains) are important agents of invasive disease (sepsis and meningitis) in young children and in adults with AIDS (4, 5). The increasing antibiotic resistance of Salmonella pathogens and high case fatality of NTS disease are stimulating the quest to find effective vaccines to assist in disease control (6–8).
There are currently two licensed commercially available S. Typhi vaccines: parenteral Vi polysaccharide and live attenuated oral strain Ty21a. More immunogenic live attenuated strains and Vi conjugate vaccines presently under development are expected to overcome the limitations of the current licensed typhoid vaccines (9–13). Not surprisingly, analogous strategies are being pursued to develop live oral and conjugate paratyphoid A and NTS vaccines (14–18).
As new Salmonella vaccines progress through clinical trials in humans, it is important to have standardized assays to evaluate their immunogenic capacity (19, 20). While the measurement of serum antibodies through antigen-binding assays (i.e., enzyme-linked immunosorbent assay [ELISA]) offers the most practical tool for monitoring the immunogenicity of different formulations and immunization schedules, regulatory agencies have urged that the functional capacities of antibodies should also be documented, in addition to their antigen recognition capacities.
For the licensure of bacterial vaccines, precedent has been set for two functional properties of serum antibodies: opsonophagocytic activity (OPA) and bactericidal activity. With pneumococcal conjugate vaccines, opsonophagocytic antibodies are accepted as correlates of protection, and for meningococcal purified polysaccharide and polysaccharide-protein conjugate vaccines, the correlate is serum bactericidal antibody (SBA). Opsonophagocytic antibodies bind to bacterial antigens and facilitate microbial uptake and killing by phagocytic cells. Bactericidal antibodies mediate direct bacterial killing in the presence of complement (21, 22).
The establishment of successful functional antibody assays requires a careful evaluation and selection of optimal parameters, such as bacterial growth conditions, the external source(s) of complement, the proportion of reagents in the reaction mixture, and incubation conditions. The source of the complement reagent has proven to be critical for SBA killing. We previously measured antibody-dependent complement-mediated SBAs in the serum samples of mice immunized with a live attenuated S. Paratyphi A vaccine using an adaptation of the Vibrio cholerae serum vibriocidal assay that employs guinea pig complement (GPC) (14). Other groups have successfully used 3- to 4-week-old baby rabbit complement (BRC) in SBA assays to evaluate S. Typhi vaccines (23, 24). Similarly, BRC was employed in an S. Typhimurium SBA assay, although very high concentrations (75%) of complement were required to mediate killing by the antibody (25). While these assays have allowed the demonstration of SBA responses against S. Typhi, S. Paratyphi A, and S. Typhimurium, no assay has been reported to determine SBA responses against S. Enteritidis (14, 23–25). Furthermore, none of these assays has been standardized to measure vaccine-induced endpoint SBA responses against Salmonella serovars.
Here, we describe assays that measure complement-mediated SBA responses induced by typhoidal (S. Typhi and S. Paratyphi A) and nontyphoidal (S. Typhimurium and S. Enteritidis) Salmonella vaccines. We document the optimal conditions, including complement source and concentration, bacterial growth phase, and target strain, and we describe an assay format to achieve endpoint SBA titers during preclinical vaccine testing.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
The bacterial strains used, described in Table 1, were grown at 37°C in Hi-Soy (HS) bacteriological medium (5 g/liter sodium chloride, 10 g/liter Soytone [Teknova, Hollister, CA], 5 g/liter Hy-Yest [Sigma-Aldrich, St. Louis, MO]). The medium was supplemented with 0.001% (wt/vol) guanine for all guaBA mutants and with 0.0001% (wt/vol) 2,3-dihydroxybenzoate (DHB) for the growth of S. Typhi strain CVD 909.
TABLE 1.
Bacterial strains used in this study
| Serovar or species | Strain | Isolation location or characteristics | Reference(s) or source |
|---|---|---|---|
| Salmonella Typhimurium | D65 | Clinical isolate from blood culture, Mali | 43, 44 |
| D23580 | Clinical isolate from blood culture, Malawi | 18, 45 | |
| CVD 1921 | I77 ΔguaBA ΔclpP | 15 | |
| CVD 1931 | D65 ΔguaBA ΔclpX | S. M. Tennant, P. Schmidlein, J. E. Galen, and M. M. Levine, unpublished data | |
| Salmonella Enteritidis | R11 | Clinical isolate from blood culture, Mali | 43, 44 |
| S01 | Clinical isolate from blood culture, Mali | 43, 44 | |
| S15 | Clinical isolate from blood culture, Mali | 43, 44 | |
| Q38 | Clinical isolate from blood culture, Mali | 43, 44 | |
| CVD 1941 | R11 ΔguaBA ΔclpP | 15 | |
| CVD 1944 | R11 ΔguaBA ΔclpX | 15 | |
| Salmonella Typhi | Ty2 | Wild-type | 46 |
| CVD 909 | Ty2 ΔaroC ΔaroD ΔhtrA Ptac-tviA | 11 | |
| CVD 910 | Ty2 ΔguaBA ΔhtrA | 26 | |
| CVD 915 | Ty2 ΔguaBA | 47 | |
| Ty21a | Ty2 ΔgalE ΔilvD ΔviaB (Vi−) H2S− | 48 | |
| Salmonella Paratyphi A | ATCC 9150 | Wild-type | American Type Culture Collection, Manassas, VA |
| CVD 1901 | ATCC 9150 ΔguaBA | 14 | |
| CVD 1902 | ATCC 9150 ΔguaBA ΔclpX | 14 | |
| Escherichia coli | Bort | O18:K1:H7, clinical isolate from blood culture, Walter Reed Army Medical Center | 49 |
Growth kinetics of target strains.
S. Typhimurium strain D65, S. Enteritidis strain R11, S. Typhi strain Ty2, and S. Paratyphi A ATCC 9150 were grown in HS bacteriological medium for 17 to 20 h overnight. The bacterial suspension was diluted 1:1,000 in 25 ml of fresh HS medium and incubated at 37°C and 250 rpm. The optical density at 600 nm (OD600) was measured every hour for 9 h.
Serum samples.
The serum samples used were obtained from mice vaccinated with live attenuated Salmonella vaccines in previous experiments performed at the Center for Vaccine Development (CVD), and the samples were maintained at −20°C. All animal studies were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Serum samples from mice immunized with attenuated Salmonella were designated immune (I), and serum samples from negative-control mice that received phosphate-buffered saline (PBS) were designated nonimmune (NI). The immune sera were pools that contained equal volumes of serum samples from 10 mice immunized with S. Paratyphi A or 12 mice immunized with S. Typhi, S. Typhimurium, or S. Enteritidis vaccine strains, unless otherwise indicated.
(i) NTS vaccines.
BALB/c mice were orally immunized with 109 CFU of strains CVD 1921 (S. Typhimurium I77 ΔguaBA ΔclpP), CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX), CVD 1941 (S. Enteritidis R11 ΔguaBA ΔclpP), or CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), or with PBS on days 0, 28, and 56, as previously described (15). The serum samples used in this study for the SBA assays were obtained at day 70 postvaccination and had anti-LPS IgG geometric mean titers (GMT) of 8,363 ELISA units (EU)/ml, 10,433 EU/ml, 6,912 EU/ml, and 2,891 EU/ml for mouse groups immunized with CVD 1921, CVD 1931, CVD 1941, and CVD 1944, respectively. The anti-LPS IgM or IgA titers were not determined.
(ii) Typhoid vaccine.
BALB/c mice were immunized intranasally (i.n.) with 108 CFU of strain CVD 910 (S. Typhi ΔguaBA ΔhtrA) or PBS on days 0 and 27, as described previously (26). The serum samples collected on day 49 (anti-LPS IgG GMT titer, 2,375 EU/ml) were used for the SBA assays.
(iii) Paratyphoid A vaccine.
BALB/c mice were immunized i.n. with 109 CFU of strain CVD 1902 (S. Paratyphi A ΔguaBA ΔclpX) or PBS on days 0, 14, and 28 (14). The serum samples collected at day 42 (anti-LPS IgG GMT titer, 14,217 EU/ml) were used for the SBA assays.
Complement resistance.
To determine the serum resistance of the target strains, we tested the survival of S. Typhimurium D65, S. Enteritidis R11, S. Typhi Ty2, and S. Paratyphi A ATCC 9150 in the presence of complement alone. Log-phase cultures were prepared by diluting an overnight culture 1:1,000 in HS medium and incubating at 37°C and 250 rpm until an OD600 of 0.4 was attained. The bacteria were then diluted in PBS to a concentration of ∼1 × 104 CFU/ml. Guinea pig complement (Cedarlane Laboratories, Burlington, NC) or 3- to 4-week-old rabbit complement (baby rabbit complement [BRC]) (Pel-Freez Biologicals, Rogers, AZ) was diluted in PBS and incubated with 10 μl of bacterial suspension at 37°C in a 96-well round-bottom plate with shaking at 115 rpm for 1 h. Viable counts of the inoculum and the bacteria postexposure were performed. The samples were tested in duplicate. The complement-resistant Escherichia coli Bort strain was grown to log phase and used as a control strain to confirm a lack of complement killing (25% BRC) in the absence of serum.
Serum bactericidal activity assay to measure antibody-dependent complement-mediated killing. (i) Optimization.
Stationary-phase Salmonella cultures were prepared by incubating bacteria in HS medium at 37°C and 250 rpm for 16 to 20 h. Log-phase cultures were prepared by diluting an overnight culture 1:1,000 in fresh HS medium and incubating at 37°C and 250 rpm until an OD600 of 0.4 or 0.8 was attained. Both log- and stationary-phase cultures were then diluted in PBS to a concentration of ∼1 × 104 CFU/ml or 1010 CFU/ml in early experiments. A master mix composed of 50% GPC or BRC in PBS was serially diluted 2-fold in PBS, and 80-μl aliquots of each dilution were added to duplicate wells of a round-bottom 96-well plate. Next, 10 μl of heat-inactivated (56°C for 20 min) pooled immune or nonimmune serum and 10 μl of bacterial suspension were added to each well and incubated at 37°C with shaking at 115 rpm for 1 h. Viable CFU counts were determined manually after exposure of the bacteria to complement.
(ii) Optimized SBA assay.
Log-phase cultures (bacteria grown to an OD600 of 0.4) of S. Typhimurium D65, S. Enteritidis S15, and S. Typhi Ty2 were prepared as described above. For S. Paratyphi A ATCC 9150, stationary-phase cultures were prepared. Pooled immune and nonimmune sera were heat inactivated at 56°C for 20 min, and 2-fold serial dilutions in normal saline were performed in a 96-well plate. Serum samples from S. Typhimurium-vaccinated mice were serially diluted as follows: 1:2,000 to 1:128,000 for postvaccination sera and 1:20 to 1:2,560 for nonimmune sera (control mice that received PBS). Serum samples from mice immunized with S. Enteritidis, S. Typhi, or S. Paratyphi A vaccines were serially diluted from 1:200 to 1:25,600. For the nontyphoidal strains, optimal SBA results were obtained by combining 25 μl of BRC (25% final concentration), 15 μl of saline, and 50 μl of diluted mouse serum; these were then incubated with 10 μl of diluted bacteria (100 to 350 CFU) at 37°C with shaking at 115 rpm for 1 h. A volume of 50 μl serum per 100-μl reaction mixture was chosen based on the format of a successful vibriocidal assay used in multiple V. cholerae clinical studies (27). For S. Typhi Ty2 and S. Paratyphi A ATCC 9150, the optimal SBA results were obtained by combining 12.5 μl of BRC (12.5% final concentration) with 27.5 μl of normal saline, 50 μl of diluted mouse serum, and 10 μl of diluted bacteria (100 to 350 CFU); these were incubated for 1 h under the same conditions, and viable CFU counts were determined. The negative control contained bacteria and complement only. The serum bactericidal antibody titer was defined as the reciprocal of the highest serum dilution that produced >50% killing in relation to the killing observed for the control wells containing bacteria and complement only (i.e., no serum) (23, 24). The titers were determined from the mean bacterial count from triplicate wells.
SBA assays with complement from other animal sources.
Optimization assays were performed using as the source of complement sera from goats (nonhemolyzed, donor herd), horses (nonhemolyzed donor herd), bovine calves (trace hemolyzed), pigs (trace hemolyzed), 3- to 4-week-old rabbits, and 8- to 12-week-old rabbits (trace-hemolyzed young rabbit serum), all purchased from Pel-Freez Biologicals. Fifty microliters of complement (50% final concentration), 30 μl of saline, and 10 μl of diluted mouse serum (from S. Typhimurium CVD 1921- or PBS-immunized mice) were combined with 10 μl of diluted S. Typhimurium D65 (100 to 350 CFU) grown to log phase (OD600, 0.4), and these were incubated for 1 h at 37°C, with shaking, as described above. The negative control contained bacteria and complement only.
LPS analysis.
Bacteria were grown to an OD600 of 0.4, 0.8, and 4.0 and adjusted to an OD600 of 2.0. LPS was isolated from S. Typhimurium D65, S. Enteritidis R11, S. Typhi Ty2, and S. Paratyphi A ATCC 9150 by pelleting 1 ml of culture, removing the supernatant, and boiling the bacterial pellet in 100 μl Laemmli lysis buffer (Bio-Rad Laboratories, Hercules, CA) for 5 min, and then adding 1/20 (vol/vol) proteinase K (20 mg/ml) to a final concentration of 1 mg/ml and incubating at 60°C for 1 h. LPS analysis was performed by Tris-glycine SDS-PAGE using 12% resolving gels. LPS silver staining was performed as described previously (28). Densitometry was performed using Quantity One software (Bio-Rad Laboratories). Briefly, the amounts of short-, medium-, and long-chain LPS were normalized to the amount of core produced for each culture.
PCR.
The presence of the rck gene was determined by PCR. Boiled lysates of Salmonella were prepared by suspending 2 to 3 colonies in 100 μl of nuclease-free water, heating at 95°C for 10 min, and then centrifuging to pellet the cell debris. PCR amplifications were performed with 2.5 U Taq DNA polymerase (Denville Scientific, Metuchen, NJ, or GenScript, Piscataway, NJ), 1× PCR buffer containing 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate (dNTP), and with 1 μM forward primer rckF (5′-TCGTTCTGTCCTCACTGC-3′) and 1 μM reverse primer rckR2 (5′-TCATAGCCCAGGATCGATG-3′). The PCR reagents were combined with boiled bacterial extract and thermocycled in a reaction volume of 20 to 50 μl, as follows: 95°C for 2 min, followed by 25 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final extension of 72°C for 5 min. The primers described above yielded an amplicon size of 474 bp.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA). A Mann-Whitney test was used to compare the percent inoculum in different serum sources and SBA titers for mice immunized with live attenuated typhoidal and nontyphoidal Salmonella vaccines versus the PBS control group. An analysis of variance (ANOVA) with Bonferroni's test for multiple comparisons was used when three or more groups were compared. Spearman's correlation coefficient was utilized to describe the correlation between the SBA and LPS IgG titers. The remaining data were analyzed using Student's t test. Two-tailed P values of <0.05 were considered statistically significant.
RESULTS
Killing of Salmonella spp. by guinea pig or baby rabbit complement alone.
To select the best source of complement for measuring SBA responses against typhoidal and nontyphoidal Salmonella vaccines, we first compared the ability of GPC, which is routinely used in vibriocidal assays (29), and of BRC, which was used in a previously reported S. Typhi SBA assay, to mediate killing of Salmonella serovars (14, 23, 24). S. Typhi Ty2, S. Paratyphi A ATCC 9150, S. Typhimurium D65, and S. Enteritidis R11 killing increased concomitantly with the amount of GPC in the assay reaction mixture when the organisms were grown to an OD600 of 0.4 (see growth curves in Fig. S1 in the supplemental material). Complement sensitivity differed among the serovars, and the order of susceptibility to killing observed at 10% GPC (middle of the range tested) was S. Typhi Ty2 being approximately equal to S. Paratyphi A 9150, followed by S. Typhimurium D65, and finally S. Enteritidis R11 (i.e., R11 was the most resistant strain) (Fig. 1A). In contrast to GPC, BRC was unable to kill S. Enteritidis, S. Typhimurium, and S. Paratyphi A in the absence of immune serum (Fig. 1B); only S. Typhi Ty2 was killed, as <100% of the inoculum survived at ≥5% BRC.
FIG 1.
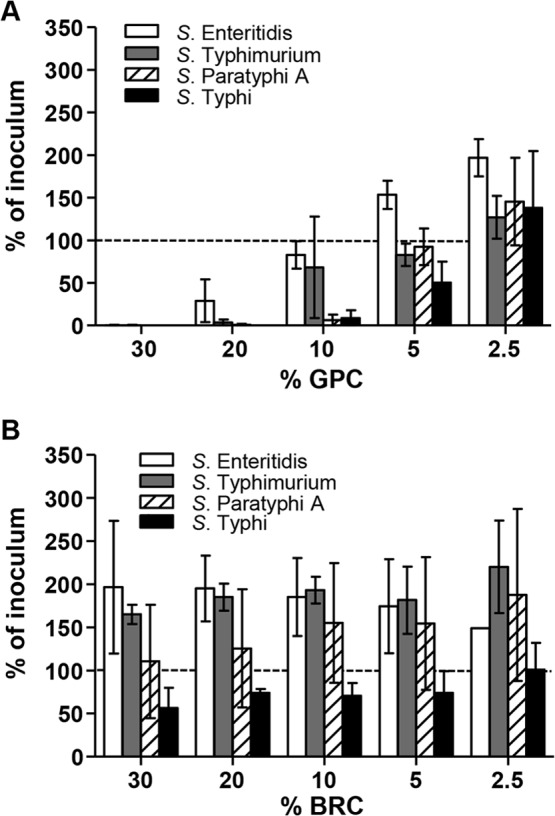
Survival of Salmonella spp. in the presence of complement alone. Survival of S. Typhimurium D65, S. Enteritidis R11, S. Typhi Ty2, and S. Paratyphi A ATCC 9150 in the presence of guinea pig complement (GPC) (A) and baby rabbit complement (BRC) (B) in the absence of immune sera. The results are the mean ± standard deviation values from at least two independent experiments. The dashed line represents 100% of inoculum as a visual reference.
In an early experiment using bacteria grown to an OD600 of 0.8, we noticed that after incubation at 37°C for 1 h, 30% BRC enhanced the growth of S. Typhimurium D65 (mean ± standard deviation [SD], 179% ± 73% survival) and S. Enteritidis R11 (138% ± 25%) but not of S. Typhi Ty2 (42% ± 14%) or S. Paratyphi A ATCC 9150 (64% ± 17%) compared to bacteria suspended in PBS (for S. Typhimurium D65, mean ± SD, 46% ± 21%, P = 0.029 [Student's t test, two-tailed]; for S. Enteritidis R11, 49% ± 28%, P = 0.014; for S. Typhi Ty2, 57% ± 57%, P = 0.678; and for S. Paratyphi A ATCC 9150, 59% ± 37%, P = 0.841). We confirmed that the complement-resistant strain E. coli Bort was not killed by BRC (data not shown).
Killing of Salmonella spp. by BRC in the presence of antibody.
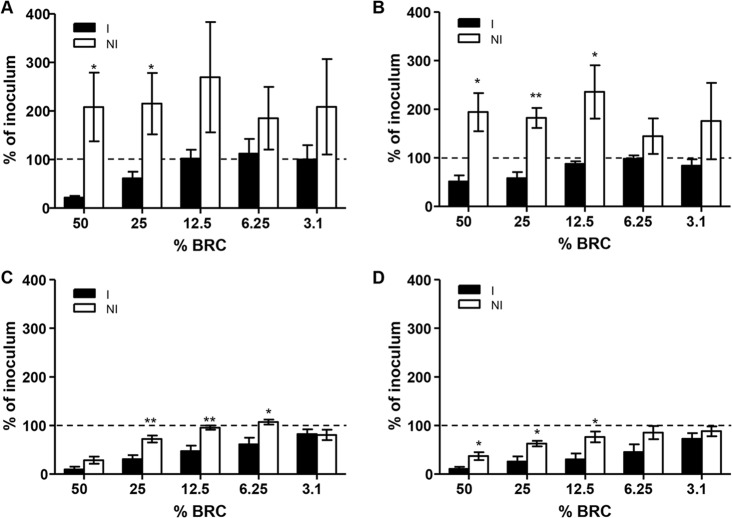
When SBA assays were performed with GPC, there was no difference in the killing of Salmonella in the presence or absence of immune serum (data not shown). Therefore, use of GPC in SBA assays was abandoned, and subsequent assays were performed using BRC. Baby rabbit complement titration with pooled serum samples from mice immunized with live attenuated Salmonella vaccines (immune) or mice that received PBS (nonimmune) showed various degrees of complement-mediated killing. There was significantly more complement-mediated killing of S. Typhimurium at 50% and 25% BRC for immune (CVD 1921-immunized mice) versus nonimmune serum (Fig. 2A). Likewise, for S. Enteritidis and S. Paratyphi A, BRC at 50%, 25%, and 12.5% showed significantly more complement-mediated killing in the presence of immune (CVD 1941- and CVD 1902-immunized mice) versus nonimmune serum (Fig. 2B and D). For S. Typhi CVD 910-immunized mice, significant differences were observed at 25%, 12.5%, and 6.25% BRC (Fig. 2C). However, for NTS and S. Typhi, the difference in complement-mediated killing was observed only when the target strain was grown to an OD600 of 0.4 but not when grown to an OD600 of 0.8, 4.0, or overnight, whereas S. Paratyphi A succumbed to complement-mediated SBA activity when grown at an OD600 of 0.4, 0.8, or 4.0, as well as overnight (results not shown). Of note, the individual serum samples included in the immune serum pool that exhibited SBA activity contained LPS-specific IgG antibody titers that ranged between 2,375 EU/ml and 14,217 EU/ml, while the nonimmune serum samples from PBS-inoculated mice lacked LPS antibodies.
FIG 2.
Complement titrations. Shown is the survival of S. Typhimurium D65 (A), S. Enteritidis R11 (B), S. Typhi Ty2 (C), all grown to log phase (OD600, 0.4), and S. Paratyphi A ATCC 9150 (D) grown to stationary phase with BRC in the presence of immune (I) compared to nonimmune (NI) sera and decreasing complement concentrations. The results are shown as the mean ± standard deviation values from more than three independent experiments. *, P < 0.05, and **, P < 0.01, by Student's t test (two-tailed). The dashed lines represent 100% of inoculum as a visual reference.
In the presence of nonimmune serum and BRC, NTS not only survived killing but grew markedly (i.e., >100% of the original inoculum was measured), whereas the survival of S. Typhi and S. Paratyphi A in the presence of nonimmune sera and complement remained at ≤100% of the inoculum. Additionally, in the presence of BRC and immune serum, there was increased survival of all the Salmonella serovars with decreasing concentrations of complement down to 6.25%. These data indicate that antibody alone is unable to kill Salmonella. As seen in Fig. 2, at 3.1% BRC (the smallest amount tested), immune serum was unable to kill S. Typhimurium (mean ± standard deviation, 100% ± 59.2% survival), S. Enteritidis (83.75% ± 26.96% survival), S. Typhi (82.25% ± 19.64% survival), or S. Paratyphi A (72.5% ± 23.1% survival).
Killing by complement from other animal sources.
In searching for less expensive and more readily available sources of complement as a possible alternative to BRC in the SBA assays, we investigated the potential use of sera from goats, horses, bovine calves, pigs, and 8- to 12-week-old rabbits. Frustratingly, only BRC from 3- to 4-week-old rabbits efficiently and consistently killed S. Typhimurium (i.e., resulted in <100% of inoculum) in the presence of immune serum (CVD 1921-immunized mice) (Fig. 3). We observed a statistically significant difference between the survival of S. Typhimurium D65 in the presence of immune serum versus serum from PBS-treated animals when using 50% BRC.
FIG 3.
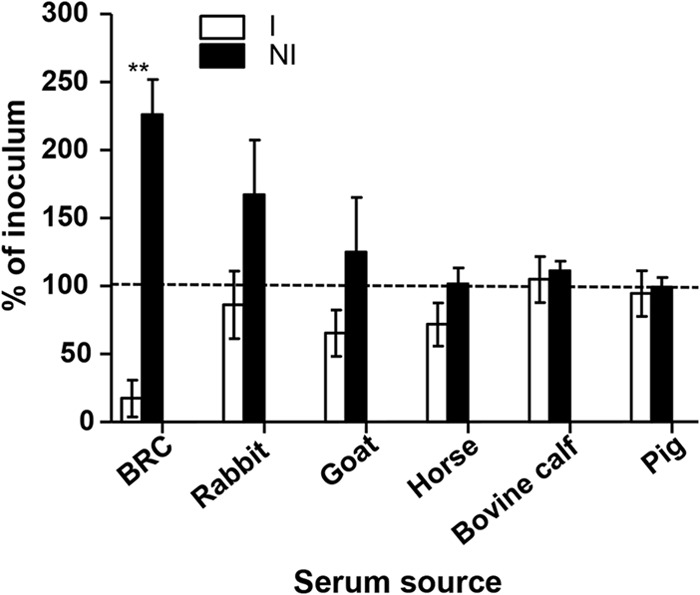
Survival of S. Typhimurium in the presence of complement from various animals and antibody. Shown is the survival of S. Typhimurium D65 in the presence of immune (I) antibody compared to nonimmune (NI) antibody with indicated complement sources. The results are the mean ± standard deviation values from more than three independent experiments. BRC is from 3- to 4-week-old rabbits. **, P < 0.01 by the Mann-Whitney test. The dashed line represents 100% of inoculum as a visual reference.
O-Antigen length of Salmonella serovars.
We postulated that the antibody-dependent complement-mediated killing of S. Typhimurium, S. Enteritidis, and S. Typhi observed at log phase but not at stationary phase might be accounted for by O-antigen length. At stationary phase, S. Typhimurium D65, S. Enteritidis R11, and S. Typhi Ty2 had >30 O-antigen repeats, whereas S. Paratyphi A ATCC 9150 had only ∼15 O-antigen repeats (Fig. 4A). The bacteria were grown to an OD600 of 0.4 or 0.8 or an OD600 of 4.0 or overnight, and we determined the amount of short, medium, and long LPS species (see Fig. S1 in the supplemental material). An example of a gel image used for the densitometry analysis can be seen in Fig. S2 in the supplemental material. S. Enteritidis, S. Typhimurium, and S. Typhi produced significantly more long O antigen at stationary phase (OD600, 4) than at log phase (OD600, 0.4 or 0.8) (Fig. 4B to D). Since the LPS profile suggests that S. Paratyphi A ATCC 9150 produces very little long O antigen even at stationary phase (Fig. 4A), we examined the quantity of medium-length LPS produced by this serovar at the log and stationary phases. S. Paratyphi A produced significantly more medium-length LPS at stationary phase than at log phase (OD600, 0.4 or 0.8) (Fig. 4E). In the early SBA experiments, we observed a positive correlation (Pearson's r = 0.8092, P = 0.0511) between bacterial survival and LPS length using 50% baby rabbit complement, 108 CFU of Salmonella (S. Typhi, S. Typhimurium, and S. Enteritidis), and immune serum (individual data not shown). S. Paratyphi A was susceptible to killing at both the log and stationary phases, presumably due to the shorter LPS length, and was therefore omitted from the correlation.
FIG 4.
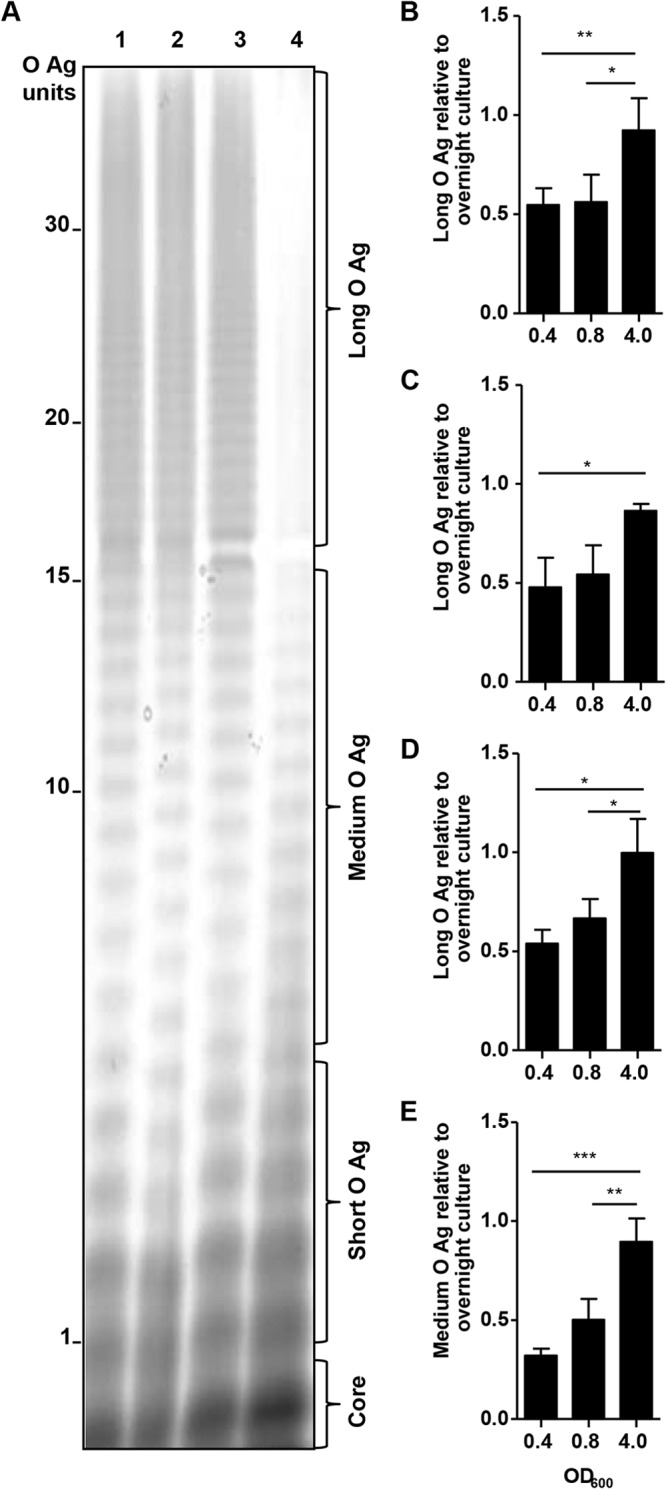
LPS produced by Salmonella serovar. (A) LPS profiles of S. Typhimurium D65 (lane 1), S. Enteritidis R11 (lane 2), S. Typhi Ty2 (lane 3), and S. Paratyphi A ATCC 9150 (lane 4) after overnight culture. Ratio of long or medium O antigen (O-Ag) at OD600 of 0.4, 0.8, and 4.0 to overnight culture for S. Typhimurium D65 (B), S. Enteritidis R11 (C), S. Typhi Ty2 (D), and S. Paratyphi A ATCC 9150 (E). The results are the mean ± standard deviation values from more than three independent experiments. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, by ANOVA, with Bonferroni's test for multiple comparison.
SBA activities of S. Enteritidis strains that possess or lack rck.
We noticed that the antibody-dependent complement-mediated killing of S. Enteritidis R11 was highly varied and did not produce consistent endpoint titers. Therefore, we hypothesized that the increased complement resistance of S. Enteritidis R11 may be due to the presence of the resistance to complement killing gene, rck. We screened 52 invasive S. Enteritidis clinical isolates from Mali by PCR for the presence of rck and found that 15% were rck positive (including R11) and 85% were rck negative. When we tested a group of rck-positive and rck-negative strains with pooled sera from mice immunized with S. Enteritidis CVD 1944, no significant differences in SBA titers were observed between the rck-positive and rck-negative strains (Fig. 5; P = 0.455, Student's t test, two-tailed). Interestingly, the highest SBA titer was measured when using strain S. Enteritidis S01 as the target, which was rck positive by PCR. The rck-negative strain S. Enteritidis S15 also yielded consistent SBA endpoint titers and therefore was chosen as the target for the optimized S. Enteritidis SBA assay.
FIG 5.
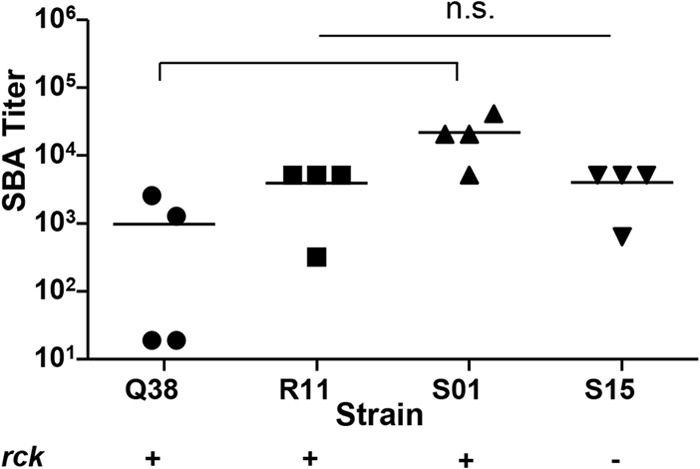
SBA titer and presence of the gene rck. SBA titers in pooled sera from CVD 1941-immunized mice against clinical invasive rck-positive or rck-negative S. Enteritidis strains. The lines represent the mean titers from at least three independent experiments. P = 0.45, Student's t test, two-tailed. n.s., nonsignificant.
Contribution of the Vi polysaccharide capsule to complement resistance.
It is well established that the Vi capsule contributes to the complement resistance of S. Typhi and is maximally expressed during the stationary phase (30). We confirmed this in our optimized SBA system with 12.5% BRC in the absence or presence of immune serum, using S. Typhi strains that varied in their expression of Vi. S. Typhi CVD 909, which produces Vi constitutively and at a higher level than does S. Typhi Ty2 (11), exhibited higher complement resistance than S. Typhi Ty2 (the wild-type strain from which it was derived) when grown to log phase (Fig. 6). S. Typhi vaccine strain Ty21a was killed in the SBA assay, as this strain does not produce Vi (not shown).
FIG 6.
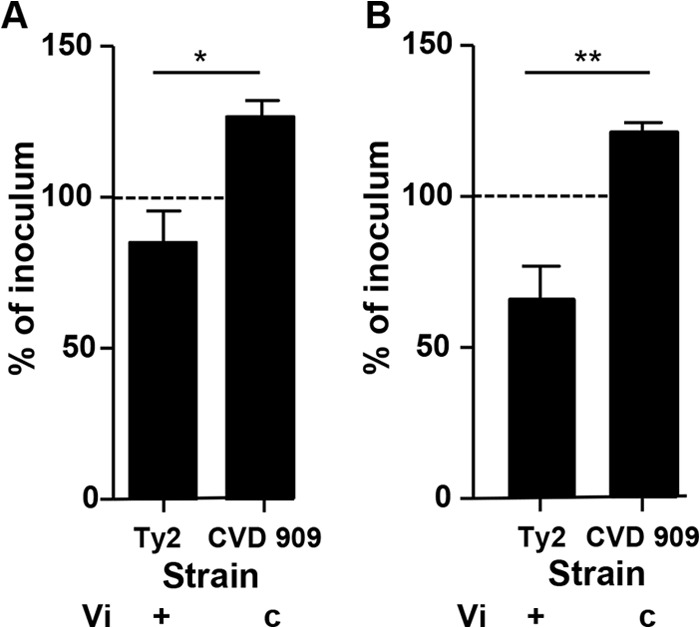
Survival of S. Typhi strains that express various levels of Vi in the presence of BRC. Shown is the survival of S. Typhi strains Ty2 and CVD 909 grown to an OD600 of 0.4 and incubated with 12.5% BRC in the absence of immune serum. The results represent the mean ± standard deviation value from at least three independent experiments. c, constitutively expressed. *, P < 0.05 and **, P < 0.01, by Student's t test, two-tailed. The dashed line represents 100% of inoculum as a visual reference.
Determination of SBA endpoint titers induced by various Salmonella vaccines.
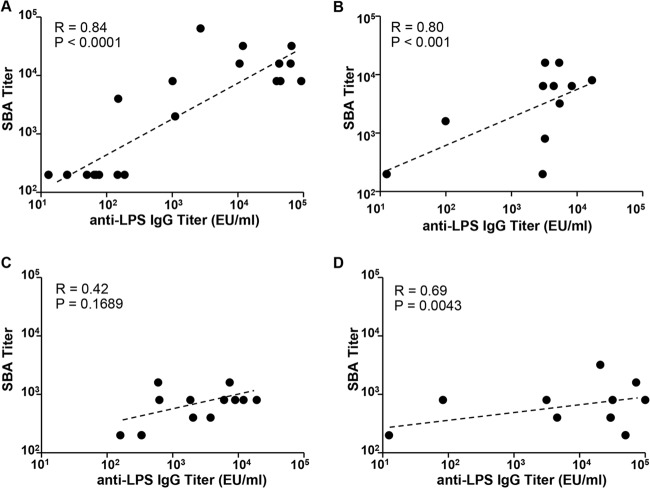
We used our optimized SBA assay (Table 2) to determine the SBA titers in individual serum samples from mice immunized with live attenuated vaccines. Serum samples from groups that received CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX), CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), CVD 910 (S. Typhi Ty2 ΔguaBA ΔhtrA), or CVD 1902 (S. Paratyphi A ΔguaBA ΔclpX) were tested against the target strains S. Typhimurium D65, S. Enteritidis S15, S. Typhi Ty2, and S. Paratyphi A ATCC 9150, respectively. As expected, significantly higher SBA titers were recorded in postvaccination serum samples than those from PBS-treated controls (Fig. 7). Furthermore, a significant correlation was seen between SBA titers and anti-LPS serum IgG titers for S. Enteritidis, S. Typhimurium, and S. Paratyphi A (Spearman's correlation coefficients, 0.80, 0.84, and 0.69; P < 0.001, 0.0001, and 0.0043, respectively) but not for S. Typhi (Spearman's correlation coefficient, 0.42; P = 0.16) (Fig. 8).
TABLE 2.
Optimized SBA assay conditions for typhoidal and nontyphoidal Salmonella
| Salmonella serovar | Growth phase | No. of CFU | % BRC |
|---|---|---|---|
| Typhimurium | Log (OD600 of 0.4) | 100–350 | 25 |
| Enteritidis | Log (OD600 of 0.4) | 100–350 | 25 |
| Typhi | Log (OD600 of 0.4) | 100–350 | 12.5 |
| Paratyphi A | Stationary | 100–350 | 12.5 |
FIG 7.
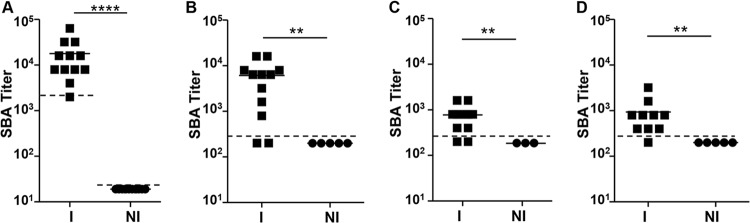
Endpoint SBA titers for mice immunized with live attenuated typhoidal and nontyphoidal Salmonella vaccines. (A) Sera from mice immunized with S. Typhimurium CVD 1931 against the target strain S. Typhimurium D65. (B) Sera from mice immunized with S. Enteritidis CVD 1944 against the target strain S. Enteritidis S15. (C) Sera from mice immunized with S. Typhi CVD 910 against the target strain S. Typhi Ty2. (D) Sera from mice immunized with S. Paratyphi A CVD 1902 against the target strain S. Paratyphi A ATCC 9150. The solid lines indicate mean titers. The dashed lines indicate the detection limit of the assay. **, P < 0.01, and ****, P < 0.0001, by the Mann-Whitney test.
FIG 8.
Correlation of SBA titer with anti-LPS serum IgG titer. SBA and anti-LPS serum titers produced by mice immunized with S. Typhimurium CVD 1931 (A), S. Enteritidis CVD 1944 (B), S. Typhi CVD 910 (C), and S. Paratyphi A CVD 1902 (D). The data were analyzed using Spearman's correlation coefficient.
Use of attenuated or reference target strains in the SBA assay.
Lastly, we investigated whether attenuated or wild-type reference strains are interchangeable for use as targets in SBA measurements. Hence, we compared antibody-dependent complement-mediated killing using S. Paratyphi A CVD 1901 (ATCC 9150 ΔguaBA) versus ATCC 9150 (wild type), S. Typhi CVD 915 (Ty2 ΔguaBA) versus Ty2 (wild type), and S. Typhimurium D23580 versus D65. CVD 1901 and CVD 915 are both attenuated strains that require exogenous guanine for growth. S. Typhimurium D23580 is a sequenced multilocus sequence type 313 (ST313) isolate and is considered the reference ST313 strain; this genotype is associated with invasive S. Typhimurium disease in sub-Saharan Africa (17, 18). Currently, little is known about invasive S. Enteritidis strains, and no reference strain exists for this serovar. We used these strains as targets in SBA assays testing pooled sera from mice immunized with S. Paratyphi A CVD 1902, S. Typhi CVD 910, or S. Typhimurium CVD 1931. The endpoint SBA titers using the alternative strains as targets were comparable to those obtained using the original wild-type strains (Fig. 9).
FIG 9.
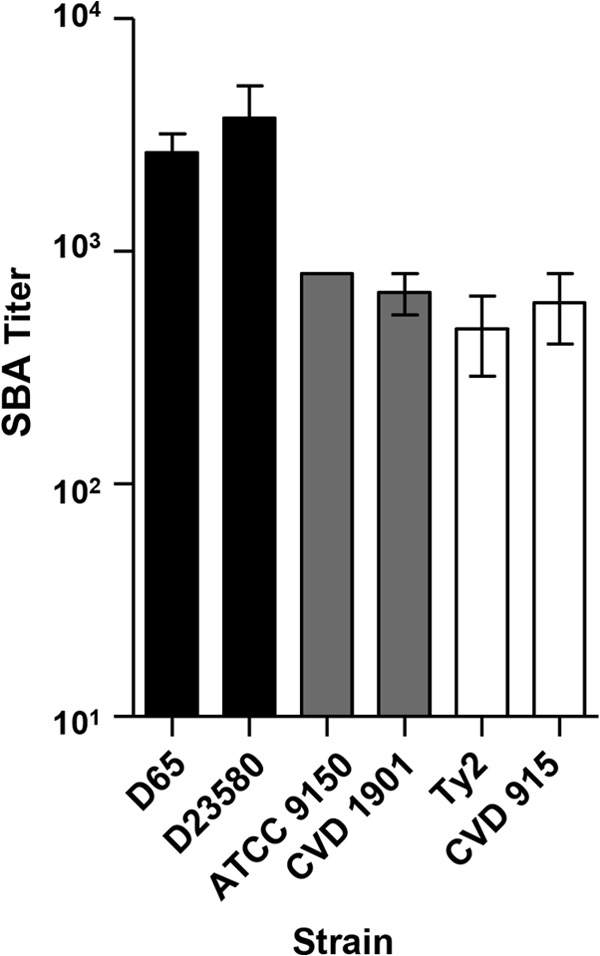
SBA titers using attenuated or reference Salmonella strains. Shown in black are the SBA titers produced by mice immunized with S. Typhimurium CVD 1931 (n = 12, pooled) using S. Typhimurium strains D65 and D23580 as target strains; in gray are the SBA titers produced by mice immunized with S. Paratyphi A CVD 1902 (n = 10, pooled) using S. Paratyphi A strains ATCC 9150 and CVD 1901 as target strains; in white are the SBA titers produced by mice immunized with S. Typhi CVD 910 (n = 12, pooled) using S. Typhi strains Ty2 and CVD 915 as target strains. The results are the mean ± standard deviation values from at least two independent experiments.
DISCUSSION
We established assays to measure SBA titers against S. Typhimurium, S. Enteritidis, S. Typhi, and S. Paratyphi A and applied these assays to measure antibodies in mice immunized with attenuated vaccine strains, with a view toward future validation for use in preclinical and clinical vaccine trials. Mice immunized with CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX) produced higher SBA titers than mice immunized with CVD 910 (S. Typhi Ty2 ΔguaBA ΔhtrA) or CVD 1902 (S. Paratyphi A ATCC 9150 ΔguaBA ΔclpX). The differences in immunization regimens likely account for these results. For example, there was a shorter immunization interval for CVD 1902 and only two immunizations for CVD 910 (at a 10-fold lower dose of 108 CFU, versus 109 CFU for other strains), as opposed to three immunizations for the other Salmonella vaccines. Differences in the handling of serum samples may have also played a role, as the functional capacities of antibodies are highly sensitive to preservation and manipulation conditions (31). Unlike SBA titers in mice immunized with CVD 1931 (S. Typhimurium), CVD 1944 (S. Enteritidis), and CVD 1902 (S. Paratyphi A), the SBA titers of mice that received CVD 910 (S. Typhi) did not correlate with serum IgG anti-LPS antibodies. The Salmonella SBA assays described here might be used to comparatively assess typhoidal and nontyphoidal Salmonella vaccines. We also intend to use them to further examine the mechanism of Salmonella serum bactericidal activity as a potential contributor to protection in vivo. With appropriate validation, these assays might provide an important tool for evaluating the immunogenicity of Salmonella vaccines in preclinical studies and clinical trials.
Although complement-dependent SBA activity was previously described in response to S. Typhi oral vaccines or wild-type infection, we report here the first SBA assays against S. Typhimurium and S. Enteritidis. Furthermore, we describe for the first time an assay format that allows one to determine the endpoint titers induced by vaccination. One lesson learned is that the component steps of the SBA assay must be tailored for different serovars. For NTS strains, the SBA assay requires bacteria grown to log phase (OD600, 0.4) and 25% BRC; for S. Typhi, bacteria are grown to log phase (OD600, 0.4) and 12.5% BRC is used; and for S. Paratyphi A, stationary-phase bacterial cultures and 12.5% BRC are optimal. In the final assay configuration, we chose the lowest concentrations of BRC that allowed us to detect killing in positive samples while limiting nonspecific killing in the absence of immune antibodies. The smallest amount of BRC possible was also sought for practical reasons (e.g., to reduce costs of testing a large number of specimens). We selected 25% BRC for the NTS SBA assays, as this was the lowest concentration that showed a significant bactericidal difference between NTS immune and nonimmune sera (Fig. 2A and B). For typhoidal Salmonella SBA assays, we chose 12.5% BRC, as this was the smallest amount that showed a significant difference in S. Typhi and S. Paratyphi A killing between immune and nonimmune sera (Fig. 2C and D).
S. Paratyphi A yielded a positive SBA result at stationary phase, because under our growth conditions, it does not produce long O antigen. In contrast, S. Typhimurium, S. Enteritidis, and S. Typhi grown to stationary phase were resistant to antibody-dependent complement-mediated killing, apparently due to the production of long O antigen or Vi (for S. Typhi only).
In our hands, BRC enhanced the growth of S. Typhimurium and S. Enteritidis but not of S. Typhi or S. Paratyphi A. S. Typhi and S. Paratyphi A either do not replicate during the assay or they replicate but then are killed by the complement, resulting in no change in the percent survival rate. In contrast, S. Typhimurium and S. Enteritidis grow significantly more in the presence of 30% BRC than in PBS. The enhanced growth might also be due to a capacity for faster growth of these serovars than for typhoidal serovars (see Fig. S1 in the supplemental material) and/or differences in nutrient utilization. NTS are well known to possess complement resistance mechanisms, and therefore, once these bacteria have grown, they are not killed by the complement.
We initially hypothesized that the combination of long O antigen and the presence of rck in S. Enteritidis strain R11 was responsible for its highly complement-resistant nature even in the presence of immune antibodies. In support of this hypothesis, conditions that resulted in long O antigen (e.g., NTS growth to stationary phase) hindered the detection of SBA, whereas SBA activity was recorded under conditions in which long O antigen was absent (log phase for NTS). Similarly, Bravo et al. (32) reported that the O-antigen length of Salmonella serovars is dependent on growth phase and influences complement resistance. Complement killing can be impeded by expression of the 17-kDa outer membrane protein encoded by the resistance to complement killing (rck) gene present in some NTS strains, which interferes with the formation of the membrane attack complex. Paradoxically, the most complement-susceptible S. Enteritidis strain, S01, is rck positive. This indicates that rck alone does not fully determine complement resistance. For example, rck-positive complement-susceptible NTS strains may not express functional Rck protein, and S. Enteritidis strain R11 likely has other non-rck complement resistance mechanisms.
Species-specific differences in complement activity have long been recognized and attributed to differences in terminal complement proteins (33–36). Our results are in agreement with these findings. While guinea pig complement readily killed Salmonella in an antibody-independent fashion, in contrast, BRC killed Salmonella in the absence of specific antibodies only at very high concentrations of complement. The precise reason for the consistency of BRC-based SBA assays is unknown, although we speculate that it might be attributed to species-specific differences in the capacity of C3b to be activated by the alternative complement pathway. With the aim of developing SBA assays that can be standardized and ultimately applied in high-throughput testing, we avoided using human complement because of the potential difficulty of finding human serum completely devoid of anti-Salmonella antibodies, based on recent reports (37). It is both time-consuming and expensive to screen for healthy donors until sufficient numbers of individuals lacking Salmonella antibodies are found. Even when these obstacles are overcome, there remains the added complication of obtaining enough complement for use in multiple studies and across laboratories. We did not attempt to use mouse complement because Siggins et al. (38) showed that mouse complement is unable to kill S. Typhimurium in the presence of human anti-Salmonella antibodies.
It is unclear from our data whether anti-Vi antibodies play a role in measured serum bactericidal activities. S. Typhi CVD 909, which expresses Vi constitutively and at a higher level than S. Typhi Ty2, was more resistant to killing than S. Typhi Ty2 in the presence or absence of immune serum. These data support previous reports that Vi contributes to serum resistance (39, 40). However, Pulickal et al. (23) found no correlation between bactericidal and anti-Vi titers in healthy Nepalese subjects, which suggests that antibodies to other components (e.g., LPS and outer membrane proteins) are responsible for SBA activity. Our data showed a positive but marginally significant correlation between anti-LPS IgG and SBA titers, suggesting the involvement of other antibodies in the observed bactericidal activity. More detailed studies will be needed to understand the mechanism of SBA activity elicited by S. Typhi vaccines and to determine the contribution of antibodies to the Vi polysaccharide, the O antigen, and outer membrane proteins to killing.
While SBA assays to assess the functionality of vaccine-induced antibodies elicited by Neisseria meningitidis capsular polysaccharide and conjugate vaccines are well established (41), these assays have been used only sporadically to assess the functional capacities of antibodies induced by Salmonella vaccines. Considering that multiple conjugate vaccines are being developed against S. Paratyphi A, S. Typhimurium, and S. Enteritidis, as well as Vi conjugates against S. Typhi, assays to monitor SBA activity will provide an invaluable tool to assist clinical vaccine development (42). Whereas the highly complement-resistant nature of serovars, like S. Typhimurium and S. Enteritidis, has been a problem, the SBA assay conditions reported here offer hope that these SBA assays will allow the immunogenicity of vaccine candidates to be monitored in a more comprehensive way, with a readout potentially related to protection. In order for the proposed SBA assay to be widely implemented in human clinical trials, safer or reference target strains will need to be used, several of which we have identified in our study. The SBA assay results that we have generated encourage further studies in order to fully standardize these assays and their application in evaluating new Salmonella vaccine candidates under clinical development, searching for functional antibody correlates of protection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Heyderman and Robert Kingsley for the kind gift of S. Typhimurium D23580.
This study was funded by the Middle Atlantic RCE Program NIAID/NIH 2 U54 AI057168 grant (M.M.L., Principal Investigator). M.A.B. was also supported by the NIH T32 AI07524 Fellowship Training Program in Vaccinology (M.M.L., Principal Investigator).
Footnotes
Published ahead of print 12 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00115-14.
REFERENCES
- 1. Crump JA. 2012. Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin. Infect. Dis. 54:1107–1109. 10.1093/cid/cis024 [DOI] [PubMed] [Google Scholar]
- 2. Buckle GC, Walker CL, Black RE. 2012. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health 2:10401. 10.7189/jogh.02.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kankwatira A, Mwafulirwa GA, Gordon MA. 2004. Non-typhoidal Salmonella bacteraemia–an under-recognized feature of AIDS in African adults. Trop. Doct. 34:198–200. 10.1177/004947550403400404 [DOI] [PubMed] [Google Scholar]
- 6. Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310–314. 10.1016/S0035-9203(00)90337-7 [DOI] [PubMed] [Google Scholar]
- 7. Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. 2007. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ 334:782. 10.1136/bmj.39118.489931.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, Barzilay EJ, Emerging Infections Program NARMS Working Group 2011. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob. Agents Chemother. 55:1148–1154. 10.1128/AAC.01333-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rondini S, Micoli F, Lanzilao L, Hale C, Saul A, Martin LB. 2011. Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin. Vaccine Immunol. 18:460–468. 10.1128/CVI.00387-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali A, An SJ, Cui C, Haque A, Carbis R. 2012. Preparation and evaluation of immunogenic conjugates of Salmonella enterica serovar Typhi O-specific polysaccharides with diphtheria toxoid. Hum. Vaccin. Immunother. 8:189–193. 10.4161/hv.18350 [DOI] [PubMed] [Google Scholar]
- 11. Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. 2000. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar Typhi oral vaccine strain CVD 909. Infect. Immun. 68:4647–4652. 10.1128/IAI.68.8.4647-4652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyon CE, Sadigh KS, Carmolli MP, Harro C, Sheldon E, Lindow JC, Larsson CJ, Martinez T, Feller A, Ventrone CH, Sack DA, DeNearing B, Fingar A, Pierce K, Dill EA, Schwartz HI, Beardsworth EE, Kilonzo B, May JP, Lam W, Upton A, Budhram R, Kirkpatrick BD. 2010. In a randomized, double-blinded, placebo-controlled trial, the single oral dose typhoid vaccine, M01ZH09, is safe and immunogenic at doses up to 1.7 × 10(10) colony-forming units. Vaccine 28:3602–3608. 10.1016/j.vaccine.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 13. Thiem V, Lin FY, Canh do G, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Schneerson R, Szu SC. 2011. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin. Vaccine Immunol. 18:730–735. 10.1128/CVI.00532-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gat O, Galen JE, Tennant S, Simon R, Blackwelder WC, Silverman DJ, Pasetti MF, Levine MM. 2011. Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines. PLoS Negl. Trop. Dis. 5:e1373. 10.1371/journal.pntd.0001373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. 2011. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect. Immun. 79:4175–4185. 10.1128/IAI.05278-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE, Levine MM. 2011. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect. Immun. 79:4240–4249. 10.1128/IAI.05484-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okoro C, Kingsley R, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 44:1215–1221. 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 19:2279–2287. 10.1101/gr.091017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin LB. 2012. Vaccines for typhoid fever and other salmonelloses. Curr. Opin. Infect. Dis. 25:489–499. 10.1097/QCO.0b013e328356ffeb [DOI] [PubMed] [Google Scholar]
- 20. Galen JE, Pasetti MF, Tennant S, Ruiz-Olvera P, Sztein MB, Levine MM. 2009. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol. Cell Biol. 87:400–412. 10.1038/icb.2009.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor PW. 1983. Bactericidal and bacteriolytic activity of serum against Gram-negative bacteria. Microbiol. Rev. 47:46–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409. 10.1086/589862 [DOI] [PubMed] [Google Scholar]
- 23. Pulickal AS, Gautam S, Clutterbuck EA, Thorson S, Basynat B, Adhikari N, Makepeace K, Rijpkema S, Borrow R, Farrar JJ, Pollard AJ. 2009. Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal. Clin. Vaccine Immunol. 16:1413–1419. 10.1128/CVI.00245-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. 2011. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect. Immun. 79:3188–3194. 10.1128/IAI.05081-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goh YS, MacLennan CA. 2013. Invasive African nontyphoidal Salmonella requires high levels of complement for cell-free antibody-dependent killing. J. Immunol. Methods 387:121–129. 10.1016/j.jim.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 26. Wang JY, Harley RH, Galen JE. 2013. Novel methods for expression of foreign antigens in live vector vaccines. Hum. Vaccin. Immunother. 9:6. 10.4161/hv.23248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benenson AS, Saad A, Mosley WH. 1968. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull. World Health Organ. 38:277–285 [PMC free article] [PubMed] [Google Scholar]
- 28. Hitchcock PJ, Brown TM. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Losonsky GA, Lim Y, Motamedi P, Comstock LE, Johnson JA, Morris JG, Jr, Tacket CO, Kaper JB, Levine MM. 1997. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin. Diagn. Lab. Immunol. 4:264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tükel Ç, Bäumler AJ. 2011. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun. 79:830–837. 10.1128/IAI.00961-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Shaughnessy CM, Cunningham AF, MacLennan CA. 2012. The stability of complement-mediated bactericidal activity in human serum against Salmonella. PLoS One 7:e49147. 10.1371/journal.pone.0049147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bravo D, Silva C, Carter JA, Hoare A, Alvarez SA, Blondel CJ, Zaldívar M, Valvano MA, Contreras I. 2008. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J. Med. Microbiol. 57:938–946. 10.1099/jmm.0.47848-0 [DOI] [PubMed] [Google Scholar]
- 33. Hänsch GM, Hammer CH, Vanguri P, Shin ML. 1981. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc. Natl. Acad. Sci. U. S. A. 78:5118–5121. 10.1073/pnas.78.8.5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwab GE, Reeves PR. 1966. Comparison of the bactericidal activity of different vertebrate sera. J. Bacteriol. 91:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gill CJ, Ram S, Welsch JA, Detora L, Anemona A. 2011. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine 30:29–34. 10.1016/j.vaccine.2011.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zollinger WD, Mandrell RE. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. 2013. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin. Vaccine Immunol. 10.1128/CVI.00289-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siggins MK, Cunningham AF, Marshall JL, Chamberlain JL, Henderson IR, MacLennan CA. 2011. Absent bactericidal activity of mouse serum against invasive African nontyphoidal Salmonella results from impaired complement function but not a lack of antibody. J. Immunol. 186:2365–2371. 10.4049/jimmunol.1000284 [DOI] [PubMed] [Google Scholar]
- 39. Hashimoto Y, Li N, Yokoyama H, Ezaki T. 1993. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella Typhi. J. Bacteriol. 175:4456–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Looney RJ, Steigbigel RT. 1986. Role of the Vi antigen of Salmonella Typhi in resistance to host defense in vitro. J. Lab. Clin. Med. 108:506–516 [PubMed] [Google Scholar]
- 41. Pace D, Pollard AJ, Messonier NE. 2009. Quadrivalent meningococcal conjugate vaccines. Vaccine 27(Suppl 2):B30–B41. 10.1016/j.vaccine.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 42. Rondini S, Lanzilao L, Necchi F, O'Shaughnessy CM, Micoli F, Saul A, MacLennan CA. 2013. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb. Pathog. 63:19–23. 10.1016/j.micpath.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 43. Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Lagos R, Nataro JP, Galen JE, Levine MM. 2008. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J. Clin. Microbiol. 46:1861–1866. 10.1128/JCM.00109-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Nataro JP, Galen JE, Levine MM. 2010. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl. Trop. Dis. 4:e621. 10.1371/journal.pntd.0000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118:1553–1562. 10.1172/JCI33998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Felix A, Pitt RM. 1951. The pathogenic and immunogenic activities of Salmonella Typhi in relation to its antigenic constituents. J. Hyg. (Lond.) 49:92–110. 10.1017/S0022172400015394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang JY, Pasetti MF, Noriega FR, Anderson RJ, Wasserman SS, Galen JE, Sztein MB, Levine MM. 2001. Construction, genotypic and phenotypic characterization, and immunogenicity of attenuated DeltaguaBA Salmonella enterica serovar Typhi strain CVD 915. Infect. Immun. 69:4734–4741. 10.1128/IAI.69.8.4734-4741.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Germanier R, Füer E. 1975. Isolation and characterization of Gal E mutant Ty 21a of Salmonella Typhi: a candidate strain for a live, oral typhoid vaccine. J. Infect. Dis. 131:553–558. 10.1093/infdis/131.5.553 [DOI] [PubMed] [Google Scholar]
- 49. Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P. 1986. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J. Infect. Dis. 154:497–503. 10.1093/infdis/154.3.497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.