Abstract
Lactobacillus plantarum is frequently isolated from the fermentation of plant material where tannins are abundant. L. plantarum strains possess tannase activity to degrade plant tannins. An L. plantarum tannase (TanBLp, formerly called TanLp1) was previously identified and biochemically characterized. In this study, we report the identification and characterization of a novel tannase (TanALp). While all 29 L. plantarum strains analyzed in the study possess the tanBLp gene, the gene tanALp was present in only four strains. Upon methyl gallate exposure, the expression of tanBLp was induced, whereas tanALp expression was not affected. TanALp showed only 27% sequence identity to TanBLp, but the residues involved in tannase activity are conserved. Optimum activity for TanALp was observed at 30°C and pH 6 in the presence of Ca2+ ions. TanALp was able to hydrolyze gallate and protocatechuate esters with a short aliphatic alcohol substituent. Moreover, TanALp was able to fully hydrolyze complex gallotannins, such as tannic acid. The presence of the extracellular TanALp tannase in some L. plantarum strains provides them an advantage for the initial degradation of complex tannins present in plant environments.
INTRODUCTION
Tannins are present in a variety of plants that are utilized as food and feed (1). Tannins seem to be a two-edged sword, since they are beneficial to health due to their chemopreventive activities against carcinogenesis and mutagenesis, but simultaneously, they may be involved in cancer formation, hepatotoxicity, or antinutritional activity (2). The molar mass of tannin molecules affects the tannin characteristics directly. It has been found that the higher the molar mass of tannin molecules, the stronger the antinutritional effects and the lower the biological activities (2). Small-molecule tannins are suggested to have fewer antinutritional effects and can be more readily absorbed.
Based on the molecular structure and origin of currently known tannins and their roles in plant life, tannins are defined as polyphenolic secondary metabolites of higher plants, and they are either galloyl esters or their derivatives, in which galloyl moieties or their derivatives are attached to a variety of polyol, catechin, and triterpenoid cores, or they are oligomeric and polymeric proanthocyanidins that can possess different interflavanyl coupling and substitution patterns (3). Gallotannins are those in which galloyl units or their metadepsidic derivatives are bound to diverse polyol, catechin, or triterpenoid units. Upon hydrolysis by acids, bases, or certain enzymes, gallotannins yield glucose and gallic acid (4).
Though tannins have toxic effects on various organisms, some microorganisms are resistant to tannins and have the ability to degrade them into oligomeric tannins and other useful derivatives, such as gallic acid or pyrogallol. Gallotannins are degraded by some bacteria, fungi, and yeasts, which can only hydrolyze the galloyl residues of galloyl esters of tannins. Tannin acyl hydrolase (EC 3.1.1.20), commonly known as tannase, catalyzes the hydrolysis of the galloyl ester bond of tannins. Tannase belongs to the superfamily of esterases. Since its discovery, tannase has found wide applications in the food, feed, beverage, pharmaceutical, and chemical industries (5). Despite the extensive interest and long history of the study of tannase, there is surprisingly little knowledge about the enzyme at the molecular level, which has become one of the critical factors that limit the large-scale application of tannase. To our knowledge, the only bacterial tannases that have been analyzed genetically are those from Staphylococcus lugdunensis (6), Lactobacillus plantarum (7, 8), and Enterobacter sp. (9). In addition, L. plantarum tannase has been biochemically and structurally characterized (7, 8, 10).
L. plantarum is a lactic acid bacterial species that is most frequently encountered in the fermentation of plant materials where tannins are abundant. These plant fermentations include several food and feed products, e.g., olives, grape must, and a variety of vegetable fermentation products. Among food lactic acid bacteria, strains from the L. plantarum group possess tannase activity (11–13). The biochemical pathway for the degradation of tannins by L. plantarum involves the action of a tannase and a gallate decarboxylase to decarboxylate the gallic acid formed by tannase action (14–16). The L. plantarum genes encoding tannase (tanBLp, formerly called tanLp1) (7) and gallate decarboxylase (lpdBCD) (16) involved in tannin degradation have been identified. However, an additional putative L. plantarum tannase sequence has been annotated in the genome of an L. plantarum strain. In this work, we have characterized the biochemical properties of this novel tannase. The presence of this tannase was analyzed among L. plantarum strains. Finally, the relative expression of both tannase genes under methyl gallate exposure was studied.
MATERIALS AND METHODS
Strains and growth conditions.
A total of 29 strains of L. plantarum were used in this study. L. plantarum strains WCFS1, NC8, and LPT 57/1 were kindly provided by M. Kleerebenzem (NIZO Food Research, The Netherlands), L. Axelsson (Norwegian Institute of Food, Fisheries and Aquaculture Research, Norway), and J. L. Ruíz-Barba (Instituto de la Grasa, CSIC; Spain), respectively. Seven strains were provided by the Spanish Type Culture Collection (CECT): L. plantarum CECT 220 (ATCC 8014), CECT 221 (ATCC 14431), CECT 223, CECT 224, CECT 749 (ATCC 10241), CECT 4645 (NCFB 1193), and the type strain L. plantarum subsp. plantarum CECT 748T (ATCC 14917; DSMZ 20174). Seven strains were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ): L. plantarum DSM 1055, DSM 2648, DSM 10492, DSM 12028, DSM 13273, DSM 20246, and the type strain of L. plantarum subsp. argentoratensis DSM 16365T. Eleven strains were isolated from grape must or wines of different wine-producing areas of Spain over the period from 1998 to 2001: L. plantarum RM28, RM31, RM34, RM35, RM38, RM39, RM40, RM41, RM71, RM72, and RM73) (17). The L. plantarum strains were routinely grown in de Man, Rogosa, and Sharpe medium (MRS) adjusted to pH 6.5 and incubated at 30°C. For the degradation assay, the L. plantarum strains were cultivated in a modified basal and defined medium described previously for L. plantarum (18). The basal medium was modified by the replacement of glucose by galactose. This defined medium was used to avoid the presence of phenolic compounds included in nondefined media. The sterilized modified basal medium was supplemented with filter-sterilized tannic acid (1 mM). The L. plantarum-inoculated medium was incubated in darkness without shaking at 30°C for 10 days. Incubated medium with cells and without phenolic compound and incubated medium without cells and with phenolic compounds were used as controls. The phenolic products were extracted from the supernatants twice with ethyl acetate (one-third of the reaction volume).
Escherichia coli DH10B was used for all DNA manipulations. E. coli BL21(DE3) was used for expression in the pURI3-TEV vector (19). E. coli strains were cultured in Luria-Bertani (LB) medium at 37°C and 140 rpm. When required, ampicillin was added to the medium at a concentration of 100 μg/ml.
PCR detection of tannase-encoding genes.
Genes encoding L. plantarum tannases (tanALp and tanBLp) were amplified by PCR using chromosomal DNA from several lactic acid bacterial strains. The tanBLp gene (1.4 kb) was amplified by using primers 951 (5′-TGATGCTGACTGGCTGGTGC) and 952 (5′-GCACAAGCCATCAATCCAGG). Oligonucleotides 953 (5′-CCTGATGAGTGGTTTGTTAG) and 954 (5′-CTTGCGTTCTGCTTCGGTATG) were used to amplify the tanALp gene (1.8 kb). The reactions were performed in a Mastercycler personal thermal cycler (Eppendorf), using 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 30 s. The amplified fragments were resolved in agarose gels.
RNA isolation, real-time (RT)-PCR, and qPCR.
For RNA isolation, L. plantarum MRS cultures were grown to an optical density at 600 nm (OD600) of 1 and then supplemented with methyl gallate at 15 mM final concentration. As a control, RNA was also isolated from cultures not supplemented with methyl gallate. After 10 min of incubation, the cultures were immediately processed for RNA extraction as previously described (20). After DNase I treatment, the absence of DNA from the RNA samples was verified by PCR. The DNA-free RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instructions. From the DNA obtained, quantitative gene expression was analyzed in an AbiPrism 7500 Fast Real Time PCR system (Applied Biosystems). Specific primer pairs were designed with the Primer Express 3.0 program to amplify internal regions of tannase genes. Oligonucleotides 1168 (5′-TGCGCTACCGTGGGATATTC) and 1169 (5′-AATCCAGGAAAATAAATCGCCTAA) were used to amplify 64 bp of tanBLp, and primers 1286 (5′-AAAGCAAGCTACGCCAAAGC) and 1287 (5′-CCCTGGGCATCCGTCTTC) were used to amplify a 56-bp fragment of tanALp. The expression level of the endogenous control gene (ldh) was assayed with primers 918 (5′-AACCGCGACAATGTTTTGATT) and 919 (5′-TTGTGAACGGCAGTTTCAGTGT). Amplifications were performed in triplicate. All quantitative-PCR (qPCR) assays amplified a single product, as determined by melting-curve analysis and by electrophoresis. A standard curve was plotted with cycle threshold (Ct) values obtained from amplification of known quantities of cDNA and used to determine the efficiency (E) as follows: E = 10−1/slope. In order to measure L. plantarum gene expression, amplification of the endogenous control gene was performed simultaneously, and its relative expression was compared with that of the target gene. Relative expression levels were calculated with the Applied Biosystems 7500 Fast System relative quantification software using the L. plantarum ldh gene as the endogenous gene and growth in the absence of methyl gallate as the growth condition calibrator.
Expression and purification of TanALp from L. plantarum ATCC 14917T.
As a peptide signal was predicted in the TanALp sequence, the gene tanALp in the locus HPREF0531_11477 from L. plantarum ATCC 14917T was PCR amplified, but lacking the 22-amino-acid peptide signal. The gene was amplified with Prime Star HS DNA polymerase (TaKaRa) by using the primers 805 (5′-GGTGAAAACCTGTATTTCCAGGGCgcttgcggacactccgaaacgaaga) and 637 (5′-ATCGATAAGCTTAGTTAGCTATtacttcaagctcttgttgacccactta) (the nucleotides pairing with the expression vector sequence are in italics, and the nucleotides pairing with the tanALp gene sequence are in lowercase). The gene was cloned into the pURI3-TEV vector, which encodes expression of a leader sequence containing a six-histidine affinity tag. The purified PCR product was then inserted into the pURI3-TEV vector by using a restriction enzyme- and ligation-free cloning strategy (19). E. coli DH10B cells were transformed, and the recombinant plasmids were isolated. Those containing the correct insert were used for transformation of E. coli BL21(DE3) cells.
E. coli cells carrying the recombinant pURI3-TEV-TanALp plasmid were grown at 37°C in LB medium containing ampicillin (100 μg/ml) and induced by adding 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After induction, the cells were grown at 22°C for 20 h and harvested by centrifugation (7,500 × g for 15 min at 4°C). The cells were resuspended in 50 mM sodium phosphate buffer, pH 7.0, containing 300 mM NaCl. Crude extracts were prepared by French press lysis of cell suspensions (three cycles at 1,100 lb/in2). The lysates were centrifuged at 17,400 × g for 40 min at 4°C. The supernatant obtained was filtered through a 0.22-μm filter (Millipore) and gently mixed for 20 min at room temperature with 1 ml Talon resin (Clontech). The resin was washed with 50 mM sodium phosphate buffer, pH 7.0, containing 300 mM NaCl and 10 mM imidazole. The recombinant His6-tagged protein was eluted with 50 mM sodium phosphate, pH 7.0, containing 300 mM NaCl and 150 mM imidazole. The eluted His6-tagged TanALp was dialyzed overnight at 4°C against 50 mM sodium phosphate buffer, pH 7.0, containing 300 mM NaCl. The purity of the enzyme was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in Tris-glycine buffer.
Tannase activity assay.
Since tannase catalyzes the hydrolysis of the galloyl ester linkage liberating gallic acid, the activity of tannase could be measured by estimating the gallic acid formed due to enzyme action (21). A method specific for the detection of gallic acid could be used for a reliable quantification of tannase activity. Inoue and Hagerman described a rhodanine assay for determining free gallic acid (22). Rhodanine reacts only with gallic acid and not with galloyl esters of other phenolics. Rhodanine reacts with the vicinal hydroxyl groups of gallic acid to give a red complex with maximum absorbance at 520 nm. Since the rhodanine assay using commercial tannic acid as the substrate gives high absorbance values due to small amounts of free gallic acid present in the preparation, methyl gallate was used as the substrate.
Gallic acid analysis in reactions was determined in triplicate by using the following assay. TanALp (10 μg) in 700 μl of 50 mM phosphate buffer, pH 6.5, was incubated with 40 μl of 25 mM methyl gallate (1 mM final concentration) for 5 min at 37°C. After incubation, 150 μl of a methanolic rhodanine solution (0.667% rhodanine in 100% methanol) was added to the mixture. After 5 min of incubation at 30°C, 100 μl of 0.5 M KOH was added. The absorbance at 520 nm was measured on a spectrophotometer. A standard curve using gallic acid concentrations ranging from 0.125 to 1 mM was prepared. One unit of tannase activity was defined as the amount of enzyme required to release 1 μmol of gallic acid per minute under standard reaction conditions.
Biochemical characterization of TanALp.
Activities of TanALp from L. plantarum ATCC 14917T were measured at 4, 20, 30, 37, 45, 55, and 65°C to determine the optimal temperature for enzymatic activity. The optimum pH value for tannase activity was determined by studying its pH dependence within the pH range between 3 and 10. Acetic acid-sodium acetate buffer was used for pH 3 to 5, citric acid-sodium citrate buffer for pH 6, sodium phosphate buffer for pH 7, Tris-HCl buffer for pH 8, glycine-NaOH buffer for pH 9, and sodium carbonate-bicarbonate for pH 10. A 100 mM concentration was used in all the buffers. The rhodanine assay was used for the optimal pH characterization of TanALp. Since the rhodanine-gallic acid complex forms only under basic conditions, after the enzymatic degradation of methyl gallate, KOH was added to the reaction mixture to ensure that the same pH value (pH 11) was achieved in all samples assayed.
For temperature stability measurements, TanALp was incubated in 50 mM phosphate buffer, pH 6.5, at 22, 30, 37, 45, 55, and 65°C for 15 min, 30 min, and 1, 2, 5, and 18 h. After incubation, the residual activity was measured as described above.
To test the effects of metals and ions on the activity of TanALp, the enzymatic activity was measured in the presence of different additives at a final concentration of 1 mM. The additives analyzed were MgCl2, KCl, CaCl2, HgCl2, ZnCl2, Triton X-100, urea, Tween 80, EDTA, dimethyl sulfoxide (DMSO), and β-mercaptoethanol. All the determinations were done in triplicate.
TanALp substrate specificity analysis by HPLC.
The activity of TanALp against 21 potential substrates was analyzed. The substrates assayed were gallic esters (methyl gallate, ethyl gallate, propyl gallate, and lauryl gallate), benzoic esters (methyl benzoate and ethyl benzoate), hydroxybenzoic esters (methyl 4-hydroxybenzoate, ethyl 4-hydroxybenzoate, propyl 4-hydroxybenzoate, and butyl 4-hydroxybenzoate), vanillic ester (methyl vanillate), dyhydroxybenzoic esters (methyl 2,4-dihydroxybenzoate, ethyl 3,4-dihydroxybenzoate or protocatechuic acid ethyl ester, and ethyl 3,5-dihydroxybenzoate), gentisic ester (methyl gentisate), salicylic ester (methyl salicylate), and ferulic esters (ferulic methyl ester and ferulic ethyl ester). Tannic acid and epigallocatechin gallate were also assayed as potential substrates. Tannase (50 μg), in phosphate buffer, pH 6.0 (50 mM), and CaCl2 (1 mM), was incubated at 37°C in the presence of the substrate (1 mM). As controls, phosphate buffers containing the reagents but not the enzyme were incubated under the same conditions. The reaction products were extracted twice with ethyl acetate; the solvent fractions were filtered through a 0.45-μm polyvinylidene difluoride (PVDF) filter and analyzed by high-performance liquid chromatography with diode array detection (HPLC-DAD). A Thermo chromatograph (Thermo Electron Corporation, Waltham, MA, USA) equipped with a P4000 SpectraSystem pump, an AS3000 autosampler, and a UV6000LP photodiode array detector were used. A gradient of solvent A (water-acetic acid, 98:2 [vol/vol]) and solvent B (water-acetonitrile-acetic acid, 78:20:2 [vol/vol/vol]) was applied to a reversed-phase Nova-pack C18 cartridge (25 cm by 4.0-mm inside diameter [i.d.]; 4.6-μm particle size) at room temperature as follows: 0 to 55 min, 80% B linear, 1.1 ml/min; 55 to 57 min, 90% B linear, 1.2 ml/min; 57 to 70 min, 90% B isocratic, 1.2 ml/min; 70 to 80 min, 95% B linear, 1.2 ml/min; 80 to 90 min, 100% linear, 1.2 ml/min; 100 to 120 min, washing, 1.0 ml/min; and reequilibration of the column under the initial gradient conditions. Samples were injected onto the cartridge after being filtered through a 0.45-μm PVDF filter. Detection of the substrates and the degradation compounds was performed spectrophotometrically by scanning from 220 to 380 nm. The identification of degradation compounds was carried out by comparing the retention times and spectral data of each peak with those of standards from commercial suppliers or by LC-DAD–electrospray ionization-mass spectrometry (ESI-MS).
Sequence data analysis.
A homology search with finished and unfinished microbial genome databases was performed with the BLAST algorithm at the National Center for Biotechnology Information server (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi). Multiple alignments were made using the Clustal W2 Program (http://www.ebi.ac.uk/Tools/msa/clustalw2/) on the EBI site after retrieval of sequences from the GenBank and Swiss-Prot databases. Computer promoter predictions were carried out at the Internet site (http://www.fruitfly.org/seq_tools/promoter.html). pI and molecular weight (MW) were analyzed on EXPASY (http://web.expasy.org/compute_pi/), signal peptide cleavage sites were analyzed on the SignalP 4.1 server site (http://www.cbs.dtu.dk/services/SignalP/), and predicted transcription terminators were analyzed at the ARNold site (http://rna.igmors.u-psud.fr/toolbox/arnold/index.php#Results).
RESULTS
Presence of a putative novel tannase-encoding gene in several L. plantarum strains.
Homology searches in genome databases allowed us to find the locus HMPREF0531_11477 in the L. plantarum ATCC 14917T genome, which encodes a 626-amino-acid-residue protein annotated as “tannase,” defined here as TanALp. Interestingly, multiple amino acid sequence alignments of TanALp from L. plantarum ATCC 14917T with TanASl tannase from S. lugdunensis and TanBLp tannase from L. plantarum revealed high sequence identity to TanASl (50%) and much lower identity to TanBLp (27%) (see Fig. S1 in the supplemental material). Moreover, TanALp shares additional features with TanASl from S. lugdunensis. Both are 67-kDa proteins and have alkaline isoelectric points (9.54 for TanASl and 9.94 for TanALp), and they present predicted signal peptides. On the other hand, TanBLp is a 50-kDa protein with an isoelectric point of 6.06, and it does not possess a signal peptide.
Based on the recently described crystal structure of TanBLp (10), the conserved sequence motif Gly-X-Ser-X-Gly typical of serine hydrolases could be easily identified in TanALp from L. plantarum ATCC 14917T (Gly-215 to Gly-219 in TanALp). From the catalytic triad identified in the structure (Ser-163, His-451, and Asp-419 in TanBLp), only serine and histidine residues are conserved in TanALp as well as in TanASl (see Fig. S1 in the supplemental material), with Asp-419 being replaced by a Gln residue in both proteins. On the other hand, the residues which make contacts with the three hydroxyl groups of gallic acid (Asp-421, Lys-343, and Glu-357 in TanBLp) are conserved in both TanALp and TanASl (see Fig. S1 in the supplemental material).
The genomes of 10 L. plantarum strains are currently available. Analyses of these genomes revealed that a copy of the tanALp gene is present only in L. plantarum ATCC 14917T and L. plantarum NC8 and is not found in the rest (L. plantarum WCFS1, JDM1, ST-III, 16, P-8, IPLA 88, UCMA 3037, and ZJ316). In the ATCC 14917T and NC8 strains, tanALp is located between the genes nox3 (encoding a NADH oxidase [accession number EFK29315]) and dapE1 (encoding succinyl-diaminopimelate desuccinylase [accession number EFK29313]) (Fig. 1). The intergenic region between these genes is 121 bp long in the L. plantarum strains devoid of tanALp; however, in the strains possessing tanALp, this region is 2,515 or 2,516 bp long in NC8 and ATCC 14917T, respectively. In L. plantarum ATCC 14917T, this region encodes a 626-amino-acid-residue protein (TanALp), which is preceded by a putative promoter, as revealed by sequence analysis. In turn, a putative transcription terminator site follows the stop codon, where a possible stem-loop structure is predicted, which would start 45 nucleotides downstream from the TAA stop codon and would have a 19-base stem and a 22-base loop. This structure may serve as a terminator for transcription (Fig. 1). The L. plantarum NC8 sequence is identical to that of ATCC 14917T with the exception of a G deletion, which produces a frameshift from Gln-529 to the end of TanALp (see Fig. S2 in the supplemental material).
FIG 1.
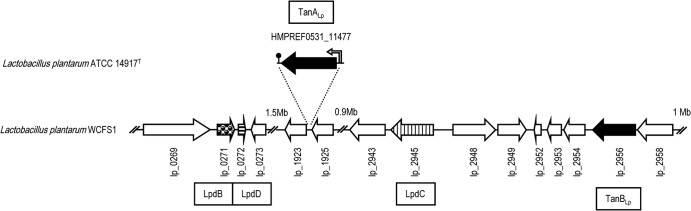
Genetic organization of the L. plantarum WCFS1 chromosomal region containing the gallate decarboxylase- and tannase-encoding genes (accession no. NC_004567; positions 243093 to 252815, 1743368 to 1746325, and 2618290 to 2635122). The insertion of the tanALp gene in L. plantarum ATCC 14917T is also represented (accession no. ACGZ02000013.1; positions c/26013 to 27893). The arrows represent genes. Genes coding for putative tannase proteins are represented by black arrows. Genes encoding gallate decarboxylase subunits (Lpd, LpdC, and LpdD) are also shown. The locations of a putative tanALp promoter (vertical bent arrow) and transcription terminator region (ball and stick) are also indicated.
In order to know the extent of the tannase genes among L. plantarum strains, the presence of the tanBLp and tanALp genes was studied in 29 L. plantarum strains isolated from different sources. To determine the presence of both genes, chromosomal DNA was extracted and PCR amplified. DNA fragments of 1.4 or 1.8 kb from tanBLp or tanALp, respectively, were PCR amplified using oligonucleotides designed on the basis of the L. plantarum ATCC 14917T sequence. All the L. plantarum strains analyzed gave the corresponding tanBLp amplicon (data not shown), which indicates that TanBLp is generally present among L. plantarum strains, as described previously (7). Apart from ATCC 14917T and NC8, two additional strains also possessed a copy of the tanALp gene, namely, L. plantarum CECT 749 and RM35 (data not shown). Since the NC8 tanALp copy is truncated, the complete sequences of the CECT 749 and RM35 strains were determined. Alignment of the resulting TanALp amino acid sequences revealed one substitution in the CECT 749 protein (Ala-107 to Asp-107) and two in the RM35 strain (Arg-563 to Lys-563, and Arg-565 to Gln-565) (see Fig. S2 in the supplemental material). It is noteworthy that these two mutated arginine residues are part of a motif (WRIR) conserved in all tannases (see Fig. S1 in the supplemental material).
Extracellular tannase activity on L. plantarum strains.
Since the sequences of the four tanALp copies present in the L. plantarum strains analyzed here differ, an activity assay was done to determine the functionality of the resulting coded proteins. In this regard, the presence of a putative signal peptide indicated that TanALp could be an extracellular protein, and in fact, an extracellular tannase produced by an L. plantarum strain has been reported (23). L. plantarum WCFS1 and the four strains possessing a tanALp copy were grown in a basal medium containing 1 mM tannic acid for 10 days. Tannic acid was chosen because it is a complex gallotannin unable to pass into the cell to be degraded by intracellular TanBLp tannase. As a control, the medium was incubated under the same conditions. From the L. plantarum culture, the cells were pelleted, and the tannic acid in the supernatant was extracted and analyzed by HPLC. Figure 2 shows that most of the chromatograms were similar to the control, and only small variations were observed among them. However, L. plantarum ATCC 14917T showed a chromatogram that clearly indicated hydrolysis of the high-molecular-weight tannins, suggesting the presence of an active extracellular tannase only in this strain.
FIG 2.
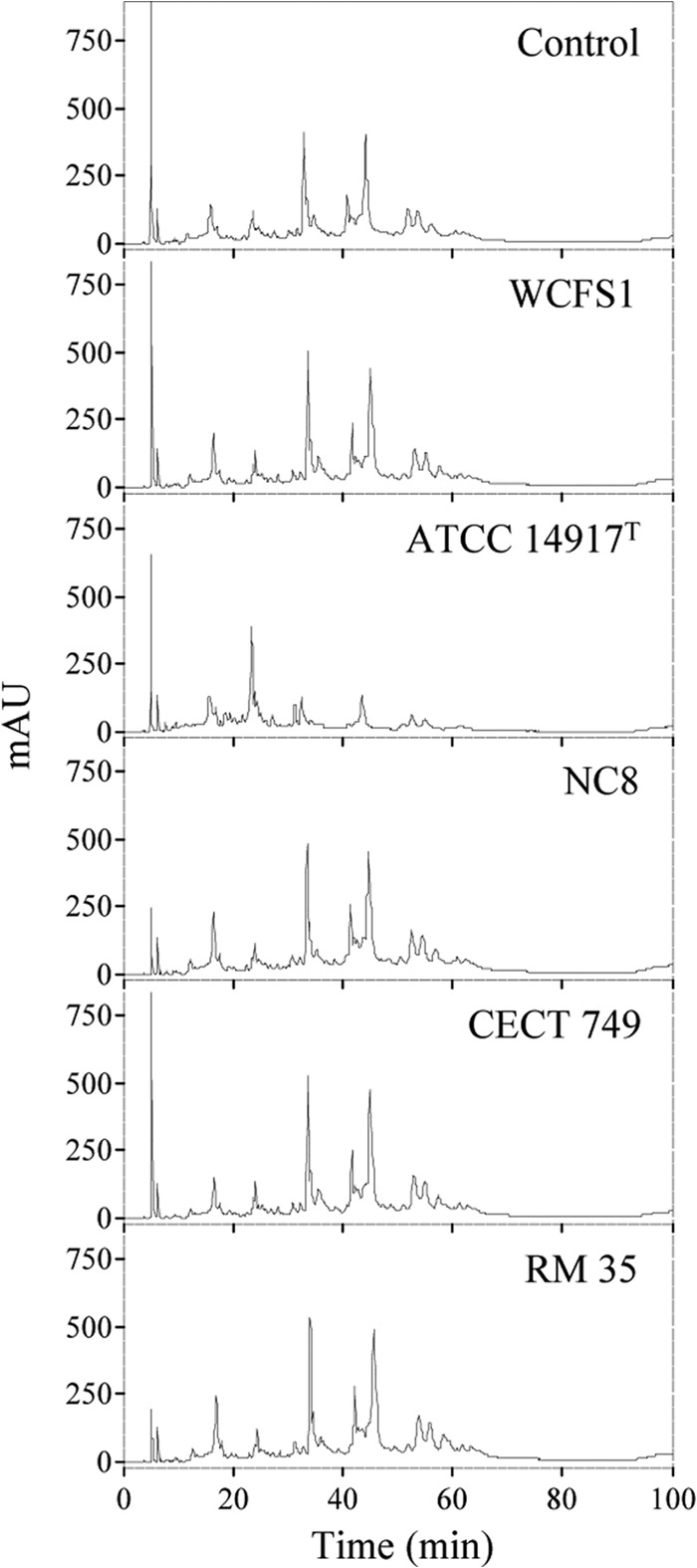
HPLC analysis of tannic acid degradation by L. plantarum cultures. Modified basal medium containing 1 mM tannic acid was inoculated with L. plantarum strains (WCFS1, ATCC 14917T, NC8, CECT 749, and RM 35) and incubated for 10 days. A noninoculated control medium was incubated under the same conditions. Detection was performed at 280 nm. AU, absorbance units.
Relative expression of L. plantarum tannase genes under methyl gallate exposure.
As tannase is involved in tannin degradation, the relative expression of both tannase-encoding genes under methyl gallate exposure was studied. Strain WCFS1 was analyzed as a model strain with only one tannase enzyme and strain ATCC 14917T as a model strain having two different active tannase proteins. L. plantarum cultures were induced for 10 min by the presence of 15 mM methyl gallate as a potential tannase substrate. The gene expression levels obtained were substantially different between the two tannase-encoding genes (data not shown), indicating the presence of two different expression patterns for the proteins. The tanALp gene, only present in L. plantarum ATCC 14917T, showed an expression level not affected by the presence of its substrate, methyl gallate. However, the tanBLp gene expression profiles were affected. In both L. plantarum strains, the presence of 15 mM methyl gallate induces about a 3-fold increase in the expression of the tanBLp gene.
Biochemical properties of TanALp from L. plantarum ATCC 14917T.
Of the L. plantarum strains possessing two tannase enzymes, only L. plantarum ATCC 14917T showed extracellular tannase activity; therefore, TanALp from this strain was biochemically characterized. The tanALp gene lacking the 22-amino-acid peptide signal sequence from L. plantarum ATCC 14917T was expressed in E. coli under the control of an inducible promoter. Cell extracts were used to detect the presence of overproduced proteins by SDS-PAGE analysis. Control cells containing the pURI3-TEV vector plasmid did not show protein overexpression; in addition, no tannase activity was observed in this control extract. However, an overproduced protein with an apparent molecular mass of around 67 kDa was observed in cells harboring pURI3-TEV-TanALp (Fig. 3). Since the cloning strategy yielded a His-tagged protein variant, L. plantarum pURI3-TEV-TanALp could be purified on an immobilized metal affinity chromatography (IMAC) resin. However, unexpectedly, the protein was scarcely purified (2.71 mg/liter), with most of the protein not being bound to the resin.
FIG 3.
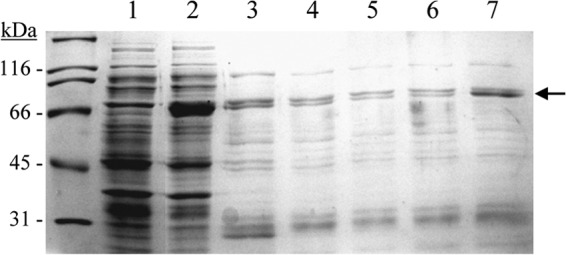
Purification of L. plantarum TanALp protein. Shown are SDS-PAGE analysis of the expression and purification of His6-TanALp and analysis of soluble cell extracts of IPTG-induced E. coli BL21(DE3)(pURI3-TEV) (lane 1) and E. coli BL21(DE3)(pURI3-TEV-TanALp) (lane 2) or fractions eluted after His affinity resin (lanes 3 to 7). The arrow indicates the overproduced and purified protein. The 12.5% gel was stained with Coomassie blue. Molecular mass markers are located on the left (SDS-PAGE Standards; Bio-Rad).
The L. plantarum ATCC 14917T TanALp enzyme, partially purified by the affinity resin, was biochemically characterized. Tannase activity was determined by using methyl gallate as the substrate. The specific activities of TanALp and TanBLp (taken as references) were determined by the rhodanine assay. TanALp has a specific activity of 39 U/mg, whereas TanBLp has 404 U/mg.
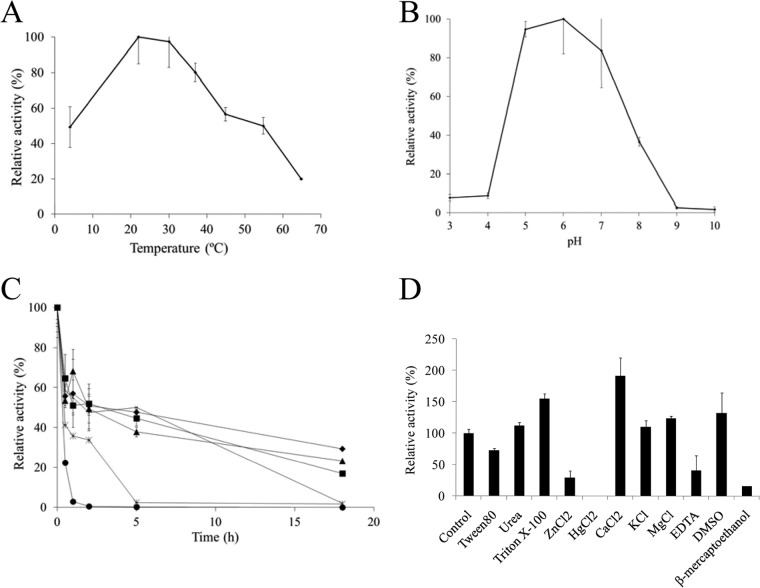
Figure 4 shows the optimum temperature and pH and the thermal stability of TanALp as determined by the rhodanine assay, with methyl gallate as the substrate. TanALp displays optimal activity within the 20 to 30°C temperature range and an optimal pH around 6. Figure 4D shows the effects of various additives (1 mM final concentration) on the enzymatic activity of TanALp. CaCl2 greatly increased and HgCl2 completely inhibited TanALp activity. In relation to TanALp substrates, similarly to TanBLp, only the esters derived from gallic and protocatechuic acids were hydrolyzed (see Fig. S3 in the supplemental material). It seems that other cinnamic acids without hydroxyl groups and with substituents other than —H or —OH at position 2 were not metabolized by TanALp or by TanBLp. Regarding the aliphatic alcohol constituent of the ester bond, a lauryl substituent could not be effectively hydrolyzed by TanALp (see Fig. S3 in the supplemental material).
FIG 4.
Some biochemical properties of TanALp protein. (A) Relative activity of TanALp versus temperature. (B) Relative activity versus pH. (C) Thermal stability of TanALp after preincubation at 22°C (diamonds), 30°C (squares), 37°C (triangles), 45°C (×), 55°C (stars), and 65°C (circles) in phosphate buffer (50 mM; pH 6.5). At the indicated times, aliquots were withdrawn and analyzed as described in Materials and Methods. The experiments were done in triplicate. The mean values and standard errors are shown. The observed maximum activity was defined as 100%. (D) Relative activity of TanALp after incubation with 1 mM concentrations of different additives. The activity of the enzyme incubated in the absence of additives was defined as 100%.
DISCUSSION
Although tannase activity has been extensively described in L. plantarum strains (12–14), the first bacterial tannase genetically identified was TanASl from S. lugdunensis (6), and then, by sequence comparison, TanBLp (Lp_2956) from L. plantarum was later identified (7). The first complete genome of an L. plantarum strain was from the WCFS1 strain. However, at present, the sequences of several L. plantarum strains are publicly available. In L. plantarum ATCC 14917T, the locus HMPREF0531_11477 was annotated as tannase and designated TanALp. TanALp from L. plantarum ATCC 14917T showed higher sequence identity to TanASl from S. lugdunensis (50%) than to L. plantarum TanBLp tannase (27%). Despite the low identity to TanBLp, TanASl and TanALp shared TanBLp motifs involved in tannase activity (10). Therefore, structural data also suggest that TanALp from L. plantarum ATCC 14917T could be an active tannase.
When the presence of both tannases was studied in 36 L. plantarum strains, it was observed that a copy of the tanBLp gene was present in all the analyzed strains, whereas tanALp was present in only four L. plantarum strains (ATCC 14917T, NC8, CECT 749, and RM35). The analysis of the TanALp protein sequences from these four strains revealed that, compared to TanALp from L. plantarum ATCC 14917T, TanALp from CECT 749 and RM35 possessed amino acid substitutions in a motif conserved in all tannases. Moreover, the sequence of tanALp from L. plantarum NC8 posses a frameshift that produces a truncated protein lacking 2 of the 3 residues of the catalytic triad (residues equivalent to His-451 and Asp-419 in TanBLp) and Asp-421, which make contacts with a hydroxyl group of gallic acid.
From the sequence analysis, TanALp from L. plantarum ATCC 14917T seems to be the only TanALp tannase in which the residues important for activity are conserved. In order to verify this hypothesis, and taking into account that TanALp seems to be an extracellular tannase, strains possessing a tanALp copy (ATCC 14917T, NC8, CECT 749, and RM35) were grown in the presence of tannic acid, a complex gallotannin unable to pass into the cell to be degraded by intracellular TanBLp tannase. As expected, the presence of an active extracellular tannase was observed only in L. plantarum ATCC 14917T. In this strain, in addition to the functionality of TanALp, the functionality of TanBLp was previously demonstrated (8). As the strain possesses two tannase proteins able to hydrolyze gallotannins, the expression of these proteins was studied under methyl gallate exposure. The presence of the substrate methyl gallate did not affect the expression of tanALp; however, it increased the expression of tanBLp. This expression behavior allows the assumption that tanBLp encodes an inducible tannase in L. plantarum ATCC 14917T, as previously observed in the WCFS1 strain under tannic acid challenge (24).
From the results obtained in this study, it seems that the two L. plantarum tannases play different physiological roles. These different functions could be partially attributed to their different biochemical properties, such as their reported substrate spectra, which seem to be very similar except that esters having a long aliphatic alcohol were not effectively hydrolyzed by TanALp. Moreover, TanALp from L. plantarum ATCC 14917T has a specific activity of 39 U/mg, 10 times lower than the specific activity calculated for TanBLp (404 U/mg) from the same strain. In addition, the optimal temperature and pH for TanALp (20 to 30°C and pH 6) differed from those described for TanBLp (40°C and pH 7 to 8) (7, 8). Interestingly, the optimal activity of TanALp is similar to the optimal tannase activity shown by cell extracts from L. plantarum ATCC 14917T (25). It is noteworthy that L. plantarum ATCC 14917T cell extracts were obtained from cultures grown in a medium devoid of possible tannase inducers, on which TanBLp tannase could not be induced. Therefore, it seems that in the absence of a substrate, the biochemical characteristics of tannase activity shown by cell extracts of L. plantarum ATCC 14917T, containing both tannase genes, are more similar to those exhibited by TanALp than to those of TanBLp. This could indicate that, in the absence of a substrate, TanALp activity predominates in L. plantarum strains having two tannase enzymes. The gene expression results indicated that TanALp is not inducible by the presence of methyl gallate; however, its basal expression level could be enough to be detected in L. plantarum cell extracts. In complex tannins, such as tannic acid, the presence of an extracellular and low-activity TanALp tannase in some L. plantarum strains could provide an enzymatic activity able to partially degrade tannic acid outside the cell. The less complex tannins originated by TanALp action could be able to induce the expression of tanBLp and pass into the cell to be degraded by TanBLp. In addition, TanALp biochemical properties are more convenient than those from TanBLp for an extracellular enzyme acting on plant substrates. Temperatures around 20 to 30°C and acidic pH could be environmental conditions present on these plant substrates.
The specific catabolic capacity of L. plantarum against gallotannins suggests that they provide a selective advantage to the species for life in environments where compounds of plant origin are abundant. The presence in some L. plantarum strains of a second active tannase provides an additional advantage. L. plantarum should be able to degrade them with TanALp and does not depend on other microorganisms for the initial degradation of these compounds. This second tannase, TanALp, is an extracellular enzyme able to hydrolyze complex gallotannins, which are unable to pass into the cell to be degraded by TanBLp. Moreover, the presence of TanALp provides L. plantarum with strains an additional response mechanism to overcome the adverse effects of tannins present in their environment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants AGL2011-22745, BFU2010-17929/BMC, Consolider INGENIO 2010 CSD2007-00063 FUN-C-FOOD (MINECO), S2009/AGR-1469 (ALIBIRD) (Comunidad de Madrid), and RM2012-00004 (Instituto Nacional de Investigación Agraria y Alimentaría). N. Jiménez is the recipient of an FPI fellowship from the MINECO.
We are grateful to M. V. Santamaría and J. M. Barcenilla.
Footnotes
Published ahead of print 7 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00324-14.
REFERENCES
- 1.Shahidi F, Naczk M. 2003. Phenolics in food and nutraceuticals. CRC Press, London, United Kingdom [Google Scholar]
- 2.Chung K-T, Wei C-I, Johnson MG. 1998. Are tannins a double-edged sword in biology and health? Trends Food Sci. Technol. 9:168–175. 10.1016/S0924-2244(98)00028-4 [DOI] [Google Scholar]
- 3.Khanbabaee K, Ree TV. 2001. Tannins: classification and definition. Nat. Prod. Rep. 18:641–649. 10.1039/b101061l [DOI] [PubMed] [Google Scholar]
- 4.Li M, Kai Y, Qiang H, Dongying J. 2006. Biodegradation of gallotannins and ellagitannins. J. Basic Microbiol. 46:68–84. 10.1002/jobm.200510600 [DOI] [PubMed] [Google Scholar]
- 5.Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Pardo-Barragán A, Rodríguez R, Contreras JC, Aguilar CN. 2012. Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Technol. 5:445–459. 10.1007/s11947-011-0608-5 [DOI] [Google Scholar]
- 6.Noguchi N, Ohashi T, Shiratori T, Narui K, Hagiwara T, Ko M, Watanabe K, Miyahara T, Taira S, Moriyasu F, Sasatsu M. 2007. Association of tannase-producing Staphylococcus lugdunensis with colon cancer and characterization of a novel tannase gene. J. Gastroenterol. 42:346–351. 10.1007/s00535-007-2012-5 [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R. 2008. Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum. ATCC 14917T. Syst. Appl. Microbiol. 31:269–277. 10.1016/j.syapm.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Curiel JA, Rodríguez H, Acebrón I, Mancheño JM, de las Rivas B, Muñoz R. 2009. Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J. Agric. Food Chem. 57:6224–6230. 10.1021/jf901045s [DOI] [PubMed] [Google Scholar]
- 9.Sharma KP, John PJ. 2011. Purification and characterization of tannase and tannase gene from Enterobacter sp. Process Biochem. 46:240–244. 10.1016/j.procbio.2010.08.016 [DOI] [Google Scholar]
- 10.Ren B, Wu M, Wang Q, Peng X, Wen H, McKinstry WJ, Chen Q. 2013. Crystal structure of tannase from Lactobacillus plantarum. J. Mol. Biol. 425:2737–2751. 10.1016/j.jmb.2013.04.032 [DOI] [PubMed] [Google Scholar]
- 11.Nishitani Y, Osawa R. 2003. A novel colorimetric method to quantify tannase activity of viable bacteria. J. Microbiol. Methods 54:281–284. 10.1016/S0167-7012(03)00063-0 [DOI] [PubMed] [Google Scholar]
- 12.Nishitani Y, Sasaki E, Fujisawa T, Osawa R. 2004. Genotypic analyses of lactobacilli with a range of tannase activities isolated from human feces and fermented foods. Syst. Appl. Microbiol. 27:109–117. 10.1078/0723-2020-00262 [DOI] [PubMed] [Google Scholar]
- 13.Vaquero I, Marcobal A, Muñoz R. 2004. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 96:199–204. 10.1016/j.ijfoodmicro.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Osawa R, Kuroiso K, Goto S, Shimizu A. 2000. Isolation of tannin degrading lactobacilli from human and fermented foods. Appl. Environ. Microbiol. 66:3093–3097. 10.1128/AEM.66.7.3093-3097.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez H, de las Rivas B, Gómez-Cordovés C, Muñoz R. 2008. Degradation of tannic acid by cell-free extracts of Lactobacillus plantarum. Food Chem. 107:664–670. 10.1016/j.foodchem.2007.08.063 [DOI] [PubMed] [Google Scholar]
- 16.Jiménez N, Curiel JA, Reverón I, de las Rivas B, Muñoz R. 2013. Uncovering the Lactobacillus plantarum WCFS1 gallate decarboxylase involved in tannin degradation. Appl. Environ. Microbiol. 79:4253–4263. 10.1128/AEM.00840-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-Arribas MV, Polo MC, Jorganes F, Muñoz R. 2003. Screening of biogenic amine production by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 84:117–123. 10.1016/S0168-1605(02)00391-4 [DOI] [PubMed] [Google Scholar]
- 18.Rozès N, Peres C. 1998. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 49:108–111. 10.1007/s002530051145 [DOI] [Google Scholar]
- 19.Curiel JA, de las Rivas B, Mancheño JM, Muñoz R. 2011. The pURI family of expression vectors: a versatile site of ligation independent cloning plasmids for producing recombinant His-fusion proteins. Protein Expr. Purif. 76:44–53. 10.1016/j.pep.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Saulnier DM, Molenaar D, de Vos WM, Gibson GR, Kolida S. 2007. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl. Environ. Microbiol. 73:1753–1765. 10.1128/AEM.01151-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller-Harvey I. 2001. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 91:3–20. 10.1016/S0377-8401(01)00227-9 [DOI] [Google Scholar]
- 22.Inoue KH, Hagerman AE. 1988. Determination of gallotannins with rhodanine. Anal. Biochem. 169:363–369. 10.1016/0003-2697(88)90296-5 [DOI] [PubMed] [Google Scholar]
- 23.Lamia A, Hamdi M. 2002. Culture conditions of tannase production by Lactobacillus plantarum. Biotech. Lett. 24:1763–1765. 10.1023/A:1020696801584 [DOI] [Google Scholar]
- 24.Reverón I, Rodríguez H, Campos H, Curiel JA, Ascaso C, Carrascosa AV, Prieto A, de las Rivas B, Muñoz R, López de Felipe F. 2013. Tannic acid-dependent modulation of selected Lactobacillus plantarum traits linked to gastrointestinal survival. PLOS One. 8:e66473. 10.1371/journal.pone.0066473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez H, de las Rivas B, Gómez-Cordovés C, Muñoz R. 2008. Characterization of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748T. Int. J. Food Microbiol. 121:92–98. 10.1016/j.ijfoodmicro.2007.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.