Abstract
Biopreservation is the science of extending the shelf life (storage time) of biological systems. The scientific field of biopreservation can be broadly classified into three distinct but interrelated research areas: Cryopreservation (storage by freezing), Desiccation (storage by drying) and Freeze-Drying (storage by freezing first and then sublimating the frozen water). Although, both freeze-frying and desiccation create products that are easier to store and transport, they have not, as yet, been successfully applied to store a variety of biological specimens. However, both these technologies have been quite successfully applied in a variety of fields including pharmaceutical sciences and food industry, as demonstrated by the easy availability of shelf-stable drugs and instant mashed potatoes! On the other hand freezing storage has a long and storied history of being used to transport biological specimen, over long distances, as far back as the time of the Pharaohs. However, the lack of portable refrigeration/freezing techniques (and the inviolate second law) limited the use of cryopreservation in every-day life, until the early 19th century. This short review will outline some of the challenges and opportunities in the fields of engineering, heat and mass transfer, biochemical and genetic adaptations in the preservation of biological systems.
CRYOPRESERVATION OVERVIEW
The initial cryopreservation studies were based on the serendipitous observation that the sperm cells were able to survive freezing in the presence of a chemical additive, in this case glycerol, whereas the sperm died after freezing in its absence (Polge et al., 1949). This class of chemical additive is called a cryoprotective agent (CPA), where dimethyl sulfoxide (DMSO), glycerol, methanol and propanediol are all examples of what are called low molecular weight membrane penetrating additives. In addition to the amount of CPA that is added to the cell suspension being cryopreserved (usually less than 1 M glycerol or DMSO), the thermal history or rate at which the cells are cooled and warmed directly impacts the post-thaw cell survival. Although much work has been done on understanding the protective effects of a variety of CPAs and thermal histories on cells, the bulk of our understanding in this area is still empirical in nature.
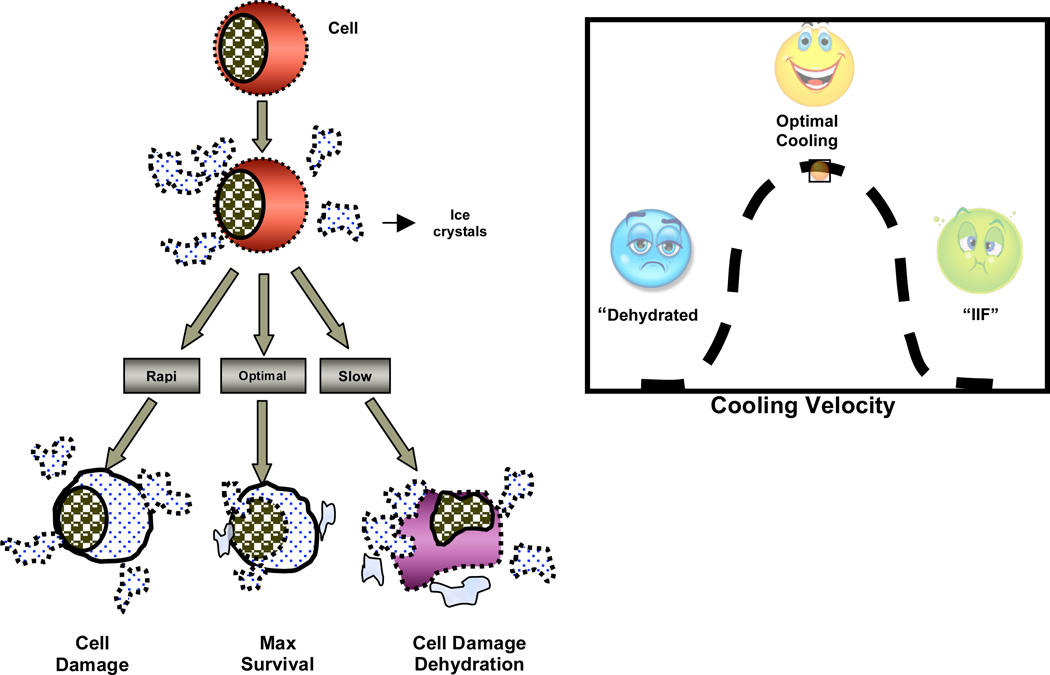
Reduced to its fundamentals, cryopreservation (freezing storage) requires that cells tolerate four non-physiological conditions: (i) exposure to molar concentrations of cryoprotective additives (CPAs), causing “osmotic” injury; (ii) cooling to subzero temperatures, causing “chilling” injury; (iii) removal or conversion of almost all liquid cell water into the solid state, or the freezing process, (iv) warming to room temperature, causing “recrystallization” or “thawing” injury.
When frozen in molar concentrations of CPAs, various types of cells exhibit survival that is strongly dependent on the cooling rate; the optimum cooling rate that yields maximum survival is dependent both on the type and concentration of CPA. Cell survival is equally dependent on warming rate; the optimum warming rate is dependent both on the CPA and its concentration, as well as on the cooling rate that preceded it.). These facts are especially relevant to tissue cryopreservation, since tissues comprise many diverse types of cells, each type with its own characteristic size, shape and permeability properties. Therefore, cooling and warming conditions that are optimum for one cell type within the tissue may be damaging to other types. Survival of frozen tissues is also dependent on cooling and warming rates, as well as on the rate and method by which CPAs are removed from cryopreserved tissues (Mazur et al. 1976; Pegg, 1996; Pegg and Karow, 1987).
Osmotic Injury
Several investigators have shown that the cell survival was affected by time of cell exposure to solutions containing CPAs before the cells were returned to isotonic conditions. The longer the time of exposure, the lower the survival. This loss of cell viability is deemed to be due to osmotic injury and several reasons for this injury have been proposed, including a) mechanical rupture of the cell membrane in hyposmotic solutions (i.e. expansion lysis); b) frictional force between water and potential membrane 'pores' causes cell membrane damage (Muldrew and McGann, 1994); c) resistance to cell shrinkage by cytoskeleton components and resulting damage to sperm cells (Meryman, 1970; Noiles et al. 1997); d) cell shrinkage causes irreversible membrane fusion change and when the cells return to isotonic condition, the cells lyse before their normal volume is recovered (Steponkus and Weist, 1979); and e) hyperosmotic stress caused by leakage/influx of non-permeating solutes (Mazur et al. 1972).
Although, the exact cause of osmotic injury is still unknown, the most obvious and common strategy used to reduce this injury is the avoidance of excessive variations in cell volume (Gao et al. 1995; Willoughby et al. 1996). Thus, optimization of CPA addition and removal requires accurate simulation of volumetric excursions during the CPA addition and removal process. When aqueous solutions are frozen, water is removed in the form of ice, causing solutions to become increasingly concentrated with decreasing temperature; the reverse occurs during thawing. When cells are frozen, they are forced to respond to these changes in solution concentrations. Cells frozen slowly undergo osmotic contraction, losing most of their cell water. When such cells are warmed rapidly, the melting of the cell suspension is equivalent to rapid dilution of the CPA that became concentrated during freezing. The rapid influx of water into cells or tissues as the extracellular milieu begins to melt can cause osmotic shock at subzero temperatures. Thus, the sensitivity to osmotic shock is a function of a cell’s permeability to water and solutes.
Another osmotic shock may occur during removal of the CPA after the cells are warmed and thawed at suprazero temperatures. This osmotic shock can be prevented by use of any one of various mono- and disaccharides as osmotic buffers (Leibo, 1984; Leibo and Oda, 1993; McWilliams et al., 1995). A key to this approach is to measure permeability of the tissue to various CPAs, so as to identify those to which the tissue being cryopreserved is most permeable and least likely to cause osmotic shock during removal, as described by Newton et al. (1998). For large cells, such as oocytes and embryos, it is now commonplace to use non-permeating compounds such as saccharides (galactose, sucrose or trehalose) as osmotic buffers to prevent osmotic shock during removal of CPAs. Rather than direct washing of thawed tissues to remove CPAs, osmotic stresses can also be alleviated by use of osmotic buffers and/or elevated temperatures. This approach has been shown to completely eliminate osmotic shock of mouse zygotes (Oda et al. 1992).
Mathematical Modeling of CPA Addition and Removal Process
Numerical predictions of volumetric excursions during the CPA addition (loading) and removal (unloading) processes in cells and tissues can be computed based on, i) microscale membrane transport formulations using irreversible thermodynamic principles (Kedem and Katchalsky, 1958) and ii) macroscale diffusion of CPAs using classical mass diffusion principles (Fick's Law) with a sink term for the CPA movement into the cells.
The model of CPA addition and removal developed by Kedem-Katchalsky (1958) assuming irreversible thermodynamic principles has also be extended to a tissue system, with the assumption that the tissue system can be modeled as a series of Krogh cylinders (He and Devireddy, 2005). Briefly, the K-K model is dependent on three cell membrane parameters: Lp or the hydraulic conductivity which measures the volume flow induced by a hydrostatic pressure difference (ΔP); w or the solute permeability which measures the solute flux induced by a concentration difference (Δp); and s or the reflection coefficient which describes the ‘relative permeability’ of the membrane to the solute. These three transport coefficients characterize the passive flux of a given solute (Js) and a given solvent (Jv) through a membrane via the K-K equations as:
Thus, the solution of the K-K equations with the appropriate parameters and the structural properties will result in the simulation of both solute and solvent flux across cell membrane and concurrently, the generation of the cell volumetric excursions at various locations within the tissue.
Macroscale Membrane Transport Formulation (Fick’s Law)
In addition, to using the irreversible thermodynamic model shown above, the cell volumetric excursions at various locations within the tissue can also be modeled using the Fick’s Law of diffusion with the uptake (addition) of CPA to the cells being modeled using a sink term while the rejection (removal) of CPA from the cells being modeled as a source term (Devireddy et al., 2000). Briefly, Fick’s Law of diffusion describes the passive movement of molecules across its concentration gradient. To complete the macroscale model, the mass diffusivity, D and the convective diffusivity, K need to be known a priori and are not always available in the published literature.
The development and implementation of the macroscale and microscale models of CPA addition and removal in tissues are necessary to obtain a qualitative and quantitative understanding of the interplay between several tissue parameters, namely cell density (which varies from 85 to 50% in native tissues to 5–10% in engineered tissue equivalents), type (penetrating and non-penetrating) of CPAs as well as the method of addition and removal (one step or multi step) of the CPA. Thus, significantly enhancing our ability to prevent damage to tissue before and after the freezing process.
Cold Shock or Chilling Injury
Cold shock or chilling injury is caused during the lowering of temperature from 37 °C to 4 °C and has been well characterized in a variety of reproductive cells and tissues (see review by Morris, 1987; Parks, 1997; Leibo et al., 1996).
Cold shock or chilling injury in sperm cells is characterized by abnormal patterns of swimming (circular or backwards), rapid loss of motility, acrosomal damage, plasma membrane damage, reduced metabolism and loss of intracellular components (Amann and Pickett, 1987; Moran et al. 1992). The sensitivity to cold shock and the amount of chilling injury varies significantly between different cell types with mouse and equine sperm being the most sensitive while human sperm cells are relatively less sensitive (Gao et al. 1997).
Cooling human oocytes to room temperature for as little as 10 minutes causes irreversible damage to the meiotic spindle (Pickering et al. 1990), and chilling them to 0°C in the presence of a CPA only exacerbates the abnormalities (Sathananthan et al. 1988). Both GV-stage and metaphase II-stage bovine oocytes exhibit similar time-dependent sensitivities to chilling to 0°C (Martino et al. 1996a). Anoxic storage of some types of intact organs may induce various types of injury (see reviews in Pegg, 1996; Pegg and Karow, 1987).
For single cells, e.g. oocytes and embryos, it is possible to “outrace” chilling injury by cooling samples at very high rates (Leibo and Oda, 1993; Martino et al. 1996b). But cooling pieces of tissue several mm in size, especially when placed into plastic ampoules, imposes constraints. It may not be possible to cool such samples fast enough to avoid chilling injury. Standard methods of equilibrium cooling at rates <1°C/min inevitably expose tissues to temperatures near 0°C for several minutes. It will be necessary, therefore, to develop alternate strategies to avoid or mitigate the chilling injury in reproductive cells and tissues. Some strategies and techniques to minimize cold shock can be gleaned from studies performed on bacteria, as described in the next section.
Cold shock - Studies on Bacteria
Bacteria differ greatly in their ability to tolerate freezing and thawing, for reasons that are only now being understood. Exposure of mesophilic bacteria to low temperatures has been shown to enhance their ability to withstand repeated freeze-thawing (“cryotolerance”) (Thammavongs et al., 1996; Panoff et al., 1997; Panoff et al., 1998). In addition, exposure of lactic acid bacteria to osmotic stress has been also shown to confer tolerance to freeze-thawing (Panoff et al. 2000). Bacteria have unique adaptations to low temperature and may have unique and currently unknown mechanisms not only to withstand prolonged exposure to freezing, but also to actually grow at sub-zero temperatures. The remarkable ability of several bacterial species to grow at temperatures as low as –8°C has been described as early as the 1930s (Haines, 1931; Tschistajakow and Botescharowa, 1938a; Tschistajakow and Botescharowa, 1938b).
When bacteria are exposed to sudden temperature downshifts, two major responses are commonly observed, changes in membrane lipid composition and production of novel proteins (Jones, 1987; Gounot, 1991). Increases in lipid unsaturation and decreases in the length of the fatty acyl chains maintain the membrane in a fluid state (Chapman, 1975; Russel, 1990). Although bacteria that can grow at low temperature appear to have enhanced potential to maintain membrane lipid fluidity (Gounot, 1991), the molecular and physiologic mechanisms underlying the differences in growth temperature minima among microbes remain largely unclear (a schematic of a hypothetical “master switch” model is shown in Fig. 1).
Fig. 1.
Master Switch Hypothesis: Hypothetical Model describing how low temperature (LT) in association with light regulates the expression of cold responsive genes. Low temperature is first perceived by putative membrane associated receptor(s) and then transmitted to the nucleus. This triggers global regulator (master switch). These transcription factors activate the promoters (red helix) of cold regulates genes (blue helix). The transcribed mRNAs are translated to different proteins that contribute to the development of freezing or cold tolerance (17).
The cold shock response has also been investigated in several gram-negative and gram-positive bacteria. The most highly conserved feature of the response is the expression of cspA homologs, having been documented in hyperthermophiles, thermophiles, mesophiles and psychrotrophs. In several species, multigene families have been identified that contain from three to nine cspA homologs. In each case, at least one of these proteins is increased in expression upon temperature downshift (Graumann et al., 1996; Mayr et al., 1996; Michel et al., 1997). Interestingly, in the case of the psychrotrophic bacterium Arhrobacter globiformis SI55, production of the protein is not limited to the cold shock period immediately following the temperature downshift, but continues during growth at low temperature (Wouters et al., 1998), suggesting that the protein functions in cold acclimation (in addition to its function during cold shock). Studies with additional psychrotrophic and psychrophilic bacteria are beginning to provide further insight on the involvement of cold-shock and cold acclimation proteins in the ability of the organisms to grow at low temperatures (see Berget et al., 1997, for a model suggesting ribosome as a temperature sensor). Most of these studies have involved the psychrotrophic food-borne pathogens Yersinia enterocolitica and Listeria monocytogenes. In Y. enterocolitica, polynucleotide phosphorylase, PNP (involved in mRNA degradation) is a cold shock protein. Moreover, PNP is required for growth at 5°C in Y. enterocolitica (Goverde et al., 1998); strains carrying transposon insertions in this gene were unable to form colonies at 5°C yet were indistinguishable from the wild type strain at 30°C. In L. monocytogenes, twelve proteins are induced following temperature downshift from 37 to 5°C, and four of these proteins continued to be expressed during cold growth, thus behaving as cold acclimation proteins (Bayles et al., 1996). Other investigators have identified cold-sensitive mutants of L. monocytogenes in which membrane fatty acid composition was markedly altered (Annous et al., 1997).
Cyanobacteria respond to a decrease in ambient growth temperature by desaturating the fatty acids of membrane lipids to compensate for the decrease in membrane fluidity at low temperatures (Murata and Ishida, 1987). Fatty acid desaturases are the enzymes that introduce the double bonds into the hydrocarbon chains of fatty acids and thus these enzymes play an important role during the process of cyanobacteria cold acclimation. The unsaturation of fatty acids occurs without de novo synthesis of fatty acids during low temperature acclimation of cyanobacteria (Sato and Murata, 1982; Wada and Murata, 1990). Most of the cyanobacterial desaturases are intrinsic membrane proteins that act on acyl-lipid substrates.
Of the four desaturases identified in the cyanobacterium Synechocystis sp. PCC 6803, three are clearly induced following a temperature downshift albeit at different rates (Los et al., 1997). In Synechococcus sp. strain PCC 7002, three desaturases, desA, desB, and desC, have been identified (Sakamoto and Bryant, 1997). The expression of all three genes was induced within five minutes of a shift from 38 to 22°C. The three genes were expressed more highly in cells grown continuously at 22°C. It has been suggested that the accumulation of desA transcripts at low temperatures might be explained by an acceleration of transcription and by stabilization of the desA mRNA (Sakamoto et al., 1998). Genetic manipulation of the desA gene has further demonstrated that the unsaturation of membrane lipids is essential for low temperature tolerance of cyanobacteria.
Interestingly, cold-sensitive Synechocystis with monounsaturated fatty acids only become cold-tolerant (insertion mutants of desA and desB were unable to grow at 15°C) by introduction of the gene for des A that allows cells to synthesize diunsaturated fatty acids (Wada and Murata, 1990; Sakamoto et al., 1998). On the other hand, directed mutations of desaturases in cold-tolerant Synechocystis that lead to production of monounsaturated fatty acids make this strain cold-sensitive (Tasaka et al., 1996). However, the temperature-sensing mechanism(s) and the control mechanism(s) regulating the expression of the desaturase genes are still unknown.
One of the most widely studied bacteria, E. coli, stops the synthesis of most proteins and ceases growth when subjected to a rapid downshift in temperature from about 37 to 15°C. A number of “cold shock” proteins, however, are synthesized and gradually (within a few hours) the bacteria resume growth. The four most abundant cold shock proteins, cspA, cspB, cspG and cspL, are members of a gene family comprising nine closely related homologs (Goldstein et al., 1990; Lee et al., 1994; Nakashima et al., 1996; Wang et al., 1999). Intriguingly, cspA, cspB and cspG are synthesized in large amounts upon cold shock under conditions that normally inhibit protein synthesis, such as amino acid starvation and treatment by inhibitory antibiotics (Etchegaray and Inouye, 1999). Each of the cspA homologs, referred to as “RNA chaperones,” bind to RNA without apparent sequence specificity. Experimental evidence indicates that cspA and its homologs act as transcription antiterminators. Moreover, they appear to act to induce cold-regulated expression of four other cold-shock proteins, RbfA, IF2, NusA and PNP. Both RbfA and IF2 are involved in the initiation of translation; nusA associates with RNA core polymerase and is involved in both transcriptional termination and antitermination; and PNP (polynucleotide phosphorylase) is a 3’ to 5’ exonuclease that interacts with Ribonulease E to degrade mRNA. Other cold shock proteins that have roles in E. coli acclimating to low temperature include csdA, H-NS, trigger factor and Hsc66 (Goldstein et al., 1990; Atlung and Ingmer, 1997; Kandror and Goldbergt, 1997). Intriguingly, studies also suggest that E. coli expressing eukaryotic bbc1 gene have enhanced tolerance against salt-stress and freezing-stress (Silbert et al., 1998).
Freezing Injury
The studies that report the effect of freezing on the cell viability in native biological systems ranging from cell suspensions to whole tissue slices to native arteries include, Mazur et al. (1972), Fisher et al. (1996), Cuono et al. (1986) and Villalba et al. (1996). In general, these studies show a low percentage of cell viability at the slower and the faster cooling rates investigated. This loss of viability is likely attributable to the two main biophysical mechanisms that occur during the freezing process in all biological systems, as described below.
All cell systems do share common cell freezing responses which may be exploited to better understand and alleviate the specific problems of cryopreservation. In particular, when ice forms in a cellular suspension, electrolytes and proteins from the original extracellular solution are left within highly concentrated unfrozen liquid fractions. The cells, which remain unfrozen at the instant of ice formation within the extracellular space, respond to the increased concentration of the unfrozen fraction by either dehydrating (transporting water out into the unfrozen liquid fraction), or by the formation of intracellular ice in the cytoplasm (Mazur, 1984). Dehydration is the attempt by the cell to reequilibrate the cytoplasmic concentration with the higher concentration extracellular unfrozen fraction by the rejection of cell water. Intracellular ice formation occurs when the super cooling (temperature below the equilibrium phase change temperature) within the cytoplasm is sufficiently large to drive the nucleation and growth of an ice crystal within the cytoplasmic compartment. In the case of dehydration, the cell can be damaged by the increase of concentration both inside and outside of the cells as the temperature drops (predicted by the phase diagram of the solution) by a form of solute effects injury (Lovelock, 1953). Intracellular ice formation is generally considered lethal if > 10–15% of the cellular water is involved (Mazur et al. 1972; McGrath, 1988; Toner, 1993).
Both IIF and long exposures to high solute concentrations are lethal to cells. So cooling rates which are either 'too high' or 'too low' can kill cells. Therefore, the 'optimal' cooling rate should and does exist between the 'high' and 'low' freezing rates. This has been confirmed experimentally for a variety of cells and the curve of cell survival plotted as a function of the cooling rate, has a characteristic inverted U-shape, as shown schematically in Fig. 2 (Mazur, 1984). Whether a prescribed cooling rate is too 'low' or too 'high', is a function of cell membrane permeability to water and the probability that any water remaining trapped within the cell at any given subzero temperature will nucleate and turn to ice. Differences in membrane permeability to water and probability of IIF result in different 'optimal' cooling rates for different cells. In general, the 'optimal' cooling rate is defined as that cooling rate which minimizes both the slow cooling and IIF injury, i.e. cool as fast as possible without forming damaging intracellular ice. Thus, to optimize and generate a firm biophysical understanding of the freezing process in any biological system, both water transport (dehydration) and intracellular ice formation (IIF) need to be experimentally determined.
Fig. 2.
Inverse ‘U’ Curve showing the relationship between freezing velocity and cell response.
Water transport and IIF can be experimentally observed in most cell systems using a cryomicroscopy system (Cosman et al. 1989; Toner et al. 1992; Devireddy et al. 1998; Berrada and Bischof, 2000). Observations of these events can then be fit to mathematical models which can then be used to predict the cell's response to freezing under arbitrary cooling conditions, and hence help to develop better cryopreservation protocols. Although both water transport and IIF have been observed and modeled in many isolated cell types during freezing, the measurement of these processes in tissue systems has only become possible in the late 90’s due to the development of two novel techniques: i) a low temperature microscopy technique (Pazhayannur and Bischof, 1997) and ii) a differential scanning calorimetry (DSC) method (Devireddy et al. 1998; Devireddy and Bischof, 1998). A combination of the microscopic and calorimetric techniques has proven to be especially useful in determining the water transport processes in a variety of heterogeneous tissue systems, including the Dunning AT-1 rat prostate tumor tissue (Devireddy et al. 1999a), the liver tissue of freeze-tolerant wood frog (Devireddy et al. 1999b, 1999c), in uterine fibroid tumor tissue (Devireddy et al. 2001). However, it should be noted that the currently available technique could only be used to generate water transport data during freezing in tissue cells. Fortunately, once the water transport parameters are known, it is possible to make useful extrapolations to the minimum amount of water that will be trapped as intracellular ice within a tissue cell - and hence obtain a conservative boundary for the amount of IIF in a tissue system (Pazhayannur and Bischof, 1997; Devireddy et al. 1999a; Devireddy et al. 2001; Devireddy and Bischof, 2003).
It is important to note, however, that tissues respond very differently to ice formation than do cell suspensions. For example, in a series of experiments with smooth muscle (Taylor and Pegg, 1983), sheep cartilage (Pegg, 1998), and rabbit kidneys (Pegg, 1987), location of ice crystals was shown to be damaging because the tissues were unable to dehydrate sufficiently to avoid intracellular ice formation. Severe ice crystal damage has also been shown to occur within blood vessels, corneal tissues, and heart valve leaflets. Under practical cooling conditions, extracellular ice crystals will usually not be in equilibrium either with respect to total volume of the tissue or with individual cells within the tissue. Consequently, any ice that has formed within the tissue itself is likely to undergo recrystallization during warming, unless the sample can be warmed at a very high rate. Cells in dilute suspensions are not affected very much by extracellular ice per se. In contrast, closely packed cells, e.g. erythrocytes present at a high concentration, are affected because the cells are unable to dehydrate sufficiently because of the neighboring cells (Pegg et al., 1984). Cells in tissues are usually closely packed, and they also have interacting connections with each other and with basement membranes. Tissues may also be traversed by fine capillaries or other blood vessels. Furthermore, tissues have three-dimensional structure that cell suspensions do not. Changes in both extracellular ice surrounding a piece of tissue, and recrystallization during warming of intracellular ice within cells of the tissue is likely to cause damage (Devireddy et al., 2003; Neidert et al., 2004).
Modeling of Biophysical Phenomena During Freezing
This section presents the mathematical equations and the biophysical parameters used to model water transport and intracellular ice formation in the presence of vascular/extracellular ice in biological systems.
Water Transport out of Embedded Tissue Cells
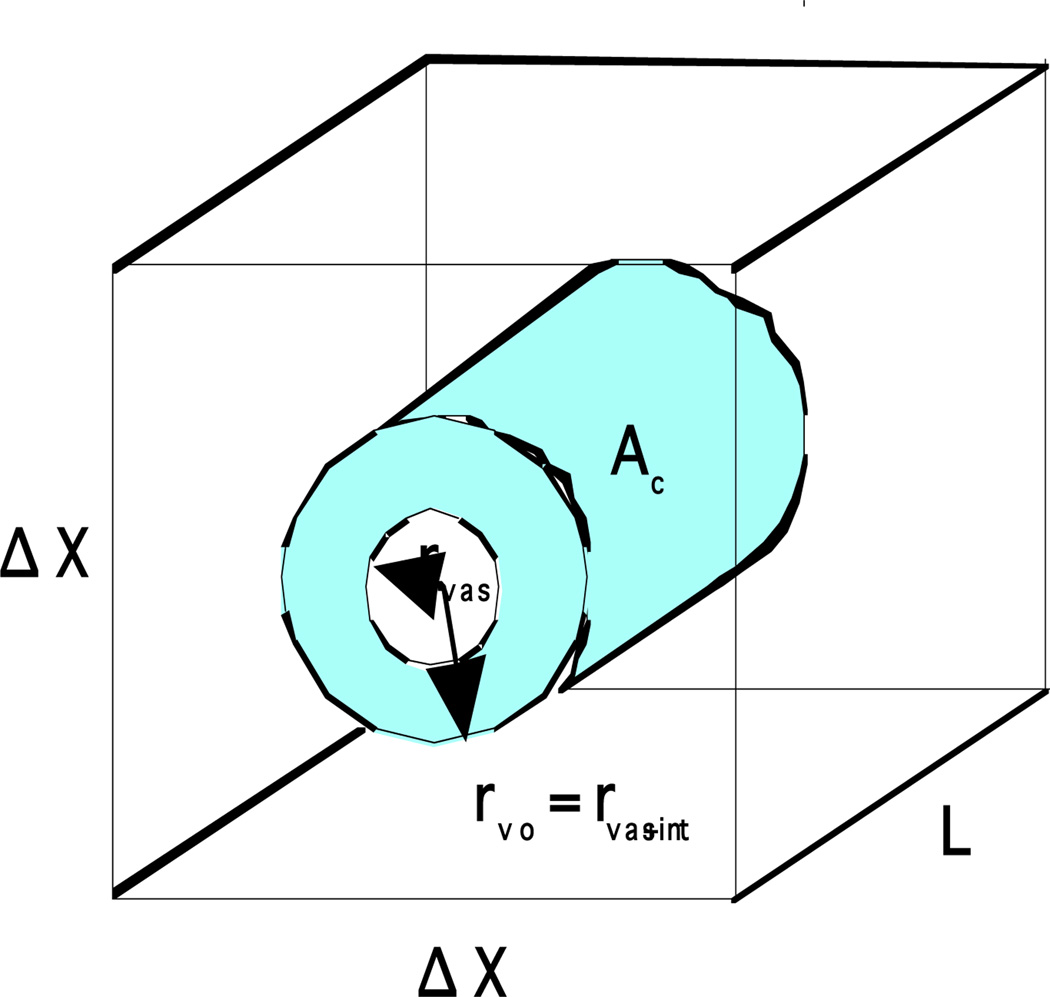
Water transport across a cell membrane during freezing in the presence of extracellular ice has been modeled by Mazur (1963) and later modified by Levin et al. (1976). During freezing of tissue systems ice tends to form first in the vascular/extracellular space of whole tissues (Love, 1966; Rubinsky et al. 1987; Bischof et al. 1993). The presence of vascular/extracellular ice creates a small highly concentrated unfrozen saline fraction which induces a chemical potential difference across the tissue cell membrane. In response to this gradient the intracellular fluid (water) is driven through the cell membrane into the vascular/extracellular space. The consequent reduction in tissue cellular volume was modeled using a Krogh cylinder approach by Rubinsky and Pegg (1988) and Pazhayannur and Bischof (1997) as,
| [1] |
with Lp, ,the tissue cell membrane permeability to water defined by Levin et al. (1976) as,
| [2] |
where, Lpg[cpa] is the reference membrane permeability at a reference temperature, TR; ELp[cpa] is the apparent activation energy (kJ/mol) or the temperature dependence of the cell membrane permeability; V is the cell volume at temperature, T; Ac is the effective membrane surface area for water transport, assumed to be constant during the freezing process; Vb is the osmotically inactive cell volume; R is the universal gas constant; B is the constant cooling rate; vw is the molar volume of water; js is the disassociation constant for salt; ns is the number of moles of salt (= Ci•(Vo-Vb), where Ci is the initial cell osmolality,0.285M); ΔHf is the latent heat of fusion of water; r is the density of water. The two unknown membrane permeability parameters of the model L pg and ELp, are determined by curve-fitting the water transport model to experimentally obtained volumetric shrinkage data during freezing.
Fig. 3 shows the Krogh cylinder model and the corresponding dimensions including the sinusoid (vascular) radius (rv), the distance between adjacent sinusoid centers (ΔX) and the axial length of the cylinder (L); In the Krogh model the cellular space with volume V is modeled as the box surrounding the cylinder (V =LΔX2 - πrv2L) and the effective membrane surface area available for water transport is assumed to be a constant, Ac = 2πrvoL, where rvo is the initial sinusoid radius. In order to use Eqn. [1] to model water transport in the tissue system several other assumptions are necessary and are described in detail by several investigators including Rubinsky and Pegg (1988) and Pazhayannur and Bischof (1997). The unknown parameters of the model (Lpg and ELp) are determined by curve-fitting the water transport model to experimentally determined water transport data, obtained using the DSC and the freeze substitution microscopy techniques as described elsewhere (Pazhayannur and Bischof, 1997; Devireddy et al., 1998).
Fig. 3.
Schematic drawing of the Krogh cylinder, a representation of generic tissue cell unit.
Intracellular Ice Formation (IIF) in Tissue Cells
Several models are reported in literature to predict the likelihood of intracellular ice formation inside cells during freezing including Toner et al. (1990), Pitt (1992) and Muldrew and McGann (1994). In this study, we propose to follow the mechanistic approach developed by Toner et al. (1990), based on the classical theory of nucleation (Turnbull and Fisher, 1949; Turnbull and Vonegut, 1952). Toner's mechanistic model, states that for a thermodynamic system composed of identical biological cells, the probability of intracellular ice formation (PIF) by surface catalyzed nucleation (SCN) in a given population of cells may be given as (Toner, 1993),
| [3] |
where the subscript 'o' refers to isotonic conditions; DT is the amount of supercooling (T-Tf), K; all the other variables are as described elsewhere (Toner, 1993). Assuming that the nucleating site is the plasma membrane and that the number of solute molecules are much lesser than the total number of water molecules, the ratio of Ns /Nso in Eqn. [3] can be written as A/Ao (Toner et al. 1990; Toner et al., 1992; Toner, 1993). In order to evaluate Eqn. [3] during freezing of cells, both the concentration and temperature dependence of the viscosity, h is needed (Toner et al. 1990). However in this study, the dependence of viscosity on the concentration of cytoplasm was neglected and a power law dependence of water viscosity on temperature, T (K) will be applied (η(T)= 0.139 • ((T/225)− 1)−1.64 × 10−3 Kg/ms) as described by Toner et al. (1992) and Toner (1993). The two unknown ice nucleation parameters (ΩSCN and κSCN) will be obtained by curve fitting Eqn. [3] to the experimentally determined PIF in rat liver tissue sections at cooling rates ranging from 10 to 50°C/min.
Toner’s intracellular ice formation model also describes the formation of intracellular ice by a volume catalyzed nucleation (VCN) mechanism when the surface catalyzed nucleation mode is suppressed by rapid cooling or in cases where cooling proceeds in the absence of extracellular ice (i.e. the intracellular fluids are supercooled to the volume catalyzed nucleation temperature). The two nucleation parameters (ΩVCN and κVCN) for volume catalyzed nucleation are similar to those described above for the surface catalyzed nucleation; however, the form of the overall probability function is slightly different (Toner et al. 1990; Toner et al. 1992; Toner, 1993):
| [4] |
Since the volume catalyzed nucleation is assumed to occur spontaneously throughout the volume of the cytosol, the PIFVCN is dependent on the volume of the cell. Since there are currently no experimental techniques to quantify the volume catalyzed nucleation parameters (ΩVCN and κVCN) in biological cells, the parameters obtained by Franks et al. (1983) for physiologic cell-sized saline droplets, i.e.ΩVCN = 6.0 × 1057 (m2s)−1 and κVCN = 1.9 × 1012 (K5) are used in this study as was done other investigators, including Toner et al. (1990), Toner et al. (1992) and Bischof et al. (1997).
By combining both the surface and volume catalyzed modes of intracellular ice formation as,
| [5] |
one obtains a general equation (Eqn. 5) which can be used to predict intracellular ice formation for a variety of cooling rates at various subzero temperatures in a population of biological cells (Toner et al. 1990; Toner et al. 1992; Toner, 1993). In summary, the thermodynamic state of the intracellular solution during freezing in the presence of extracellular/vascular ice can be characterized using a set of water transport (Lpg and ELp) and ice nucleation (ΩSCN and κSCN and ΩVCN and κVCN) parameters.
Thawing Injury
Many biological systems can survive the thawing process provided the ice formed does not recrystallize. Thus, thawing injury will be minimized using very high (>100 °C/min) thawing rates.
VITRIFICATION OVERVIEW
During traditional cryopreservation techniques, tissues are exposed to large magnitudes of chemical and thermal excursions from "physiological" which can have dramatic affects on both material properties and cell viability (Mazur, 1970). Large chemical gradients develop during the loading and unloading of cryoprotectants (CPAs). In addition, freezing and thawing leads to large overall temperature (and associated phase) changes which can drastically rearrange cellular and extracellular water/ice and thereby damage the extracellular matrix structure (Bischof, 2000; Devireddy et al., 2003; Neidert et al., 2004). Since the formation of ice crystals can lead to negative effects on both viability and mechanical properties, current work is aimed at investigating alternatives.
One possibility is the use of glass forming sugars such as Trehalose, and/or Sucrose to help the extracellular compartment and matrix to reach a partially or totally vitrified state (i.e. no crystals). It may also be possible to attempt freeze drying (i.e. removal of moisture content in the frozen sample) of the vitrified samples if stabilizing sugars are present. In fact, it may also be possible to dry the tissues (under appropriate conditions) even in the absence of freezing, as evident in various anhydrobiots, e.g., brine shrimp or cyanobacteria (Potts, 1999). The effect of vitrification and/or freeze-drying on tissue structure and mechanical properties as well as cell viability would certainly need to be established and optimized. These preservation technologies of the future could completely revolutionize storage, banking and transport (and hence use) of tissues.
The primary motivation for investigating the viability of vitrification as an alternative process of banking and storage of tissues is the ability of vitrified samples to maintain structural integrity during and after the freezing process. Several studies show that vitrification leads to a dramatic increase in patency of vascular grafts and human skin over traditional cryopreservation methods (Song et al., 2000; Brockbank et al., 2000; Fujita et al., 2000). Vitrification is the solidification of a liquid into an amorphous or glass like state. This is different than the traditional cryopreservation process where crystallization and growth of ice is considered as a natural by product of the technique (a comparison of cryopreserved and vitrified veins cryosubstituted at −90 °C is shown in Fig. 4). It is postulated that the liquid to glass transformation (vitrification) is unlikely to have any adverse biological effects as opposed to the liquid to crystal transformation (cryopreservation). Thus, the improved post-thaw viability of samples that have been vitrified as opposed to cryopreserved samples.
Fig. 4.
Morphology of frozen (A) and vitrified (B) veins cryosubstituted at −90 °C. The structure of the frozen sample is noticeably distorted due to the presence of variable sized ice crystals. In marked contrast, the vitrified sample appears to be free of ice with the normal structural features of the tunica intima (I), the tunica media (M) and tunica adventitia (A) clearly discernible. Reproduced from Song et al. (2000).
As noted by Luyet and his colleagues in the 1950s, vitrification is a matter of allowing insufficient time for the freezing of the sample during cooling to temperatures very far below its nominal freezing point (i.e. the imposition of a very high cooling rate to a very low sub zero temperature). For example, the cooling rates required to vitrify pure water is in the order of 106 to 109 °C/min. The simplest way to supress or prevent the crystallization of ice in solutions (i.e. vitrify samples at practicable cooling rates) is the use of chemical compounds (cryoprotectants) in very high concentrations.
Fig. 5 shows the supplemented phase diagram for a generic chemical compound in water (redrawn from Fahy et al., 1984). As might be expected, different concentrations of the chemical compound result in different regions of behavior in the phase diagram. In region I, cooling to any temperature below the equilibrium freezing/melting temperature (Tm) results in the formation of ice crystals due to the presence of impurities or heterogeneous nucleating agents. In the absence of impurities, homogeneous nucleation of ice occurs at a well defined temperature (Th). Thus, there is no clear way to supress the formation of ice crystals in region I (with the exception of imposing impracticable high cooling rates) and this region is comparable to traditional cryopreservation processes.
Fig. 5.
Generic supplemented phase diagram. See text, for discussion. Redrawn from Fahy et al. (1984).
At higher concentrations (region II), cooling rates achievable by quenching of small samples allow the apparent vitrification of the sample (Boutron, 1986; MacFarlane, 1987). In this region, the sample remains transparent to the eye rather than white and opaque after cooling to a temperature below the glass transition temperature (Tg). Nevertheless, it is likely that ice crystal nuclei will form in such solutions, even if the nuclei are unable to grow to visible size (Fahy, 1988). Thus, region II is called "doubly unstable" because the ice formed is not only thermodynamically unstable but is also unstable by virtue of Tg < Th. There are two disadvantages to using high cooling rates in combination with concentrations shown in region II. The first is that the required cooling rates are not applicable to large organs or tissues and the second is the manifestation of ice nuclei during the rewarming process along the curve labeled Td. This is the curve for devitrification or the formation of ice from previously vitrified liquid. However, it should be noted, that many biological systems can survive some devitrification provided the ice formed does not recrystallize. Thus, the use of fast thawing rates. At the high concentration limit of region II, Tg ∼ Th, and the heterogeneous nucleation can be transcended in much the same way as the homogeneous nucleation is transcended in region II. Thus, in Region III, the formation of a vitreous state intervenes to make ice crystallization impossible (i. e. both the homogeneous and heterogeneous nucleation of ice are supressed).
The concentrations which is equal to the boundary between region II and III is referred to as CV, i.e. the lowest concentration supporting apparently complete vitrification. At CV, Tg is achieved without encountering Th. In general, CV ranges from 40 to 60% weight to volume ratio (Fahy, 1988). The concentrations at which devitrification is longer observed determines the boundary between region III and region IV. Concentrations in region IV would be very stable in terms of ice formation and are ideal for vitrification of samples but produce overwhelming chemical toxicity to living systems. Hence region III and particularly the lower concentration limit of region III, forms the best compromise between potential chemical toxicity and the tendency to devitrify during thawing. Therefore, this region is of great relevance and interest for practical preservation of macroscopic systems, although, systems of sufficiently small size and of sufficiently great resistance to devitrification can survive rapid cooling and thawing in more dilute solutions (Fahy, 1988).
Vitrification Strategies
To reduce CV several strategies can be adopted, some of which are outlined here:
Use of High Pressures: Hydrostatic pressure is indistinguishable from solutes, in that the relationship between Tm and Th is the same for pressure as it is for solutes [Fahy et al., 1984; Fahy, 1988). But the injurious effects of pressure for biological systems limits the applicablity of high pressures to < 1000 atms; which translates in water to a solute concentration of 23% w/w Me2SO or 29% w/w of glycerol (Fahy et al., 1988). Thus the application of hydrostatic pressure enables the replacement of potentially toxic chemical compound required for vitrification by a non-toxic pressure. This effect is seen in Fig. 2 as the lowering of Th and an elevation of Tg, causing CV to occur at a lower concentration;
Depression of the liquid to crystal transition temperature (Tm) with the addition of membrane stabilizing sugars like trehalose, sucrose, glucose, raffinose and stachyose (Crowe et al., 1996; Oliver et al., 1998; Crowe et al., 1998)
Chemical control of ice crystal growth using natural or synthetic versions of anti-freeze proteins, AFPs, described later in this review (Harrison et al., 1987).
FREEZE-DRYING OVERVIEW
The use of freeze-drying as a method for storage of allograft valves was reported as early as 1953 by Pate and Sawyer (1953). However, freeze-drying fell into disuse due to the calcification and failure of a majority of implanted valves (Moore et al., 1975). However, the freeze-drying technique has been widely used in pharmaceutical (Rey, 1992) and food industries (Franks, 1994), since it provides increased stability or rapid solubility. One of the primary motives for the study of freeze-drying process is that it is the only preservation method which permits the ambient temperature, long-term storage of biological materials (Rey, 1992; Franks, 1994). Given its light weight and acceptance by regulatory authorities for therapeutic products, dried storage is ideal for long term preservation of artificial tissue scaffolds. Since freeze drying in mammalian systems has only reported reasonable viability after rehydration in one cell system and no tissue systems to date (Kang and Ikada, 1999), current researchers are pursuing it mainly from the perspective of scaffold production and storage. The use of freeze-dried scaffolds has the potential to significantly reduce the time necessary for creation of an artificial tissue and is certainly a credible path of investigation as a partial solution to the storage and banking question in tissue engineered products.
FUTURE STRATEGIES – LEARNING FROM NATURE
Insights from Studies on Plants
Many of the basic insights into freezing injury have come from studies with plants (Hughes and Dunn, 1996; Thomashow, 1998; Thomashow, 1999; Breton et al., 2000; Black and Pritchard, 2002). For instance, plants of tropical origin are typically killed by the slightest freeze, while plants from temperate and polar environments can survive freezing at high subzero temperatures (Thomashow, 1998; Thomashow, 1999). Moreover, most temperate and polar plants increase in freezing tolerance in response to low nonfreezing temperatures, a process referred to as “cold acclimation” or “cold hardening”. Nonacclimated rye plants are killed upon freezing at about −5°C, but after cold acclimation, can survive freezing down to about −30°C.
Steponkus et al., (1993) report that the membrane systems of the cell are the primary site of freezing injury and that freeze-induced membrane damage results primarily from the severe dehydration associated with freezing. For example, if the freezing temperature is −10°C, the unfrozen liquid will have an osmolarity in excess of 5 and typically, greater than 90% of the osmotically active water will have moved out of the cell. Although freezing injury is thought to result primarily from membrane lesions caused by cellular dehydration, additional factors may also contribute to freezing-induced cellular damage. There is evidence that freeze-induced production of reactive oxygen species contributes to membrane damage (McKersie and Bowley, 1997) and that extracellular ice can form adhesions with cell walls and membranes and cause cell rupture (Olien and Smith, 1997). In addition, there is evidence that protein denaturation occurs in plants at low temperature (Guy et al., 1998), which could potentially result in cellular damage.
Most of what is known about freezing tolerance mechanisms has come from the study of the cold acclimation response. These studies have shown that the key function of cold acclimation is to stabilize membranes against freezing-injury. Multiple mechanisms appear to be involved in this stabilization. The best documented are changes in lipid composition (Hughes and Dunn, 1996; Breton et al., 2000; Black and Pritchard, 2002). However, the accumulation of “compatible solutes,” such as proline and simple sugars which typically occur with cold acclimation, are likely to contribute to the stabilization of membranes as these molecules can protect membranes (as well as proteins) against freeze-induced damage in vitro (Strauss and Hauser, 1986; Anchirdoguy et al., 1987) and in vivo (Nanjo et al., 1999). There is also emerging evidence that certain cold-inducible hydrophilic novel and LEA (late embryogenesis abundant) polypeptides also participate in the stabilization of membranes against freeze-induced injury (Hughes and Dunn, 1996; Breton et al., 2000; Black and Pritchard, 2002).
Several groups have identified a family of transcriptional activator proteins, known as CBF1, CBF2 and CBF3 (or DREB1b, DREB1c and DREB1a, respectively), that control the expression of a battery of cold-responsive genes in Arabidopsis including the COR66, COR15a, COR47 and COR78 (Gilmour et al., 1996; Gilmour et al., 1998; Lin and Thomashow, 1992; Stockinger et al., 1997; Steponkus et al., 1998). As discussed above, freezing tolerance must include tolerance to severe dehydration stress (Steponkus et al., 1993). Thus, one might expect that some of the genes that are important for freezing tolerance would also be important in dehydration tolerance. Indeed, many of the COR, LEA and other cold-responsive genes discovered in plants are also induced upon dehydration stress. Moreover, studies do indicate that the cold-responsive genes impart both freezing and drought (high salinity) tolerance, as well (reviewed in Kasuga et al., 1999).
Antifreeze Proteins
Antifreeze proteins (AFPs) that have been identified and best studied are from fish and insects but they have also been described in plants and bacteria. A hallmark characteristic of these proteins is “thermal hysteresis” activity: the proteins decrease the temperature at which ice is formed, but do not affect the melting point of the solution. This effect results from the AFPs binding to the surface of ice nuclei and inhibiting ice crystal growth. In addition, AFPs affect the shape of the ice crystals that form when temperatures drop below the freezing point of the solution and are potent inhibitors of ice recrystallization (they inhibit the coalescing of small ice crystals into large ice crystals). Significantly, AFPs are highly diverse in amino acid sequence and structure (Ewart et al., 1999).
In fish, there are four classes of AFPs: type I are alanine-rich a-helixes: type II are cystine-rich globular proteins; type III proteins form a compact b-sheet structure; and type IV proteins are thought to form a four-helix bundle. Two AFPs from insects, spruce budworm and mealworm beetle, are rich in threonine and cytosine and have been shown to form b-helixes. In winter rye plants, six AFPs ranging in molecular mass from 16 to 35 kD have been shown to accumulate in the extracellular fluids of cold acclimated plants (Ewart et al., 1999). One of the rye AFPs has been purified and shown to have both endochitinase and antifreeze activity (the protein alters the shape of ice crystals and has a low level of thermal hysteresis activity). In contrast, a purified endochitinase from tobacco, a freezing sensitive plant, was devoid of antifreeze activity.
In addition, the functions of the AFPs appear to differ among organisms. In some cases, the AFPs have a role in frost avoidance. In polar fish living in waters that are about two degrees below freezing, the AFPs, through their thermal hysteresis activity, contribute to keeping the blood serum in the liquid state. However, in organisms that do not avoid freezing, like cold-acclimated rye plants, AFP function is presumably different. In the case of extracellular AFPs, it is possible that the inhibition of ice recrystallization or the effects that AFPs have on ice crystal shape might mitigate against physical damage caused by ice and thereby enhance freezing tolerance. Intracellular AFPs, which do not come into direct contact with ice, might have fundamentally different roles. Indeed, it has been shown that certain AFPs (antifreeze glycoproteins) can inhibit leakage of ions from liposomes during thermotropic phase transitions (Antikainen and Griffith, 1997). Thus, some AFPs might have important roles in stabilization of membranes at low and freezing temperatures (Carpenter and Crowe, 1988; Chao et al., 1996).
CONCLUSION
Temperature affects the structure of all large biomolecules, because of which a vast amount of fine tuning of the properties of “molecules of life” has occurred during evolution. The fact that organisms are capable of carrying out highly similar metabolic reactions and information transfers (DNA to RNA to protein) over a wide range of temperature (from −80°C to over 100°C) is a testament to the pervasiveness and success of biochemical adaptational processes (Johnston and Bennet, 1996; Hochaka and Somero, 2002; Helmreich, 2002; Black and Pritchard, 2002). By studying the traits or character states of organisms that are universally conserved in the face of environmental (temperature) challenges, it should be possible to develop a sense of “what really matters” in the design of biological systems in our universe.
Acknowledgements
NIH grant (R21 DK091852) support from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) and from the Department of Mechanical Engineering at Louisiana State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK or the NIH.
References
- Amann RP, Pickett BW. Principal of cryopreservation and a review of cryopreservation of stallion spermatozoa. J Equine Vet Sci. 1987;7:145–173. [Google Scholar]
- Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24:324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antikainen M, Griffith M. Antifreeze protein accumulation in freezing-tolerance cereals. Physiol. Plant. 1997;99:423–432. [Google Scholar]
- Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- Bayles DO, Annous BA, Wilkinson BJ. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl. Environ. Microbiol. 1996;62:1116–1119. doi: 10.1128/aem.62.3.1116-1119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Normand P, Potier P. CapA, a cspA-like gene that encodes a cold acclimation protein in the psychrotrophic bacterium Arthrobacter globiformis SI55. J. Bacteriol. 1997;179:5670–5676. doi: 10.1128/jb.179.18.5670-5676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrada MS, Bischof JC. A determination of biophysical parameters related to freezing of an ELT-3 cell line; Adv. Heat and Mass Transfer in Biotechnology. 2000;368:41–48. [Google Scholar]
- Bischof JC, Christov K, Rubinsky B. A morphological study of cooling rate response in normal and neoplastic human liver tissue: cryosurgical implications. Cryobiology. 1993;30:482–492. doi: 10.1006/cryo.1993.1049. [DOI] [PubMed] [Google Scholar]
- Bischof JC, Ryan CM, Yarmush RG, Yarmush YL, Toner M. Ice formation in isolated human hepatocytes and human liver tissue. ASAIO J. 1997;43:271–278. [PubMed] [Google Scholar]
- Bischof JC. Quantitative measurement and prediction of biophysical changes during freezing in tissues. Ann. Rev. Biomed. Eng. 2000;2:257–288. doi: 10.1146/annurev.bioeng.2.1.257. [DOI] [PubMed] [Google Scholar]
- Black M, Pritchard HW. Desiccation and survival in plants: Drying without dying. New York: CABI Publishing; 2002. [Google Scholar]
- Boutron P. Comparison with the theory of the kinetics and extent of ice crystallization and of the glass-forming tendency in aqueous cryoprotective solutions. Cryobiology. 1986;23:88–102. doi: 10.1016/0011-2240(86)90022-2. [DOI] [PubMed] [Google Scholar]
- Breton G, Danyluk J, Ouellet F, Sarhan F. Biotechnological applications of plant freezing associated proteins. Biotechnology Annual Review. 2000;6:57–100. doi: 10.1016/s1387-2656(00)06019-1. [DOI] [PubMed] [Google Scholar]
- Brockbank KGM, Lightfoot FG, Song YG, Taylor MJ. Interstitial ice formation in cryopreserved homografts: a possible cause of tissue deterioration and calcification in vivo . J. Heart Valve Dis. 2000;9:200–206. [PubMed] [Google Scholar]
- Carpenter JF, Crowe JH. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988;25:244–255. doi: 10.1016/0011-2240(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Chao H, Davies LL, Carpenter JF. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J. Exp. Biol. 1996;199:2071–2076. doi: 10.1242/jeb.199.9.2071. [DOI] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidly characteristics of lipids and cell membranes. Quart. Rev. Biophys. 1975;8:185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Cosman MD, Toner M, Kandel J, Cravalho EG. An integrated cryomicroscopy system. Cryo-Letters. 1989;10:17–38. [Google Scholar]
- Crowe JH, Hoekstra FA, Nguyen KHN, Crowe LM. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Carpenter J, Crowe LM. The role of vitrification in anhydrobiosis. Ann. Rev. Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- Cuono CB, Langdon R, Birchall N, McGuire J. Simple economical skin cryopreservation. Surg Forum. 1986;37:114–115. [Google Scholar]
- Devireddy RV, Raha D, Bischof JC. Measurement of water transport during freezing in cell suspensions using a differential scanning calorimeter. Cryobiology. 1998;36:124–155. doi: 10.1006/cryo.1997.2071. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Bischof JC. Measurement of water transport during freezing in mammalian liver tissue - Part II: The use of differential scanning calorimetry. ASME J of Biomech Eng. 1998;120:559–569. doi: 10.1115/1.2834745. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Smith DJ, Bischof JC. Mass transfer during freezing in rat prostate tumor tissue. AIChE J. 1999a;45:639–654. [Google Scholar]
- Devireddy RV, Barratt PR, Storey KB, Bischof JC. Liver freezing response of the freeze tolerant wood frog, Rana sylvatica, in the presence and absence of glucose. I. Experimental Measurements. Cryobiology. 1999b;38:310–326. doi: 10.1006/cryo.1999.2175. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Barratt PR, Storey KB, Bischof JC. Liver freezing response of the freeze tolerant wood frog, Rana sylvatica, in the presence and absence of glucose. II. Mathematical modeling Cryobiology. 1999c;38:327–338. doi: 10.1006/cryo.1999.2176. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Bhowmick S, Neidert MR, Tranquillo RT, Bischof JC. Determination of loading and unloading of cryoprotective agents in tissues and tissue-equivalents. Annals of Biomedical Eng. 2000;28:S–124. [Google Scholar]
- Devireddy RV, Coad JE, Bischof JC. Microscopic and calorimetric assessment of freezing processes in uterine fibroid tissue. Cryobiology. 2001;42:225–243. doi: 10.1006/cryo.2001.2327. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Neidert MR, Bischof JC, Tranquillo RT. Cryopreservation of collagen-based tissue-equivalents - Part I: Effect of freezing in the absence of cryoprotective agents. Tiss. Eng. 2003;9:1089–1100. doi: 10.1089/10763270360728008. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Bischof JC. Recent Advances in Cryobiology Using Calorimetry. In: Kakac S, Smirnov H, Mila MR, editors. Low Temperature and Cryogenic Refrigeration. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. pp. 265–294. [Google Scholar]
- Etchegaray JP, Inouye M. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J Bacteriol. 1999;181:1827–1830. doi: 10.1128/jb.181.6.1827-1830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart KV, Lin Q, Hew CL. Structure, function and evolution of antifreeze proteins. Cell. Mol. Life Sci. 1999;55:271–283. doi: 10.1007/s000180050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Fahy GM. Vitrification. 1988:113–146. ASME BED-Vol. 10, HTD-Vol. 98. [Google Scholar]
- Fisher RL, Hasal SJ, Sanuik JT, Hasal KS, Gandolfi AJ, Brendel K. Cold- and cryopreservation of dog liver and kidney slices. Cryobiology. 1996;33:163–171. doi: 10.1006/cryo.1996.0016. [DOI] [PubMed] [Google Scholar]
- Franks F, Mathias SF, Galfre P, Webster SD, Brown D. Ice nucleation and freezing in undercooled cells. Cryobiology. 1983;20:298–309. doi: 10.1016/0011-2240(83)90018-4. [DOI] [PubMed] [Google Scholar]
- Franks F. Long-term stabilization of biologicals. Vol. 12. Biotechnology-NY: 1994. pp. 253–256. [DOI] [PubMed] [Google Scholar]
- Fujita T, Takami Y, Ezoe K, et al. Successful preservation of human skin by vitrification. J. Burn Care and Rehab. 2000;21:304–309. doi: 10.1067/mbc.2000.107745. [DOI] [PubMed] [Google Scholar]
- Gao DY, Liu J, Liu C, McGann LE, Watson PF, Kleinhans FW, Mazur P, Critser ES, Critser JK. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 1995;10:1109–1122. doi: 10.1093/oxfordjournals.humrep.a136103. [DOI] [PubMed] [Google Scholar]
- Gao D, Mazur P, Critser JE. Fundamental Cryobiology of Mammalian Spermatozoa. In: APaC Karow JE, editor. Reproductive Tissue Banking. Academic Press; 1997. pp. 263–328. [Google Scholar]
- Gilmour SJ, Lin C, Thomashow MF. Purification and properties of Arabidopsis thaliana COR (cold- regulated) gene polypeptides COR15am and COR6.6 expressed in Escherichia coli . Plant Physiol. 1996;111:293–299. doi: 10.1104/pp.111.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Pollitt NS, Inouye M. Major cold shock protein of Escherichia coli . Proc. Natl. Acad. Sci. USA. 1990;87:283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounot AM. Bacterial life at low temperature: physiological aspects and biotechnological implications. J. Appl. Bacteriol. 1991;71:386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Goverde RLJ, Huis in’t Veld JHJ, Kusters JG, Mooi FR. The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5°C) Mol. Microbiol. 1998;28:555–569. doi: 10.1046/j.1365-2958.1998.00816.x. [DOI] [PubMed] [Google Scholar]
- Graumann P, Shcröder K, Schmid R, Marahiel MA. Cold shock stress-induced proteins in Bacillus subtilis . J. Bacteriol. 1996;178:4611–4619. doi: 10.1128/jb.178.15.4611-4619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C, Haskell D, Li QB. Association of proteins with the stress 70 molecular chaperones at low temperature: evidence for the existence of cold labile proteins in spinach. Cryobiology. 1998;36:301–314. [Google Scholar]
- Haines RB. Growth of microorganisms on chilled and frozen meat. J. Soc. Chem. Ind. 1931;50:223–227. [Google Scholar]
- Harrison K, Hallet J, Burcham TS, Feeney RE, Kerr WL, Yeh L. Ice growth in supercooled solutions of antifreeze glycoprotein. Nature. 1987;328:241–243. doi: 10.1038/328241a0. [DOI] [PubMed] [Google Scholar]
- Hays LM, Feeney RE, Crowe LM, Crowe JH, Oliver AE. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. 1996;93:6835–6840. doi: 10.1073/pnas.93.13.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Devireddy RV. An Inverse Approach to Determine the Permeability of Cryoprotective Agents and Water in Artificial Tissues. Ann. Biomed. Eng. 2005;33:709–718. doi: 10.1007/s10439-005-1511-x. [DOI] [PubMed] [Google Scholar]
- Helmreich EJM. The biochemistry of cell signaling. New York: Oxford University Press; 2002. [Google Scholar]
- Hochachka PW, Somero GN. Biochemical adaptation – Mechanism and process in physiological evolution. New York: Oxford University Press; 2002. [Google Scholar]
- Hughes MA, Dunn MA. The molecular biology of plant acclimation to low temperature. J. Exp. Bot. 1996;47:291–305. [Google Scholar]
- Johnston IA, Bennett AF. Animals and temperature – Phenotypic and evolutionary adaptation. New York: Cambridge University Press; 1996. [Google Scholar]
- Jones PG, Van Bogelen RA, Neidhardt FC. Induction of proteins in response to low temperatures in Escherichia coli . J. Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror O, Goldberg AL. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc. Natl. Acad. Sci. USA. 1997;94:4978–4981. doi: 10.1073/pnas.94.10.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HW, Tabata Y, Ikada Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials. 1999;20:1339–1344. doi: 10.1016/s0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotech. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes Biochim Biophys Acta. 1958;27:229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Xie A, Jiang W, Etchegaray J, Jones PG, Inouye M. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol. Microbiol. 1994;11:833–839. doi: 10.1111/j.1365-2958.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Leibo SP. A one-step method for direct non-surgical transfer of frozen-thawed bovine embryos. Theriogenology. 1984;21:767–790. doi: 10.1016/0093-691x(84)90022-0. [DOI] [PubMed] [Google Scholar]
- Leibo SP, Oda K. High survival of mouse zygotes and embryos cooled rapidly or slowly in ethylene glycol plus polyvinylpyrrolidone. Cryoletters. 1993;14:133–144. [Google Scholar]
- Leibo SP, Martino A, Kobayashi S, Pollard JW. Stage-dependent sensitivity of oocytes and embryos to low temperatures; Anim. Reprod Science. 1996;42:45–53. [Google Scholar]
- Levin RL, Cravalho EG, Huggins CG. A membrane model describing the effect of temperature on the water conductivity of Erythrocyte membranes at subzero temperatures. Cryobiology. 1976;13:415–429. doi: 10.1016/0011-2240(76)90097-3. [DOI] [PubMed] [Google Scholar]
- Lin C, Thomashow MF. DNA sequence analysis of a complementary DNA for cold-regulation Arabidopsis gene cor15 and characterization of the COR15 polypeptide. Plant Physiol. 1992;99:519–525. doi: 10.1104/pp.99.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los DA, Ray MK, Murata N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp PCC 6803. Mol. Microbiol. 1997;25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- Love RM. The freezing of animal tissue. In: Meryman HT, editor. Chapter 7 in Cryobiology. London: Academic Press; 1966. [Google Scholar]
- Lovelock JE. The haemolysis of human red blood cells by freezing and thawing. Biochim Biophys Acta. 1953;10:424–426. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- MacFarlane DR. Physical aspects of vitrification in aqueous solutions. Cryobiology. 1987;24:181–195. [Google Scholar]
- Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Devel. 1996a;45:503–512. doi: 10.1002/(SICI)1098-2795(199612)45:4<503::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod. 1996b;54:1059–1069. doi: 10.1095/biolreprod54.5.1059. [DOI] [PubMed] [Google Scholar]
- Michel V, Lehoux I, Depret G, Anglade P, Labadie J, Hebraud M. The cold shock response of the psychrotrophic bacterium Pseudomonas fragi involves four low-molecular-mass nucleic acid-binding proteins. J. Bacteriol. 1997;179:7331–7342. doi: 10.1128/jb.179.23.7331-7342.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Kaplan T, Lechner S, Scherer S. Identification and purification of a family of dimeric major cold shock protein homologs from the psychrotrophic Bacillus cereus WSBC 10201. J. Bacteriol. 1996;178:2916–2925. doi: 10.1128/jb.178.10.2916-2925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J. Gen. Physiology. 1963;47:347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: The freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mazur P, Leibo SP, Chu EHY. A two-factor hypothesis of freezing injury. Exp Cell Res. 1972;71:345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- Mazur P, Kemp JA, Miller RH. Survival of fetal rat pancreases frozen to -78° and −196°C. Proc Natl Acad Sci. 1976;73:4105–4109. doi: 10.1073/pnas.73.11.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Freezing of living cells: mechanisms and implications. Amer J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- McGrath JJ. In: Membrane transport properties In Low Temperature Biotechnology: Emerging Applications and Engineering Contributions. McGrath JJ, Diller KR, editors. 1988. pp. 273–330. ASME BED-Vol. 10, HTD-Vol. 98. [Google Scholar]
- McKersie BD, Bowley SR. Active oxygen and freezing tolerance in transgenic plants. In: Li PH, Chen THH, editors. Plant Cold Hardiness. Molecular Biology, Biochemistry, and Physiology. New York: Plenum Press; 1997. pp. 203–214. [Google Scholar]
- McWilliams RB, Gibbons WE, Leibo SP. Osmotic and physiological responses of mouse and human ova to mono- and disaccharides. Hum Reprod. 1995;10:1163–1171. doi: 10.1093/oxfordjournals.humrep.a136112. [DOI] [PubMed] [Google Scholar]
- Meryman HT. The exceeding of a minimum tolerable cell volume in hypertonic suspension as a cause of freezing injury. In: Wolstenholme GEW, O’Connor M, editors. The Frozen Cell. London: Churchill; 1970. pp. 51–67. [Google Scholar]
- Moore CH, Martelli V, Al-Janabi N, Ross DN. Analysis of homograft valve failure in 311 patients followed up to 10 years. Ann. Thorac. Surg. 1975;20:274–281. doi: 10.1016/s0003-4975(10)64219-1. [DOI] [PubMed] [Google Scholar]
- Moran DM, Jasko DJ, Squires EL, Amann RP. Determination of temperature and cool rate induced cold shock in stallion spermatozoa. Theriogenology. 1992;38:999–1012. doi: 10.1016/0093-691x(92)90114-7. [DOI] [PubMed] [Google Scholar]
- Morris GJ. Direct chilling injury. In: Grout BWW, Morris GJ, editors. The Effects of Low Temperatures on Biological Systems. London: Edward Arnold; 1987. pp. 120–146. [Google Scholar]
- Muldrew K, McGann LE. The osmotic rupture hypothesis of intracellular freezing. Biophys J. 1994;66:532–541. doi: 10.1016/s0006-3495(94)80806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Nishida I. Lipids of blue-green algae (cyanobacteria) In: Stumpf PK, editor. The Biochemistry of Plants. San Diego: Academic Press; 1987. pp. 315–347. [Google Scholar]
- Neidert MR, Devireddy RV, Tranquillo RT, Bischof JC. Cryopreservation of Collagen-Based Tissue-Equivalents - Part II: Improved Freezing in the Presence of Cryoprotective Agents. Tiss. Eng. 2004;10:23–32. doi: 10.1089/107632704322791664. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Kanamaru K, Mizuno T, Horikoshi K. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli . J. Bacteriol. 1996;178:2994–2997. doi: 10.1128/jb.178.10.2994-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana . FEBS Lett. 1999;461:205–210. doi: 10.1016/s0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- Newton H, Fisher J, Arnold JRP, Pegg DE, Faddy MJ, Gosden RG. Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod. 1998;13:376–380. doi: 10.1093/humrep/13.2.376. [DOI] [PubMed] [Google Scholar]
- Noiles EE, Thompson KA, Storey BT. Water permeability, Lp, of the mouse sperm plasma membrane and its activation energy are strongly dependent on interaction of the plasma membrane with the sperm cytoskeleton. Cryobiology. 1977;35:79–92. doi: 10.1006/cryo.1997.2033. [DOI] [PubMed] [Google Scholar]
- Oda K, Gibbons WE, Leibo SP. Osmotic shock of fertilized mouse ova. J Reprod Fertil. 1992;95:737–747. doi: 10.1530/jrf.0.0950737. [DOI] [PubMed] [Google Scholar]
- Olien CR, Smith MN. Ice adhesions in relation to freeze stress. Plant Physiol. 1977;60:499–503. doi: 10.1104/pp.60.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AE, Crowe LM, Crowe JH. Methods for dehydration-tolerance: depression of the phase transition temperature in dry membranes and carbohydrate vitrification. Seed Sci. Res. 1998;8:211–221. [Google Scholar]
- Panoff JM, Thammavongs B, Gueguen M, Boutibonnes P. Cold stress responses in mesophilic bacteria. Cryobiology. 1998;36:75–83. doi: 10.1006/cryo.1997.2069. [DOI] [PubMed] [Google Scholar]
- Panoff JM, Corroler D, Thammavongs B, Boutibonnes P. Differentiation between cold shock proteins and cold acclimation proteins in a mesophilic gram-positive bacterium, Enterococcus faecalis JH2–2. J. Bacteriol. 1997;179:4451. doi: 10.1128/jb.179.13.4451-4454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoff JM, Thammavongs B, Gueguen M. Cryoprotectants lead to phenotypic adaptation to freeze-thaw stress in Lactobacillus delbrueckii ssp. bulgaricus CIP 101027T. Cryobiology. 2000;40:264–269. doi: 10.1006/cryo.2000.2240. [DOI] [PubMed] [Google Scholar]
- Parks JE, Ruffing NA. Factors affecting low temperature survival of mammalian oocytes. Theriogenology. 1992;37:59–73. [Google Scholar]
- Pate JW, Sawyer PN. Freeze-dried aortic grafts. A preliminary report of experimental evaluation. Am. J. Surg. 1953;82:3–13. doi: 10.1016/0002-9610(53)90293-1. [DOI] [PubMed] [Google Scholar]
- Pazhayannur PV, Bischof JC. Measurement and simulation of water transport during freezing in mammalian liver tissue. ASME J of Biomech Eng. 1997;119:269–277. doi: 10.1115/1.2796091. [DOI] [PubMed] [Google Scholar]
- Pegg DE, Karow AM. The Biophysics of Organ Cryopreservation. New York: Plenum Publ; 1987. [Google Scholar]
- Pegg DE. Mechanisms of freezing damage. Symp Soc Exp Biol. 1987;41:363–378. [PubMed] [Google Scholar]
- Pegg DE. Cryopreservation: a perspective. In: Hervé P, Rifle G, Vuitton D, Dureau G, Bechtel P, Justrabo E, editors. Organ Transplantation and Tissue Grafting. John Libbey & Co; 1996. pp. 375–378. [Google Scholar]
- Pegg DR. Cryopreservation of cells in situ: experiments with ovine articular cartilage. Cryobiology. 1998;37:381–382. [Google Scholar]
- Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54:102–108. doi: 10.1016/s0015-0282(16)53644-9. [DOI] [PubMed] [Google Scholar]
- Pitt RE. Thermodynamics and intracellular ice formation. In: Steponkus PL, editor. Advances in Low-Temperature Biology. Vol. 1. London: JAI Press LTD; 1992. pp. 63–99. [Google Scholar]
- Polge C, Smith AU, Parkes AS. “Revival of spermatozoa after vitrification and dehydration at low temperatures”. Nature. 1949;Vol. 164:666–676. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- Potts M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999;34:319–328. [Google Scholar]
- Rey LR. Basic aspects and future trends in the freeze-drying of pharmaceuticals. Dev. Biol. Stand. 1992;74:3–8. [PubMed] [Google Scholar]
- Rubinsky B, Lee CY, Bastacky J, Hayes TL. The mechanism of freezing process in biological tissue. Cryo-Lett. 1987;8:370–381. [Google Scholar]
- Rubinsky B, Pegg DE. A mathematical model for the freezing process in biological tissue. Proc R Soc Lond Series B: Biol Sci. 1988;234:343–358. doi: 10.1098/rspb.1988.0053. [DOI] [PubMed] [Google Scholar]
- Russell NJ. Cold adaptation of micro-organisms. Phil. Trans. R. Soc. Lond. B. 1990;326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Bryant DA. Temperature-regulated mRNA accumulation and stabilization for fatty acid desaturase genes in the cyanobacterium Synechococcus sp. strain PCC 7002. Mol. Microbiol. 1997;23:1281–1292. doi: 10.1046/j.1365-2958.1997.3071676.x. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Trounson A, Freeman L, Brady T. The effects of cooling human oocytes. Hum. Reprod. 1988;3:968–977. doi: 10.1093/oxfordjournals.humrep.a136827. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Shen G, Higashi S, Murata N, Bryant DA. Alteration of low-temperature susceptibility of the cyanobacterium Synechococcus sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch. Microbiol. 1998;169:20–28. doi: 10.1007/s002030050536. [DOI] [PubMed] [Google Scholar]
- Sato N, Murata N. Lipid biosynthesis in the blue-green alga, Anabaena variabilis. II. Fatty acids and lipid molecular species. Biochim. Biophys. Acta. 1982;710:279–289. [Google Scholar]
- Silberg JJ, Hoff KG, Vickery LE. The Hsc66-Hsc20 chaperone system in Escherichia coli: chaperone activity and interactions with the DnaK-DnaJ-GrpE system. J. Bacteriol. 1998;180:6617–6624. doi: 10.1128/jb.180.24.6617-6624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YC, Hagen P, Lightfoot FG, Taylor MJ, Smith AC, Brockbank KGM. In vivo evaluation of the effects of a new ice-free cryopreservation process on autologous vascular grafts. J. Invest. Surg. 2000;13:279–288. doi: 10.1080/08941930050206300. [DOI] [PubMed] [Google Scholar]
- Steponkus PA, Weist SC. Freeze-thaw induced lesions in the plasma membrane. In: Lyons JM, Graham D, Raison JK, editors. Low Temperature Stress in Crop Plants: The Role of the Membrane. New York: Academic Press; 1979. pp. 231–254. [Google Scholar]
- Steponkus PL, Uemura M, Webb MS. Membrane destabilization during freeze-induced dehydration. Curr. Topics Plant Physiol. 1993;10:37–47. [Google Scholar]
- Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF. Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA. 1998;95:14570–14575. doi: 10.1073/pnas.95.24.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C- repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G, Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA. 1986;83:2422–2426. doi: 10.1073/pnas.83.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohba T, Okhi K, Murata N. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Pegg DE. The effect of ice formation on the function of smooth muscle tissue following storage at −21°C or −60°C. Cryobiology. 1983;20:36–40. doi: 10.1016/0011-2240(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Thammavongs B, Corroler D, Panoff JM, Auffray Y, Boutibonnes P. Physiological response of Enterococcus faecalis JH2-2 to cold shock: growth at low temperatures and freezing/thawing challenge. Lett. Appl. Microbiol. 1996;23:398–402. doi: 10.1111/j.1472-765x.1996.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998;118:1–7. doi: 10.1104/pp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Toner M, Cravalho EG, Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J. Appl. Phys. 1990;67:1582–1593. [Google Scholar]
- Toner M, Tompkins RG, Cravalho EG, Yarmush ML. Transport phenomena during freezing of isolated hepatocytes. AIChE J. 1992;38(10):1512–1522. [Google Scholar]
- Toner M, Cravalho EG, Stachecki J, Fitzgerald T, Tompkins RG, Yarmush ML, Armant DR. Nonequilibrium freezing of one-cell mouse embryos. Membrane integrity and developmental potential; Biophys J. 1993;64:1908–1921. doi: 10.1016/S0006-3495(93)81562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner M. Nucleation of ice crystals in biological cells . In: Steponkus PL, editor. Vol. 2. London: JAI Press LTD; 1993. pp. 1–52. [Google Scholar]
- Tschistajakow FM, Botescharowa GL. The effect of low temperatures on microorganisms. II (in Russian) Mikrobiologiya (USSR) 1938;7:498–524. [Google Scholar]
- Tschistajakow FM, Botescharowa GL. The effect of low temperatures on microorganisms. IV (in Russian) Mikrobiologiya (USSR) 1938;7:838–842. [Google Scholar]
- Turnbull D, Fisher JC. Rate of nucleation in condensed systems. J. Chem. Phys. 1949;17:71–73. [Google Scholar]
- Turnbull D, Vonegut B. Nucleation catalysis. Ind. Eng. Chem. 1951;44:1292–1298. [Google Scholar]
- Villalba R, Benitz J, No-Lowis ED, Rioja LF, Gómez-Villagrán JL. Cryopreservation of human skin with propane-1,2 diol. Cryobiology. 1996;33:525–529. doi: 10.1006/cryo.1996.0056. [DOI] [PubMed] [Google Scholar]
- Wada H, Murata N. Temperature-induced changes in the fatty acids composition of the cyanobacterium, Synechocystis PCC 6803. Plant Physiol. 1990;92:1062–1069. doi: 10.1104/pp.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yamanaka K, Inouye M. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 1999;181:1603–1609. doi: 10.1128/jb.181.5.1603-1609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters JA, Sanders J-W, Kok J, de Vos WM, Kuipers OP, Abee T. Clustered organization and transcriptional analysis of a family of five csp genes of Lactococcus lactis MG1363. Microbiol. 1998;144:2885–2893. doi: 10.1099/00221287-144-10-2885. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, Mazur P, Peter AT, Critser JK. Osmotic tolerance limits and properties of murine spermatozoa. Biol Reprod. 1996;55:715–727. doi: 10.1095/biolreprod55.3.715. [DOI] [PubMed] [Google Scholar]