Abstract
Bacillus thuringiensis has been widely used as a biopesticide, primarily for the control of insect pests, but some B. thuringiensis strains specifically target nematodes. However, nematicidal virulence factors of B. thuringiensis are poorly investigated. Here, we describe virulence factors of nematicidal B. thuringiensis DB27 using Caenorhabditis elegans as a model. We show that B. thuringiensis DB27 kills a number of free-living and animal-parasitic nematodes via intestinal damage. Its virulence factors are plasmid-encoded Cry protoxins, since plasmid-cured derivatives do not produce Cry proteins and are not toxic to nematodes. Whole-genome sequencing of B. thuringiensis DB27 revealed multiple potential nematicidal factors, including several Cry-like proteins encoded by different plasmids. Two of these proteins appear to be novel and show high similarity to Cry21Ba1. Named Cry21Fa1 and Cry21Ha1, they were expressed in Escherichia coli and fed to C. elegans, resulting in intoxication, intestinal damage, and death of nematodes. Interestingly, the effects of the two protoxins on C. elegans are synergistic (synergism factor, 1.8 to 2.5). Using purified proteins, we determined the 50% lethal concentrations (LC50s) for Cry21Fa1 and Cry21Ha1 to be 13.6 μg/ml and 23.9 μg/ml, respectively, which are comparable to the LC50 of nematicidal Cry5B. Finally, we found that signaling pathways which protect C. elegans against Cry5B toxin are also required for protection against Cry21Fa1. Thus, B. thuringiensis DB27 produces novel nematicidal protoxins Cry21Fa1 and Cry21Ha1 with synergistic action, which highlights the importance of naturally isolated strains as a source of novel toxins.
INTRODUCTION
Bacillus thuringiensis is a Gram-positive, spore-forming bacterium which is extensively used for biological control of insects and nematodes (1, 2). B. thuringiensis produces an array of virulence factors that contribute to its pathogenic effect. These virulence factors include exotoxins, extracellular proteases, enhancins, chitinase, and collagenase, which breach the epithelial cells of the insect gut (3). However, the major virulence factors of B. thuringiensis (4) are pesticidal proteins called Cry and Cyt produced during sporulation as a crystal inclusions.
These crystal proteins are pore-forming toxins lethal to insects and nematodes but nontoxic to vertebrates, which makes B. thuringiensis a safe and an effective pesticide that has been successfully used for many years (1, 4). While annotated Cry toxins are quite diverse in terms of sequence similarity (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/toxins2.html), they exhibit specific activity against insects of the orders Lepidoptera, Diptera, Coleoptera, Hymenoptera, Homoptera, Orthoptera, and Mallophaga (4), and they are also toxic to nematodes (5, 6). In contrast, Cyt toxins have shown mainly dipteran specificity, being able to kill mosquitoes and black flies (7). However, considering the diversity and amount of nematode species in soil, which is also ubiquitously inhabited by B. thuringiensis, nematodes also have to be considered the target for B. thuringiensis and its toxins (5). In support of this, several families of Cry proteins (Cry5, Cry6, Cry12, Cry13, Cry14, Cry21, and Cry55) were shown to be toxic to a number of free-living and parasitic nematodes (5, 8), but the full spectrum of nematicidal Cry toxins as well as their host targets is far from completion. Also, considering the growing problem of pest resistance to existing toxins, there is a high demand for new toxins (9).
Naturally isolated Bacillus strains have often been used as a source of new Cry toxins (see, for example, reference 10). Given that B. thuringiensis and nematodes coexist and coevolve in the natural environment, they undergo reciprocal changes, one of which is increased pathogen virulence (11). Therefore, naturally isolated B. thuringiensis strains may serve as a reservoir of novel Cry toxins, some of which may be used for the production of biological pesticides. In support of this, we have previously isolated several nematicidal Bacillus strains from environmental samples (12). One of the strains, B. thuringiensis DB27, was isolated from dung beetles and exhibits extreme toxicity to the model nematode Caenorhabditis elegans (12). Previously, we investigated C. elegans transcriptional response to B. thuringiensis DB27 infection (13) and elucidated the mechanisms of C. elegans resistance (14). However, the molecular mechanisms of the high virulence of B. thuringiensis DB27 for C. elegans are still unknown.
Here, we show that B. thuringiensis DB27 produces novel plasmid-encoded Cry protoxins (Cry21Fa1 and Cry21Ha1), which act synergistically to kill free-living and animal-parasitic nematodes via intestinal damage. We determined the 50% lethal concentrations (LC50s) for Cry21Fa1 (13.6 μg/ml) and Cry21Ha1 (23.9 μg/ml) and found that they are comparable to the LC50 of nematicidal Cry5B. Additionally, we show that C. elegans conserved pathways provide protection against multiple pore-forming toxins.
MATERIALS AND METHODS
Bacterial and nematode strains.
The following strains were provided by the Caenorhabditis Genetics Center (University of Minnesota): C. elegans wild-type Bristol (N2), Oscheius carolinensis, Pelodera strongyloides, and Panagrellus redivivus. C. elegans pmk-1(km25), jun-1(gk551), kgb-1(um3), xbp-1(zc12), bre-2(ye31), and bre-3(ye26) mutants were also used in this study. Nematodes were maintained on nematode growing medium (NGM) agar plates with Escherichia coli OP50 as a food source and stored at 20°C. Strongyloides papillosus was maintained as described previously (15) and was kindly provided by Adrian Streit. B. thuringiensis DB27 was isolated by our group (12). Its plasmid-cured strain was generated in the current study. E. coli JM103 and protein expression vector pQE9 were provided by Raffi Aroian.
Nematode killing assays. (i) Vegetative cells.
B. thuringiensis DB27 was grown overnight in a shaking incubator at 30°C in Luria-Bertani (LB) broth. A 40-μl volume of the culture was spread to the edges of 6-cm-diameter NGM plates, and plates were incubated for 12 to 14 h at 25°C before the assay. A total of 20 adult worms were placed into each plate in three to six independent replicates and were monitored for survival. Every 6 h (before bacteria on a plate started sporulation), worms were transferred to freshly prepared plates to ensure exposure to vegetative cells. Microscopy was used to monitor the state of bacteria on a plate.
(ii) Mixture of vegetative cells and spores.
B. thuringiensis DB27 was grown overnight in a shaking incubator at 30°C in Luria-Bertani (LB) broth. An 80-μl volume of the culture was spread to the edges of 6-cm-diameter NGM plates, and plates were incubated for 24 h at 25°C before the assay. A total of 20 adult worms were placed into each plate in three to six independent replicates and were monitored for survival.
(iii) Spores.
B. thuringiensis DB27 was grown overnight in a shaking incubator at 30°C in Luria-Bertani (LB) broth. An 80-μl volume of the culture was spread to the edges of 6-cm-diameter NGM plates, and plates were incubated for 24 h at 25°C before the assay. A total of 20 adult worms were placed into each plate in three to six independent replicates and were monitored for survival. Survival assays were repeated multiple times and conducted at 25°C.
Chemotaxis assays.
Chemotaxis assays were modified from previous studies (16). Briefly, 25 μl of overnight B. thuringiensis DB27 suspension was placed 0.5 cm away from the edge of a 6-cm-diameter petri dish filled with NGM. The same amount of E. coli OP50 was placed on the opposing side and acted as the counterattractant. Approximately 50 to 200 J4/adult-stage C. elegans individuals were placed between the two bacterial spots. All nematodes used were previously fed on E. coli OP50. Plates were then sealed with Parafilm and stored at room temperature in the dark. After defined periods, the number of nematodes found in each bacterial spot was recorded. A chemotaxis index was used to score the response of the nematodes and was calculated as follows: number of nematodes in the test bacteria − number of nematodes in control bacteria/total number of nematodes counted (16). This gave a chemotaxis score ranging from −1.0 (total repulsion from test bacteria) to 1.0 (total attraction toward test bacteria). A score of around 0 means there were equal numbers of nematodes in all bacterial spots. Five plates were used per replicate, and the procedure was repeated five times.
Pulse-chase experiment.
Pulse-chase experiments were conducted to find the minimum time required for B. thuringiensis DB27 to establish infection in C. elegans. Plates for pulse-chase experiments were prepared in the same way as for the nematode killing assay (mixture of vegetative cells and spores). Larval stage 4 (L4)-synchronized worms were exposed to B. thuringiensis DB27 for a defined period of time, washed five times with phosphate-buffered saline (PBS) buffer to remove surface bacteria, and then shifted to E. coli OP50 plates. Survival was scored after 24 h.
Plasmid isolation, gel electrophoresis, and plasmid curing.
Plasmids of B. thuringiensis DB27 were extracted following the protocol of Reyes-Ramírez and Ibarra (17). Plasmids were resolved using 0.7% agarose gel, following a previously published protocol (18). Plasmid curing was performed by growing B. thuringiensis DB27 at 42°C with small amounts (0.0002%) of SDS in culture medium. Plasmid-cured derivatives were selected based on changes in colony morphology after plating on LB plates and verified by plasmid profiling.
Coomassie stain and EM of crystals.
B. thuringiensis sporulation medium (the recipe can be found at http://www.bgsc.org) was used to produce large amounts of spore-crystal mixtures. B. thuringiensis DB27 spore-crystal mixtures were collected by centrifugation and washed three times with 1 M NaCl and ice-cold distilled water. The washed spore-crystal mixtures were resuspended in 1 ml of distilled water, and 10 μl of each sample was dropped onto a glass slide. Samples were fixed in 1% OsO4, air-dried overnight, and then coated with gold. The scanning electron microscopy (SEM) observation was conducted on a Hitachi S-800 microscope (Hitachi, Japan), following the instructions for the device. For light microscopy, spore-crystal mixtures were spread on a glass slide, heat fixed, stained with Coomassie blue (0.133% Coomassie blue–50% acetic acid), and observed under ×100 magnification using immersion oil.
Solubilization and SDS-PAGE profiling of crystal proteins.
Spore-crystal mixtures were collected and washed as described above. The spore-crystal pellet was resuspended in solubilization buffer (50 mM Na2CO3, 25 mM dithiothreitol [DTT], pH 10.5) and incubated at 37°C for 2 h. Insoluble remainings were removed by centrifugation, and the solubilized proteins from the supernatant were analyzed using SDS-PAGE.
Toxin cloning, protein expression, killing assay, and synergism assays.
The Cry21 genes were PCR amplified from genomic DNA of B. thuringiensis DB27, digested with BamHI restriction enzyme, and ligated into the BamHI site of expression vector pQE9, generating pQE9(Cry21) plasmids for expression of His-tagged proteins. Recombinant plasmids were electroporated into E. coli JM103, which is used for Cry toxin expression (5). Bacteria were grown at 37°C to midlog phase (optical density at 600 nm [OD600] = 0.6 to 0.8), and expression of Cry21 was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). After induction, bacteria were grown at 30°C for 4 h and then 30 μl of bacterial culture was spread in the center of enriched nematode growth (ENG) plates (19) containing 1 mM IPTG (ENG-IC plates). Plates were incubated overnight at 25°C and then used for nematode toxicity assays. Synchronized nematodes at the adult stage (20 per plate) were transferred to toxicity plates and monitored for survival and intoxication. Nematodes were transferred to fresh plates every day and were considered dead when they failed to respond to touch. E. coli JM103 transformed with empty pQE9 vector was used as a control. The expression of Cry21 protoxin was verified by SDS-PAGE.
For synergism assays, E. coli protoxin-expressing cultures were grown and induced as described above. After induction, the OD600 of each culture, including the empty vector control, was measured and adjusted to 2.0. To determine synergism between two proteins, the respective protoxin-expressing E. coli cultures were mixed in equal amounts (100 μl plus 100 μl) and 30 μl of bacterial culture was spread in the center of ENG plates and used for toxicity assays. Given that the final amount of protoxin-expressing E. coli in synergism plates was 50%, empty-vector E. coli culture mixed with each of the protoxin-expressing E. coli cultures (Cry21 plus vector) was used for comparison and each was termed a single protoxin treatment. When all three protoxins were used, three E. coli cultures were mixed in equal amounts.
Protein purification.
Bacteria were grown at 37°C to midlog phase (OD600 = 0.6 to 0.8), and then IPTG was added at 1 mM final concentration to induce the expression of Cry21Fa1. Since Cry21Fa1 yield is higher at lower temperatures, after IPTG was added, the temperature was reduced to 25°C. Expression was carried out during a 10-h time period, and then cells were harvested by centrifugation (6,328 × g for 20 min). Cry21Ha1 was expressed similarly with the exception that expression was carried out during a 6-h time period at 30°C. The bacterial pellet was resuspended in buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.5 mM β-mercaptoethanol, 4 mM MgCl2, a protease inhibitor mix [Roche], phenylmethylsulfonyl fluoride [PMSF], DNase I), sonicated, and centrifuged at 35,000 rpm for 1 h at 4°C using an ultracentrifuge (Beckman). The supernatant was applied onto a nickel-nitrilotriacetic acid (Ni-NTA) column (GE Healthcare). Bound protein was eluted from the column with a linear gradient of 0 to 0.5 M imidazole in a buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.5 mM β-mercaptoethanol). Fractions were analyzed by SDS-PAGE and Western blotting using anti-His antibodies. Cry21-containing fractions were pooled, dialyzed against buffer (20 mM Tris-HCl, 10 mM NaCl, 0.5 mM β-mercaptoethanol), and loaded on an anion exchange column (Mono Q; GE Healthcare). A linear gradient of 0 to 3 M KCl in the loading buffer was used to elute bound Cry21. The Cry21 fractions were identified by SDS-PAGE and Western blotting, pooled, and dialyzed against 20 mM Tris-HCl–150 mM NaCl–0.5 mM β-mercaptoethanol buffer. The final step was size exclusion chromatography (Superdex 200; GE Healthcare) performed using 20 mM Tris-HCl–150 mM NaCl–0.5 mM β-mercaptoethanol. Fractions were analyzed by SDS-PAGE and Western blotting, pooled, and concentrated. Western blotting was performed with standard procedures as described elsewhere (20). Briefly, protein samples were separated in 8% SDS-PAGE gels and then transferred to nitrocellulose membranes (GE Healthcare). The membrane was incubated overnight with a 1:1,000 dilution of anti-His antibodies. Horseradish peroxidase-conjugated secondary antibody was used at 1:5,000. The signal was visualized with enhanced chemiluminescence (Bio-Rad).
Liquid assay with purified Cry21 protoxins.
Purified protoxins were used for C. elegans single-well toxicity assays as described in reference 19 to determine the effect of known concentrations of protoxin on single nematodes. L4 nematodes were individually placed into the wells of 96-well microtiter plates. Each well contained S medium (prepared as described in reference 19), 3 μl of a saturated OP50 culture as a standard food, the desired dose of purified Cry21 protoxin, and 2 μl of 8 mM FUDR (5-fluorodeoxyuridine). The final volume in each well was 120 μl. Wells containing buffer instead of protoxin were used as a control. To calculate LC50 values, L4 C. elegans hermaphrodites were subjected to a single-well assay and incubated in a humid chamber for 5 days at 25°C. Nematodes that did not show movement when touched were considered dead. Experiments were repeated at least three times per dose, and representative data were used to generate a semilog plot. The fraction of dead worms was plotted as a function of protoxin concentration using a semilog plot. Dead or intoxicated nematodes were not observed in buffer-containing control wells. The data were fitted to a line by the least-squares method, and LC50 was calculated from the line fit. Probit analysis (Minitab) was used to calculate 95% fiducial limits. The data (total number dead/number tested) are as follows: for Cry21Fa1, 22/24 at 58 μg/ml, 20/30 at 29 μg/ml, 20/48 at 14.5 μg/ml, 10/30 at 7.25 μg/ml, 6/36 at 3.1 μg/ml, and 3/36 at 1.6 μg/ml; and for Cry21Ha1, 22/24 at 80 μg/ml, 16/26 at 40 μg/ml, 10/28 at 20 μg/ml, 8/32 at 10 μg/ml, and 4/32 at 5 μg/ml.
To determine the potential synergism between Cry21Ha1 and Cry21Fa1 protoxins, nematodes were exposed to different protein ratios (1:1, 1:2, and 2:1) of Cry21Ha1/Cry21Fa1 mixtures in a single-well assay and observed LC50 values were calculated as described above. The theoretical (expected) LC50 values were calculated according to Tabashnik's equation (21), assuming a simple additive effect. The theoretical LC50 value is the harmonic mean of the intrinsic LC50 values of the components weighted by the ratio used in the mixture. The synergism factor (SF) was calculated by dividing the expected toxicity by the observed toxicity of the mixture. SF values greater than 1 indicate synergism.
Statistical analysis.
Kaplan-Meier nonparametric comparison and a log-rank test (Minitab) were used for statistical analysis of survival curves based on the number of survivors at the sampled time points. In cases where multiple replicates were examined in one experiment, the average survival rate at each time point was determined. Bonferroni correction was applied when multiple comparisons were performed. Statistical significance was set at P ≤ 0.05. Log-rank statistical analysis of survival curves is shown in Table S1 in the supplemental material.
Nucleotide sequence accession numbers.
The nucleotide sequences for Cry21Fa1, Cry21Ga1, and Cry21Ha1 in strain DB27 have been deposited in the GenBank database (accession no. KF701307, KF771885, and KF771886, respectively) and designated Cry21Fa1, Cry21Ga1, and Cry21Ha1 by the Bacillus thuringiensis Toxin Nomenclature Committee.
RESULTS
B. thuringiensis DB27 kills C. elegans via intestinal damage.
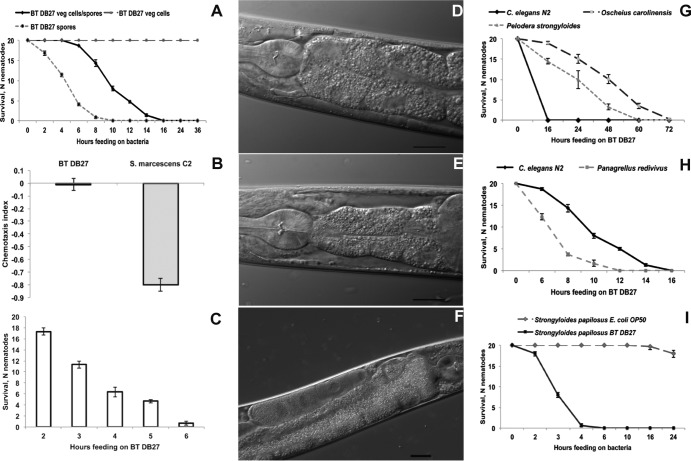
The B. thuringiensis DB27 strain was isolated previously and potentially uses novel virulence factors, since C. elegans bre mutants resistant to B. thuringiensis Cry5B toxin are susceptible to B. thuringiensis DB27 (12). B. thuringiensis DB27 showed remarkable toxicity for C. elegans, killing 100% of worms in just 16 h (Fig. 1A) (12, 14). Microscopic examination of the bacterial culture used in this assay revealed the presence of a mixture of vegetative cells and spores (see Fig. S1A in the supplemental material). To elucidate which stages of B. thuringiensis DB27 are virulent to nematodes, the assay plates were prepared with pure vegetative cells or spores for verification by microscopy (see Fig. S1B and C). Interestingly, survival of the worms was not affected on pure vegetative cells (Fig. 1A) even after 36 h, while pure spores showed toxicity even higher than that seen with the mixture of spores and vegetative cells (Fig. 1A). These findings suggest that virulence factors are produced during sporulation but not by vegetative cells. Although the spores were the most virulent, we used in all subsequent assays mixed cultures of spores and vegetative cells to prevent starvation (22), which might affect the outcome of the assay.
FIG 1.
B. thuringiensis DB27 (BT DB27) kills diverse nematodes via intestinal infection. (A) C. elegans survival on monoxenic cultures of B. thuringiensis DB27. Pure spores of B. thuringiensis DB27 are significantly (P < 0.0001) more toxic to C. elegans than a mixture of spores and vegetative cells. Vegetative (veg) cells alone are not virulent. (B) B. thuringiensis DB27 did not repel C. elegans in a chemotaxis assay, in contrast to Serratia marcescens, used as a positive control. (C) C. elegans survival after a short exposure to B. thuringiensis DB27 (pulse-chase). The x axis shows the pulse (time of exposure to the pathogen). The y axis shows the number of nematodes (scored 24 h postinfection) that recovered after a given pulse. (D to F) C. elegans intestinal changes caused by B. thuringiensis DB27. Compared to control worms on OP50 (D), worms exposed to B. thuringiensis DB27 exhibit intestinal shrinkage (E) and accumulation of bacteria in the gut (F). Bar, 20 μm. (G to I) Other nematodes show levels of susceptibility that differ from that of B. thuringiensis DB27. (G) O. carolinensis and P. strongyloides are more resistant (P < 0.0001) to B. thuringiensis DB27-mediated killing than C. elegans. (H) P. redivivus is significantly (P < 0.001) more susceptible than C. elegans. (I) Animal-parasitic nematode S. papillosus is killed rapidly by B. thuringiensis DB27 compared to control E. coli OP50. For survival curves, the number of worms alive (N nematodes) is plotted as a function of time. The data shown are means ± standard errors of the means.
Additionally, we noticed that C. elegans did not avoid B. thuringiensis DB27 in the chemotaxis assay, showing equal preferences for OP50 and B. thuringiensis DB27 (Fig. 1B).
To study whether B. thuringiensis DB27 is able to establish infection in C. elegans, we performed pulse-chase experiments, where worms were exposed to B. thuringiensis DB27 for a defined period of time and then shifted to E. coli OP50. We found that a 6-h pulse was sufficient to establish a lethal infection in nearly 100% of worms (Fig. 1C). After 3 h, nearly 40% of worms were already infected, suggesting that B. thuringiensis DB27 relies on an active infection process as part of its virulence mechanism. This is further supported by our microscopy observations. Compared to control worms on E. coli OP50 (Fig. 1D), B. thuringiensis DB27-infected worms exhibited strong intestinal changes, namely, intestinal shrinkage and dissociation from body walls (Fig. 1E). Furthermore, we could also show a dramatic accumulation of B. thuringiensis DB27 spores and cells in the C. elegans intestine (Fig. 1F), which is consistent with the pulse-chase experiments and confirms that B. thuringiensis DB27 relies on an active infection process. Similar levels of intestinal destruction and accumulation of spores in the intestine were reported previously for another nematicidal B. thuringiensis isolate (23). Together, these results show that B. thuringiensis DB27 infects the C. elegans intestine, leading to intestinal damage and subsequent death of the nematode.
B. thuringiensis DB27 is pathogenic to diverse nematodes.
To determine the specificity of B. thuringiensis DB27 virulence, we exposed several other nematodes to the pathogen. As shown in Fig. 1G and H, the nematodes Oscheius carolinensis, Pelodera strongyloides, and Panagrellus redivivus were also killed by B. thuringiensis DB27, although they showed different degrees of susceptibility. While O. carolinensis and P. strongyloides were more resistant to B. thuringiensis DB27 than C. elegans (Fig. 1G), P. redivivus was significantly more susceptible (Fig. 1H). In addition, we found that the free-living stage of the animal-parasitic nematode Strongiloides papillosus was also highly susceptible to B. thuringiensis DB27, being killed in as little as 4 h (Fig. 1I). Thus, B. thuringiensis DB27 is toxic to a number of free-living and also animal-parasitic nematodes, indicating its potential application as a nematicidal agent.
Role of plasmids in B. thuringiensis DB27 virulence.
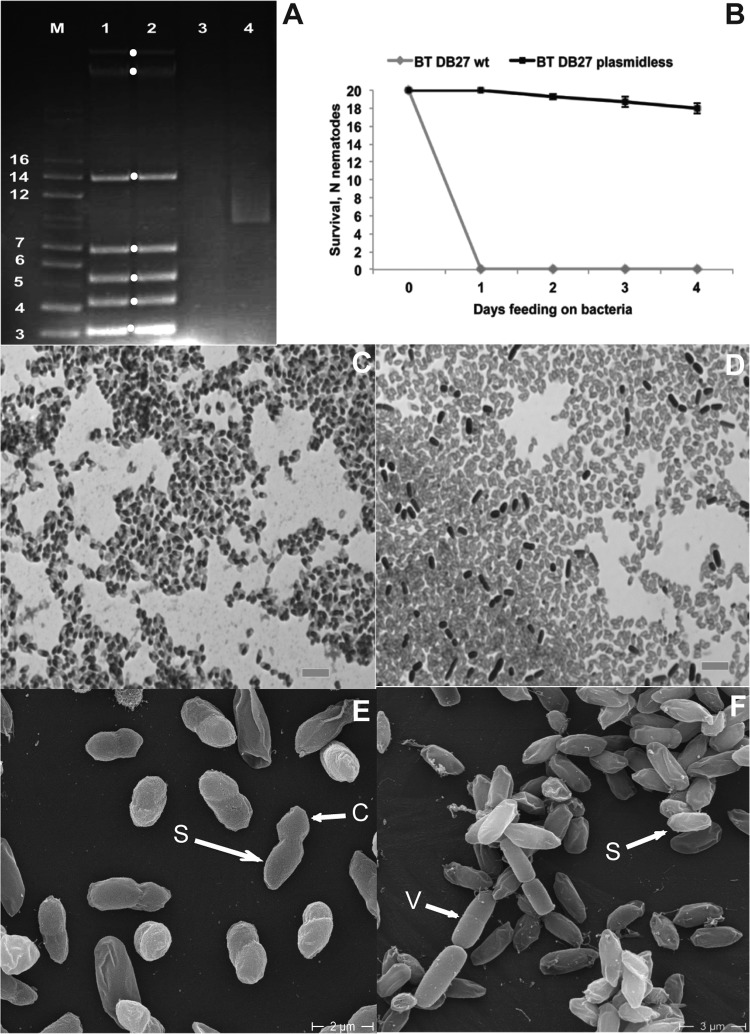
Given that B. thuringiensis major virulence factors are often encoded by plasmids (24), we next explored the role of B. thuringiensis DB27 plasmids in pathogenicity against nematodes. First, we extracted and gel-separated plasmids of B. thuringiensis DB27 and found that it harbored seven plasmids, ranging in size from 3 to above 16 kilobases (Fig. 2A). Next, we generated several plasmid-cured variants of B. thuringiensis DB27, some of which lost all seven plasmids (Fig. 2A). When we exposed C. elegans to those plasmid-cured variants, we found that they lost their virulence completely (Fig. 2B), suggesting that nematicidal factors are encoded by plasmids.
FIG 2.
B. thuringiensis DB27 virulence factors are plasmid-associated Cry protoxins. (A) Plasmid profile of B. thuringiensis DB27 (lanes 1 and 2) and its plasmid-cured derivative (lanes 3 and 4). B. thuringiensis DB27 has seven plasmids (marked with dots), while the plasmid-cured variant lost all of them. M, marker (kb). Considering that the marker is represented by linear DNA fragments and the state of the plasmids (linear, circular, or supercoiled) is not known, it cannot be used as a determinant of the exact plasmid size. (B) C. elegans survival on the B. thuringiensis DB27 plasmid-cured variant is not affected (P < 0.0001) compared to that of the wild-type (wt) strain. (C and D) Light microscopy images (100×) of Coomassie-stained spore-crystal mixtures of wild-type B. thuringiensis DB27 (C) and its plasmidless derivative (D). Crystals (stained in black in panel C) show a strong association with the spore (SCA phenotype). No crystal inclusions are formed by plasmid-cured derivative. Rod-like black structures not attached to spores are vegetative cells. Bars, 100 μm. (E and F) Scanning electron microscopy images of spore-crystal mixtures of wild-type B. thuringiensis DB27 (E) and its plasmidless derivative (F). Arrows point to spores (S), crystals (C), and vegetative cells (V).
B. thuringiensis Cry toxins are almost exclusively encoded by plasmids (24) and are therefore potential nematicidal candidates produced by B. thuringiensis DB27. To explore that idea further, we tested whether B. thuringiensis DB27 produces Cry toxins. Light microscopy of Coomassie-stained spore-crystal mixtures indicated the presence of Cry protein crystals in B. thuringiensis DB27 (Fig. 2C) but not in a plasmid-cured derivative (Fig. 2D). Interestingly, crystals showed a strong association with spores, the phenotype previously described as spore-crystal association (SCA) (25). SEM images confirmed this phenotype (Fig. 2E) and showed striking similarity to the SCA of a rare filamentous B. thuringiensis strain (26). In contrast to wild-type B. thuringiensis DB27, the plasmid-cured variant did not produce any Cry protein crystals, as shown in light microscopy (Fig. 2D) and scanning EM images (Fig. 2F). To further confirm this phenotype, we solubilized proteins from spore-crystal mixtures of B. thuringiensis DB27 and its plasmid-cured variant and resolved them in an SDS-PAGE gel. While the B. thuringiensis DB27 profile revealed a dominant protein of around 130 kDa (see Fig. S2 in the supplemental material), which corresponds to the size of some Cry protoxins, the profile of the plasmid-cured variant showed no proteins at all (see Fig. S2). These findings confirm our microscopy results showing that the plasmid-cured variant does not produce Cry protoxins. Together, these results indicate that B. thuringiensis DB27 virulence factors are plasmid-encoded Cry protoxins, which also agrees with the fact that sporulating cultures show high virulence activity (Fig. 1A). Consistent with this, vegetative cells that did not kill nematodes (Fig. 1A) did not produce any Cry proteins, as verified by SDS-PAGE (see Fig. S3); spores that showed the highest virulence also yielded the largest amount of Cry proteins (see Fig. S3); and spore/vegetative cell mixtures showed an intermediate level of killing and an intermediate amount of Cry proteins (see Fig. S3). Thus, there is a correlation between the amounts of Cry protoxins produced by different stages and the levels of nematode lethality.
Candidate virulence factors identified by genome sequencing.
To gain further insight into B. thuringiensis DB27 virulence factors, we sequenced the genome of this bacterium (27) and analyzed the genome sequence for the presence of potential virulence factors. Multiple proteases, enterotoxins, cytotoxins, collagenase, and chitinase were found (see Table S2 in the supplemental material). However, neither Cyt nor Vip (vegetative insecticidal protein) toxins implicated in B. thuringiensis insecticidal activity (7, 28) were found in the B. thuringiensis DB27 genome. Besides the circular chromosome, whole-genome sequencing revealed the presence of seven plasmids, ranging in size from 4 to 200 kb (See Table S3 in the supplemental material), which agrees with the observed plasmid profile (Fig. 2A). Given that our results suggest the nematicidal factors to be plasmid-encoded Cry protoxins, we concentrated specifically on plasmid-encoded factors. Indeed, we found several Cry-like toxins belonging to nematicidal families to be located on plasmids (See Table S3). Specifically, the 200-kb plasmid harbored a Cry-like toxin which showed similarity to the Cry21Ba1 toxin. In addition, the 8-kb and 6-kb plasmids also carried Cry-like toxins, both of which are similar to Cry21Ba1. Although a BLAST search identified sequence similarity of all three toxins to Cry21Ba1 toxin, more-detailed sequence comparisons revealed that all three proteins were different and that only the C-terminal part was conserved (see Fig. S4). Considering the low degree of sequence similarity to known Cry toxins, all three proteins were identified by the Cry toxin nomenclature committee as novel and were assigned new official names (See Table S3). Based on their sequence similarity, the newly identified Cry21 proteins are potentially nematicidal. However, they probably differ in the sensitivity spectrum of nematodes and the extent of toxicity.
Novel Cry21 protoxins show synergistic nematicidal activity.
To test the potential role of these proteins in toxicity to C. elegans, we cloned them individually into the unique BamHI restriction site of the pQE9 E. coli protein expression vector. E. coli transformed with pQE9-Cry21 plasmids produced a Cry protein of above 130 kDa (not shown), which corresponds to the size of some Cry protoxins. This also suggests that the 130-kDa protein from the B. thuringiensis DB27 spore-crystal mixture corresponds to Cry21 protoxins, given the similarities in size.
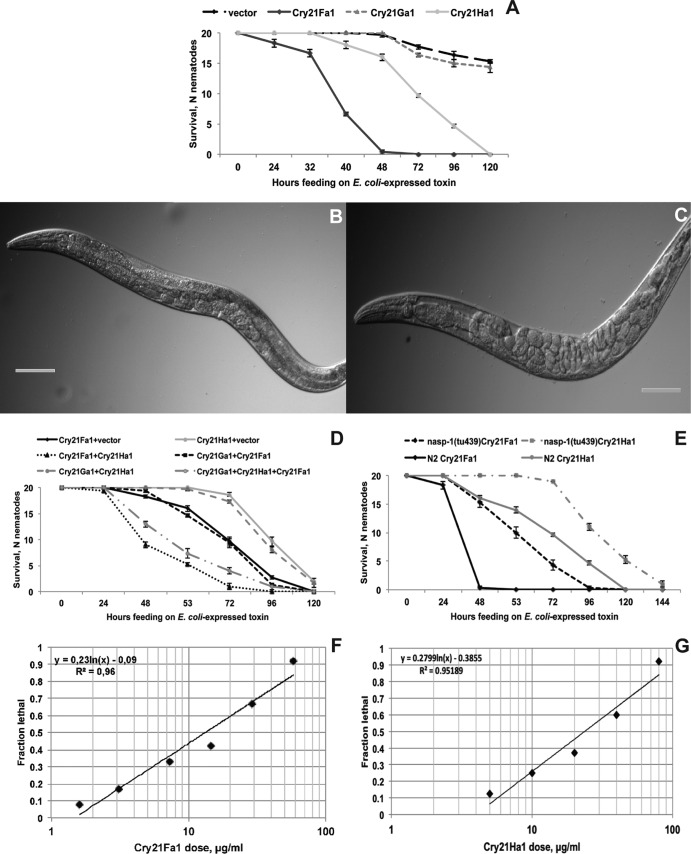
When we fed C. elegans with E. coli expressing the three protoxins individually, we observed different degrees of intoxication (Fig. 3A). Cry21Fa1 was the most effective protoxin, killing worms in around 48 h. Cry21Ha1 showed moderate toxicity and caused 100% lethality in 5 days. In contrast, Cry21Ga1 did not show any obvious toxicity to C. elegans even after 5 days (Fig. 3A). Note that longer exposure times were not feasible in these experiments since even E. coli with empty vector is toxic to C. elegans on rich medium such as ENG. Worms fed with Cry21Fa1 or Cry21Ha1 protoxin exhibited classical intoxication phenotypes, such as slow movement, pale appearance, reduction in body size, and, finally, death of the worms. Additionally, in contrast to nematodes fed on E. coli with an empty vector (Fig. 3B), worms exposed to the E. coli-expressed protoxin exhibited intestinal shrinkage and damage (Fig. 3C), in similarity to worms fed with B. thuringiensis DB27 (Fig. 1E).
FIG 3.
Cry21 protoxins are the nematicidal virulence factors of B. thuringiensis DB27. (A) C. elegans survival on E. coli which expresses individual Cry21 protoxins. Cry21Fa1 and Cry21Ha1 significantly (P < 0.0001) reduce C. elegans survival compared to the vector control. The Cry21Ga1 effect is similar (P > 0.05) to that of the vector control. (B and C) C. elegans fed with E. coli-expressed Cry21Fa1 protoxin exhibits dramatic intestinal shrinkage and destruction (C) compared to vector-fed control worms (B). Bars, 100 μm. (D) C. elegans survival upon exposure to combinations of E. coli clones that express different Cry21 protoxins. C. elegans survival is significantly (P < 0.0001) reduced when worms are exposed to the combination of Cry21Fa1 and Cry21Ha1 protoxins compared to exposure to each toxin individually. Combining Cry21Ga1 with either Cry21Fa1 or Cry21Ha1 does not significantly (P > 0.05) change the survival compared to that seen with the individual protoxins. The combination of all three proteins is almost as toxic as the combination of Cry21Fa1 and Cry21Ha1, suggesting that Cry21Ga1 has no synergistic effect. (E) The nasp-1 mutant is significantly more resistant to Cry21Fa1 (P < 0.0001) and Cry21Ha1 (P < 0.001) protoxins than the wild-type strain. The data shown are means ± standard errors of the means. (F and G) C. elegans dose-dependent lethality to purified Cry21Fa1 (F) and Cry21Ha1 (G) protoxins in a liquid assay. A semilog plot of animals that died versus the concentration of toxin is shown. The data were fitted to a line by the least-squares method. The LC50s for Cry21Fa1 (13.6 μg/ml) and for Cry21Ha1 (23.9 μg/ml) were calculated from the line fits. The data can be found in Materials and Methods.
Given that Cry toxins often show synergistic action (29), we next fed C. elegans with mixtures of E. coli clones expressing different protoxins. As shown in Fig. 3D, combining Cry21Fa1 and Cry21Ha1 protoxins resulted in significantly higher C. elegans mortality compared to that seen with the single protoxins, suggesting that the two protoxins might act synergistically. A Cry21Ga1 combination with Cry21Fa1 and/or Cry21Ha1 did not increase C. elegans lethality compared to single protoxins (Fig. 3D), indicating that Cry21Ga1 (alone or in combination with other protoxins) is not involved in C. elegans killing. Interestingly, the C. elegans nasp-1(tu439) mutant, which is resistant to B. thuringiensis DB27-mediated killing, also exhibits increased resistance to the Cry21Fa1 and Cry21Ha1 protoxins (Fig. 3E). Thus, Cry21Fa1 and Cry21Ha1 are important nematicidal factors produced by B. thuringiensis DB27 that show potential synergistic action.
Quantitative effect of Cry21Fa1 and Cry21Ha1 on C. elegans.
To quantify Cry21 protoxin actions, Cry21Fa1 and Cry21Ha1, which showed toxicity to C. elegans in a feeding experiment (Fig. 3A), were purified as a His-tagged proteins using affinity chromatography, verified by Western blotting (see Fig. S5 in the supplemental material), and used to intoxicate C. elegans in liquid assays. The purified protoxins showed clear dose-dependent action (Fig. 3F and G and Materials and Methods). While 58 μg/ml of Cry21Fa1 protoxin was sufficient to kill 100% of worms in 5 days, the estimated concentration of Cry21Fa1 that kills 50% of worms is 13.6 μg/ml (calculated LC50). For Cry21Ha1, the LC50 is 23.9 μg/ml. Thus, Cry21Fa1 is almost twice as toxic as Cry21Ha1, which agrees with the results of the feeding experiment (Fig. 3A). While these values are very close to the LC50 of another nematicidal toxin, Cry5B (12.6 μg/ml) (30), direct comparison is not possible due to experimental differences in the toxin purification. When Cry21Ha1 and Cry21Fa1 protoxins were combined at different ratios (Table 1), all the observed LC50 values of protoxin mixtures were lower than the expected LC50 values and the mixtures exhibited clear synergistic activity. Specifically, the Cry21Fa1/Cry21Ha1 combination with a ratio of 2:1 exhibited the best synergistic activity (LC50 6.1 μg/ml), representing a 2.6 reduction in the LC50 value compared to the expected LC50 (15.88 μg/ml) (Table 1).
TABLE 1.
Toxicity to C. elegans of Cry21Fa1 and Cry21Ha1 single protoxins and their mixtures
| Cry21Fa1/Cry21Ha1 ratio | LC50 (μg/ml) |
Synergism factorc | |
|---|---|---|---|
| Observeda | Expectedb | ||
| 1:0 | 13.6 (10.07–19.6) | ||
| 0:1 | 23.9 (18.07–33.25) | ||
| 1:1 | 8.7 (6.21–11.98) | 17.33 | 1.99 |
| 1:2 | 7.98 (4.75–12.76) | 19.08 | 2.39 |
| 2:1 | 6.1 (3.88–8.56) | 15.88 | 2.6 |
LC50s were determined experimentally; 95% fiducial limits determined by Probit analysis are given in parentheses.
Theoretical LC50s were calculated by using Tabashnik's equation and assuming a simple additive effect.
Synergism factors were calculated by dividing the expected LC50 by the observed LC50.
C. elegans requires conserved defense pathways against pore-forming toxins.
Considering that multiple C. elegans pathways involved in the defense against nematicidal Cry5B toxin have been previously described (31–33), we wanted to test whether they are important for the defense against Cry21 protoxins. First, we tested C. elegans bre mutants that lack the receptor for Cry5B (32). Interestingly, those mutants showed slightly increased but not significantly different survival compared to wild-type animals when exposed to E. coli-expressed Cry21 protoxins (Fig. 4A). This result is consistent with previous studies and agrees with the fact that bre mutants do not show cross-resistance to multiple Cry toxins (32). This finding also indicates that Cry21Fa1 and Cry21Ha1 protoxins may require a different and/or additional receptor or epitope in comparison to the Cry5B toxin. The p38 mitogen-activated protein kinase (MAPK) pathway and the c-Jun N-terminal kinase (JNK) MAPK pathway were previously shown to play a central role in the C. elegans response to Cry5B pore-forming toxin (33). Consistent with this, we found that animals carrying mutations in the p38 MAPK pathway (pmk-1) or in a downstream transcription factor of JNK MAPK pathway jun-1 were all hypersusceptible to Cry21Fa1 protoxin (Fig. 4B). JNK MAPK pathway mutant kgb-1 was slightly, but not significantly, more susceptible to Cry21Fa1 protoxin (Fig. 4B). This surprising result suggests that the role of kgb-1 in C. elegans defense against pore-forming toxins might be toxin dependent, and more-detailed investigation will be needed to elucidate the molecular mechanisms of the differentially protective role of kgb-1. Additionally, we found that xbp-1 mutants, which are sensitive to Cry5B (31), are also more susceptible to Cry21Fa1 than wild-type worms (Fig. 4B), confirming the protective role of the xbp-1 pathway against pore-forming toxins. Thus, C. elegans requires some conserved pathways for defenses against multiple Cry pore-forming toxins.
FIG 4.
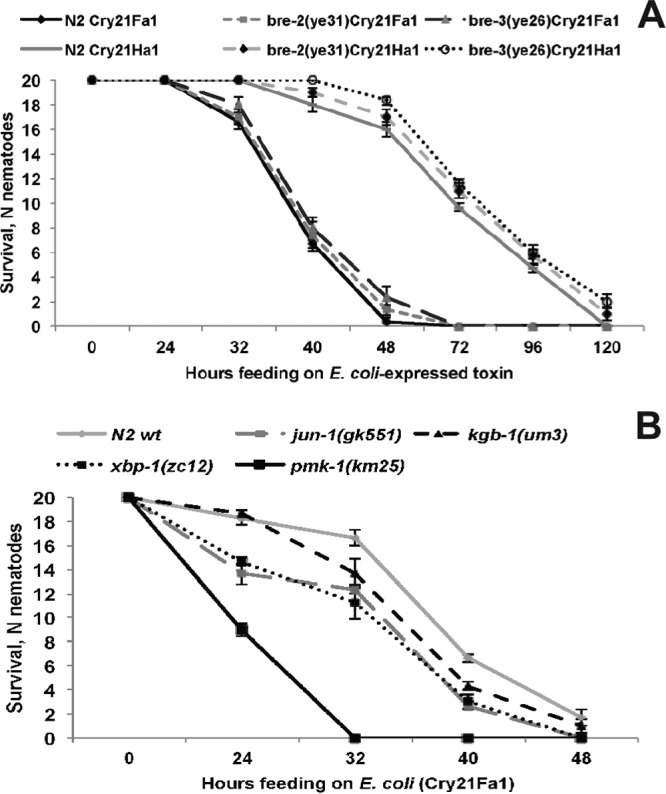
(A) bre-2 and bre-3 mutants are as susceptible to Cry21Fa1 and Cry21Ha1 protoxins as wild-type worms. (B) C. elegans jun-1 (P < 0.05), xbp-1 (P < 0.05), and pmk-1 (P < 0.0001) mutants are hypersensitive to Cry21Fa1 protoxin compared to wild-type worms. Survival of the kgb-1 mutant is not significantly (P > 0.05) different from wild-type survival. The data shown are means ± standard errors of the means.
DISCUSSION
In the present study, we characterized novel virulence factors of the highly nematicidal B. thuringiensis DB27 strain. Combining plasmid curing, whole-genome sequencing, and a candidate gene approach, we identified three proteins related to Cry21Ba1 toxin as potential nematicidal factors. Previous methods of Cry toxin identification are clearly dominated by PCR-based techniques (34). While these methods proved to be useful, they have certain limitations. Therefore, whole-genome sequencing and genome mining become reasonable alternatives for the identification of novel Cry toxins (8, 35). Applying these techniques, we found that three different plasmids encode three Cry-like proteins. All three proteins appeared to be novel Cry protoxins and were assigned new official names.
In contrast to previous reports showing that the genes encoding Cry toxins are located on large plasmids (24), which is also true for Cry21Fa1 protoxin produced by B. thuringiensis DB27, we found that two other protoxins, Cry21Ga1 and Cry21Ha1, are encoded by small 8- and 6-kb plasmids, respectively. Interestingly, another nematicidal toxin, Cry55Aa1, was also encoded by a relatively small 17.7-kb plasmid (8). Whether this unusual location has any functional consequences is not clear yet and remains to be elucidated.
While all three novel proteins belong to the Cry21 family of nematicidal toxins, only two, Cry21Fa1 and Cry21Ha1, showed activity against C. elegans. Whereas they are potent as single protoxins, they also showed synergistic activity against C. elegans. Synergism between Cry toxins and Cyt toxins has been described previously (7, 29). Additionally, enzymes such as chitinase and collagenase and different proteases have been shown to have an enhancing effect on Cry toxin efficiency (3, 36). Given that whole-genome sequencing of B. thuringiensis DB27 revealed the presence of multiple enzymes with potential enhancing properties, we do not exclude their involvement in B. thuringiensis DB27 virulence, but their role awaits further investigation. At this stage, it is not known why Cry21Ga1 is not active against C. elegans. It is possible that Cry21Ga1 functions in combination with other toxins and/or enzymes. In addition to this, there are many other examples in which Cry toxins do not show pesticidal activity (37). Thus, the exact target host and molecular function of Cry21Ga1 in B. thuringiensis DB27 pathogenicity will require further investigation.
B. thuringiensis strains very often carry multiple plasmids with different Cry toxins. B. thuringiensis DB27 carries seven plasmids, two of which harbor nematicidal Cry21 protoxins with synergistic activity. This trait may provide a strong selective advantage to the pathogen. First, loss of one of the toxins does not completely eliminate its virulence. Second, the synergistic action of two toxins facilitates faster host killing than is seen with strains with a single toxin. Third, the presence of multiple toxins drastically reduces the probability of the targeted host developing resistance (38). Consistent with this, several rounds of mutagenesis were needed in order to isolate a C. elegans nasp-1 mutant resistant to B. thuringiensis DB27 (12), while multiple alleles of five bre mutants resistant to Cry5B toxins were isolated in a single mutagenesis screen (30).
Previous studies have shown that C. elegans exhibits avoidance behavior when challenged with different pathogens (39). B. thuringiensis was one of the pathogens that C. elegans strongly avoided (40). Other nematode-pathogenic Bacillus spp., such as B. nematocida, evolved strategies to attract nematodes (41). Interestingly, in the case of B. thuringiensis DB27, worms showed neither repulsion from nor attraction to the bacteria. The absence of host-aversive behavior very likely benefits the pathogen via increasing the chances of successful infection.
Interestingly, EM and light microscopy revealed that B. thuringiensis DB27 spores and crystals have strong associations. Crystals are normally located outside the exosporium and are separated from spores after lysis of the mother cell. However, in a few strains, such as B. thuringiensis subsp. finitimus strains (42) and B. thuringiensis subsp. oyamensis strain LBIT-113 (43), the parasporal crystals are located between the exosporium and the spore coat and continue to adhere to the spore after mother cell lysis. This phenotype has been previously described as spore-crystal association (SCA) (25). While many studies have concentrated on identification of genes responsible for this phenotype (25, 26, 44), the functional significance of SCA is not yet clear. Considering that Cry proteins are not stable in the environment, the exosporium may be used as a protective membrane. At the same time, SCA may be used as a secure strategy to deliver the spores together with toxins to the host gut, which is not guaranteed when crystals are separated from spores. Given that B. thuringiensis spores (45) and vegetative cells (46) have been shown to enhance the toxicity of Cry proteins, SCA thus ensures that the two components are always present together to achieve fast killing of the host. Therefore, B. thuringiensis DB27 SCA may be an important advantageous strategy combined with the production of several novel Cry21 protoxins acting synergistically to ensure efficient host killing.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Jürgen Berger for taking EM pictures. We thank the Caenorhabditis Genetic Centre (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for providing strains. We thank James Lightfoot and Joshua Yim for proofreading the manuscript.
This research was supported by Max Planck Society and by the DFG-funded RTG1708 (Molecular principles of bacterial survival strategies).
Footnotes
Published ahead of print 14 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00464-14.
REFERENCES
- 1.Bravo A, Likitvivatanavong S, Gill SS, Soberon M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41:423–431. 10.1016/j.ibmb.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Aroian RV. 2012. Bacterial pore-forming proteins as anthelmintics. Invert. Neurosci. 12:37–41. 10.1007/s10158-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. 2010. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 18:189–194. 10.1016/j.tim.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 4.Bravo A, Gómez I, Porta H, García-Gómez BI, Rodriguez-Almazan C, Pardo L, Soberón M. 2013. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb. Biotechnol. 6:17–26. 10.1111/j.1751-7915.2012.00342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei J-Z, Hale K, Carta L, Platzer E, Wong C, Fang S-C, Aroian RV. 2003. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. U. S. A. 100:2760–2765. 10.1073/pnas.0538072100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui F, Scheib U, Hu Y, Sommer RJ, Aroian RV, Ghosh P. 2012. Structure and glycolipid binding properties of the nematicidal protein Cry5B. Biochemistry 51:9911–9921. 10.1021/bi301386q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soberón M, López-Díaz JA, Bravo A. 2013. Cyt toxins produced by Bacillus thuringiensis: a protein fold conserved in several pathogenic microorganisms. Peptides 41:87–93. 10.1016/j.peptides.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 8.Guo S, Liu M, Peng D, Ji S, Wang P, Yu Z, Sun M. 2008. New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl. Environ. Microbiol. 74:6997–7001. 10.1128/AEM.01346-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffitts JS, Aroian RV. 2005. Many roads to resistance: how invertebrates adapt to Bt toxins. Bioessays 27:614–624. 10.1002/bies.20239 [DOI] [PubMed] [Google Scholar]
- 10.Swiecicka I, Bideshi DK, Federici BA. 2008. Novel isolate of Bacillus thuringiensis subsp. thuringiensis that produces a quasicuboidal crystal of Cry1Ab21 toxic to larvae of Trichoplusia ni. Appl. Environ. Microbiol. 74:923–930. 10.1128/AEM.01955-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte RD, Makus C, Hasert B, Michiels NK, Schulenburg H. 2010. Multiple reciprocal adaptations and rapid genetic change upon experimental coevolution of an animal host and its microbial parasite. Proc. Natl. Acad. Sci. U. S. A. 107:7359–7364. 10.1073/pnas.1003113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rae R, Iatsenko I, Witte H, Sommer RJ. 2010. A subset of naturally isolated Bacillus strains show extreme virulence to the free-living nematodes Caenorhabditis elegans and Pristionchus pacificus. Environ. Microbiol. 12:3007–3021. 10.1111/j.1462-2920.2010.02278.x [DOI] [PubMed] [Google Scholar]
- 13.Sinha A, Rae R, Iatsenko I, Sommer RJ. 2012. System wide analysis of the evolution of innate immunity in the nematode model species Caenorhabditis elegans and Pristionchus pacificus. PLoS One 7:e44255. 10.1371/journal.pone.0044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iatsenko I, Sinha A, Rödelsperger C, Sommer RJ. 2013. New role for DCR-1/Dicer in Caenorhabditis elegans innate immunity against the highly virulent bacterium Bacillus thuringiensis DB27. Infect. Immun. 81:3942–3957. 10.1128/IAI.00700-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhardt AG, Mayer WE, Streit A. 2007. The free-living generation of the nematode Strongyloides papillosus undergoes sexual reproduction. Int. J. Parasitol. 37:989–1000. 10.1016/j.ijpara.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, Weller AM, Dieterich C, Sommer RJ. 2008. Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J. Exp. Biol. 211:1927–1936. 10.1242/jeb.014944 [DOI] [PubMed] [Google Scholar]
- 17.Reyes-Ramírez A, Ibarra JE. 2008. Plasmid patterns of Bacillus thuringiensis type strains. Appl. Environ. Microbiol. 74:125–129. 10.1128/AEM.02133-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kado CI, Liu ST. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischof LJ, Huffman DL, Aroian RV. 2006. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods Mol. Biol. 351:139–154 [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Russel DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21.Tabashnik BE. 1992. Evaluation of synergism among Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 58:3343–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laaberki M-H, Dworkin J. 2008. Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J. Bacteriol. 190:6197–6203. 10.1128/JB.00623-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgonie G, Claeys M, Leyns F, Arnaut G, DeWaele D, Coomans AV. 1996. Effect of nematicidal Bacillus thuringiensis strains on free-living nematodes 1. Light microscopic observations, species and biological stage specificity and identification of resistant mutants of Caenorhabditis elegans. Fundam. Appl. Nematol. 19:391–398 http://hdl.handle.net/1854/LU-189235 [Google Scholar]
- 24.Kronstad JW, Schnepf HE, Whiteley HR. 1983. Diversity of locations for Bacillus thuringiensis crystal protein genes. J. Bacteriol. 154:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji F, Zhu Y, Ju S, Zhang R, Yu Z, Sun M. 2009. Promoters of crystal protein genes do not control crystal formation inside exosporium of Bacillus thuringiensis ssp. finitimus strain YBT-020. FEMS Microbiol. Lett. 300:11–17. 10.1111/j.1574-6968.2009.01743.x [DOI] [PubMed] [Google Scholar]
- 26.Ammons DR, Reyna A, Granados JC, Ventura-Suárez A, Rojas-Avelizapa LI, Short JD, Rampersad JN. 2013. A novel Bacillus thuringiensis Cry-like protein from a rare filamentous strain promotes crystal localization within the exosporium. Appl. Environ. Microbiol. 79:5774–5776. 10.1128/AEM.01206-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iatsenko I, Corton C, Pickard DJ, Dougan G, Sommer RJ. 20 February 2014. Draft genome sequence of highly nematicidal Bacillus thuringiensis DB27. Genome Announc. 10.1128/genomeA.00101-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG. 1996. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. U. S. A. 93:5389–5394. 10.1073/pnas.93.11.5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue J-L, Cai Q-X, Zheng D-S, Yuan Z-M. 2005. The synergistic activity between Cry1Aa and Cry1c from Bacillus thuringiensis against Spodoptera exigua and Helicoverpa armigera. Lett. Appl. Microbiol. 40:460–465. 10.1111/j.1472-765X.2005.01712.x [DOI] [PubMed] [Google Scholar]
- 30.Marroquin LD, Elyassnia D, Griffitts JS, Feitelson JS, Aroian RV. 2000. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics 155:1693–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischof LJ, Kao C-Y, Los FCO, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. 2008. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 4:e1000176. 10.1371/journal.ppat.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffitts JS, Haslam SM, Yang T, Garczynski SF, Mulloy B, Morris H, Cremer PS, Dell A, Adang MJ, Aroian RV. 2005. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307:922–925. 10.1126/science.1104444 [DOI] [PubMed] [Google Scholar]
- 33.Kao C-Y, Los FCO, Huffman DL, Wachi S, Kloft N, Husmann M, Karabrahimi V, Schwartz J-L, Bellier A, Ha C, Sagong Y, Fan H, Ghosh P, Hsieh M, Hsu C-S, Chen L, Aroian RV. 2011. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 7:e1001314. 10.1371/journal.ppat.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguera PA, Ibarra JE. 2010. Detection of new cry genes of Bacillus thuringiensis by use of a novel PCR primer system. Appl. Environ. Microbiol. 76:6150–6155. 10.1128/AEM.00797-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye W, Zhu L, Liu Y, Crickmore N, Peng D, Ruan L, Sun M. 2012. Mining new crystal protein genes from Bacillus thuringiensis on the basis of mixed plasmid-enriched genome sequencing and a computational pipeline. Appl. Environ. Microbiol. 78:4795–4801. 10.1128/AEM.00340-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X, Chen L, Huang Q, Zheng J, Zhou W, Peng D, Ruan L, Sun M. 2013. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl. Environ. Microbiol. 79:460–468. 10.1128/AEM.02551-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roh JY, Park HW, Je YH, Lee DW, Jin BR, Oh HW, Gill SS, Kang SK. 1997. Expression of mosquitocidal crystal protein genes in non-insecticidal Bacillus thuringiensis subsp. israelensis. Lett. Appl. Microbiol. 24:451–454. 10.1046/j.1472-765X.1997.00038.x [DOI] [Google Scholar]
- 38.Georghiou GP, Wirth MC. 1997. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 63:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulenburg H, Ewbank JJ. 2007. The genetics of pathogen avoidance in Caenorhabditis elegans. Mol. Microbiol. 66:563–570. 10.1111/j.1365-2958.2007.05946.x [DOI] [PubMed] [Google Scholar]
- 40.Hasshoff M, Böhnisch C, Tonn D, Hasert B, Schulenburg H. 2007. The role of Caenorhabditis elegans insulin-like signaling in the behavioral avoidance of pathogenic Bacillus thuringiensis. FASEB J. 21:1801–1812. 10.1096/fj.06-6551com [DOI] [PubMed] [Google Scholar]
- 41.Niu Q, Huang X, Zhang L, Xu J, Yang D, Wei K, Niu X, An Z, Bennett JW, Zou C, Yang J, Zhang KQ. 2010. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc. Natl. Acad. Sci. U. S. A. 107:16631–16636. 10.1073/pnas.1007276107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojciechowska J, Lewitin E, Revina L, Zalunin I, Chestukhina G. 1999. Two novel delta-endotoxin gene families cry26 and cry28 from Bacillus thuringiensis ssp. finitimus. FEBS Lett. 453:46–48. 10.1016/S0014-5793(99)00650-X [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Meza JE, Ibarra JE. 1996. Characterization of a novel strain of Bacillus thuringiensis. Appl. Environ. Microbiol. 62:1306–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Ji F, Shang H, Zhu Q, Wang P, Xu C, Deng Y, Peng D, Ruan L, Sun M. 2011. Gene clusters located on two large plasmids determine spore crystal association (SCA) in Bacillus thuringiensis subsp. finitimus strain YBT-020. PLoS One 6:e27164. 10.1371/journal.pone.0027164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson D, Oppert B, McGaughey W. 1998. Spore coat protein synergizes Bacillus thuringiensis crystal toxicity for the Indianmeal moth. Curr. Microbiol. 36:278–282. 10.1007/s002849900310 [DOI] [PubMed] [Google Scholar]
- 46.Kho MF, Bellier A, Balasubramani V, Hu Y, Hsu W, Nielsen-LeRoux C, McGillivray SM, Nizet V, Aroian RV. 2011. The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans. PLoS One 6:e29122. 10.1371/journal.pone.0029122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.