Abstract
Immature myeloid cells in bone marrow are a heterogeneous population of cells that, under normal conditions, provide tissues with protective cell types such as granulocytes and macrophages. Under certain pathological conditions, myeloid cell homeostasis is altered and immature forms of these cells appear in tissues. Murine immature myeloid cells that express CD11b and Ly6C or Ly6G (two isoforms of Gr-1) have been associated with immunosuppression in cancer (in the form of myeloid-derived suppressor cells) and, more recently, infection. Here, we found that CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells accumulated and persisted in tissues of mice infected with Salmonella enterica serovar Typhimurium (S. Typhimurium). Recruitment of CD11b+ Ly6Chi Ly6G− but not CD11b+ Ly6Cint Ly6G+ cells from bone marrow into infected tissues depended on chemokine receptor CCR2. The CD11b+ Ly6Chi Ly6G− cells exhibited a mononuclear morphology, whereas the CD11b+ Ly6Cint Ly6G+ cells exhibited a polymorphonuclear or band-shaped nuclear morphology. The CD11b+ Ly6Chi Ly6G− cells differentiated into macrophage-like cells following ex vivo culture and could present antigen to T cells in vitro. However, significant proliferation of T cells was observed only when the ability of the CD11b+ Ly6Chi Ly6G− cells to produce nitric oxide was blocked. CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection could also present antigen to T cells in vivo, but increasing their numbers by adoptive transfer did not cause a corresponding increase in T cell response. Thus, CD11b+ Ly6Chi Ly6G− immature myeloid cells recruited in response to S. Typhimurium infection exhibit protective and immunosuppressive properties that may influence the outcome of infection.
INTRODUCTION
Salmonellae are a leading and increasing cause of morbidity and mortality in humans worldwide (1, 2). Infections with salmonellae range in severity from self-limiting gastroenteritis to typhoid fever. Nontyphoidal salmonellae such as Salmonella enterica serovar Typhimurium (S. Typhimurium) are a significant cause of inflammatory enterocolitis and death due to food-borne illness and an emerging cause of invasive bacteremia in immunocompromised hosts. Typhoidal salmonellae such as Salmonella enterica serovar Typhi (S. Typhi) cause systemic infections characterized by bacterial penetration of the intestinal barrier and extraintestinal dissemination to the liver and spleen, where the microorganisms survive and replicate in professional phagocytes. Septic shock and death can occur if systemic infections with salmonellae are left untreated (1, 2).
Much of what is known about immunity to salmonellae comes from experimental infection of mice with S. Typhimurium, which has served as a useful model for the human disease caused by S. Typhi (3, 4). In humans and animal hosts, S. Typhimurium induces acute immunosuppression and delays the onset of protective immune responses (3–6). Immunity that eventually develops against S. Typhimurium requires humoral and cell-mediated immune responses (3). T cells, particularly gamma interferon (IFN-γ)-producing CD4+ T cells, play a critical role in the clearance of S. Typhimurium (3), but T cell responses to S. Typhimurium are thwarted during infection by mechanisms that, despite recent progress (7–12), are not well understood.
During early stages of infection, macrophages and neutrophilic granulocytes are critical for controlling spread and growth of S. Typhimurium (3). These cells are produced by the differentiation of immature myeloid cells in infected tissues and bone marrow (13–15). Although immature myeloid cells play a critical role in the protective host response to infection (14), several early studies have linked nitric oxide (NO)-producing macrophage precursors to immunosuppression in salmonellosis (16). Murine immature myeloid cells express surface CD11b and Gr-1 (13) and have been found in spleens of mice infected with S. Typhimurium (17, 18). CD11b+ Gr-1+ immature myeloid cells are a heterogeneous population of cells that can be further divided based on the surface expression of Ly6C and Ly6G, two isoforms of Gr-1 that are differentially expressed on neutrophilic granulocytes, inflammatory monocytes, and some populations of dendritic cells (13, 14, 19–21).
Here, we found that large numbers of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells accumulated and persisted in tissues of mice infected with S. Typhimurium. We characterized these cells and found that recruitment of CD11b+ Ly6Chi Ly6G− but not CD11b+ Ly6Cint Ly6G+ cells from the bone marrow depended on chemokine receptor 2 (CCR2). Splenic CD11b+ Ly6Chi Ly6G− cells differentiated into macrophage-like cells following ex vivo culture and could present antigen to both CD4+ and CD8+ T cells in vitro. However, robust proliferation of the T cells was observed only when the ability of the CD11b+ Ly6Chi Ly6G− cells to produce NO was blocked. CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection could also present antigen to T cells in vivo, but increasing the number of these cells by adoptive transfer did not cause a corresponding increase in T cell response. Thus, CD11b+ Ly6Chi Ly6G− immature myeloid cells provide a balance of immunosuppressive and protective functions in the host response to S. Typhimurium. The tipping of this balance may be an important factor influencing the outcome of infection.
MATERIALS AND METHODS
Bacteria.
S. Typhimurium strain IR715 (22), which is a spontaneous, nalidixic acid-resistant derivative of S. Typhimurium strain 14028 (American Type Culture Collection), was used as the wild-type strain. Bacteria were grown aerobically for 12 to 18 h at 37°C in 3 ml of Luria-Bertani (LB) broth (250 rpm) or on LB agar plates using standard microbiological techniques.
Mice.
C57BL/6J and 129X1/SvJ mice (both H-2b haplotype) were purchased from The Jackson Laboratory. These strains of mice have been used as model hosts to study acute and persistent salmonellosis, respectively (4). F1 (C57BL/6J × 129X1/SvJ) hybrid mice, which can accept cells from either C57BL/6J or 129X1/SvJ mice, were bred at Stony Brook University, Division of Laboratory Animal Resources. B6.129S4-Ccr2tm1Ifc/J (Ccr2−/−), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), and B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-II) mice (all H-2b haplotype) were purchased from The Jackson Laboratory and bred at Stony Brook University, Division of Laboratory Animal Resources. Ccr2−/− mice lack chemokine receptor CCR2. OT-I mice are T-cell-receptor (TCR) transgenic mice in the C57BL/6J strain background that produce CD8+ T cells specific for amino acid residues 257 to 264 of chicken egg ovalbumin (OVA257–264) bound to class I major histocompatibility complex (MHC) (H-2Kb). OT-II mice are TCR transgenic mice in the C57BL/6J strain background that produce CD4+ T cells specific for amino acid residues 323 to 339 of chicken egg ovalbumin (OVA323–339) bound to class II MHC (I-Ab). The Institutional Animal Care and Use Committee at Stony Brook University approved all procedures and experiments using mice.
Mouse infections.
Mouse infections were performed using naive, 8- to 12-week-old sex-matched C57BL/6J, 129X1/SvJ, and Ccr2−/− mice. Briefly, mice were inoculated intragastrically with S. Typhimurium (5 × 107 CFU for C57BL/6J and Ccr2−/− mice and 5 × 108 CFU for 129X1/SvJ mice) suspended in 0.1 ml of phosphate-buffered saline (PBS). To improve the consistency of intragastric infections, food (but not water) was removed 6 to 8 h prior to inoculation. Immediately following inoculation, food was provided ad libitum. Tenfold serial dilutions of the inoculum were plated on LB agar to confirm the inoculum titer. At indicated times after inoculation, target organs (i.e., spleen, liver, mesenteric lymph nodes, and bone marrow) were harvested and processed to obtain single-cell suspensions. At each time point, we controlled for the effect of age by preparing samples from two age-matched mice left uninfected. At the end of the experiment, the results were combined and reported as the uninfected control. Bacterial loads were determined by lysing the cells with Triton X-100 (0.05%) and plating for CFU on LB agar containing nalidixic acid (50 μg/ml). Mice infected with S. Typhimurium were euthanized when moribund or at the termination of the experiment.
Cell staining and analysis by flow cytometry.
Conjugated monoclonal antibodies and reagents described here were purchased from BioLegend unless indicated otherwise. Routinely, cells were stained in the presence of Fc block (anti-mouse CD16/32 antibody; clone 93) using anti-mouse antibodies specific for Gr-1 (clone RB6-8C5), Ly6C (clone HK1.4), Ly6G (clone 1A8), CD11b (clone M1/70), F4/80 (clone CI:A3-1), and I-Ab (clone AF6-120.1). In T cell assays, cells were stained in the presence of Fc block using anti-mouse antibodies specific for CD4 (clone RM4-5), CD8β (clone YTS156.7.7), and CD90.2 (clone 30-H12) to identify T cells; anti-mouse antibodies specific for Vα2 (clone B20.1) and Vβ5.1/5.2 (clone MR9-4; BD Biosciences) to identify the T cell receptor expressed by OT-I and OT-II T cells; and anti-mouse antibody specific for CD69 (clone H1.2F3) to measure T cell activation. Data were acquired and analyzed using a BD FACSCalibur flow cytometer (BD Biosciences) with BD CellQuestPro (BD Biosciences) and FlowJo (Tree Star) software or a BD FACSAria cell sorter with BD FACSDiva software (BD Biosciences).
CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cell purification and analysis.
For all experiments that used purified CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells, splenocytes harvested from 129X1/SvJ mice infected with S. Typhimurium for 21 to 28 days were used as a source of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells. Following treatment of the splenocytes with ACK lysing buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA) to lyse red blood cells, magnetic cell separation (MACS) technology (Miltenyi Biotec) was used to enrich for CD11b+ cells. Throughout this process and all other manipulations of the cells, antibiotics (i.e., penicillin, streptomycin, and gentamicin) were present to kill all bacteria. Enriched populations of CD11b+ cells were stained in the presence of Fc block using antibodies specific for CD11b, Ly6C, and Ly6G, as described above. A BD FACSAria cell sorter with BD FACSDiva software was used to purify the CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells. In preparation for morphological analysis, 1 × 105 purified CD11b+ Ly6Chi Ly6G− or CD11b+ Ly6Cint Ly6G+ cells were centrifuged onto glass microscope slides that were subsequently air dried. The cells were then fixed using methanol, stained using Reastain Quick-Diff (Reagena), and visualized by light microscopy.
Nitrite assay.
Purified CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells were seeded into flat-bottom 96-well tissue culture plates at 1 × 106 cells per well. After 16 h of incubation at 37°C in 5% CO2, we measured the levels of nitrite in supernatant using the Griess reagent system (Promega).
Ex vivo culture of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells.
Purified CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells were seeded into 24-well tissue culture plates at 1 × 105 cells per well using medium formulated for the ex vivo culture of bone marrow-derived macrophages (Dulbecco's modified Eagle medium [DMEM] with GlutaMAX-I [Invitrogen] supplemented with 20% heat-inactivated fetal bovine serum [Atlanta Biologicals], 20% L-cell conditioned medium [a source of macrophage colony-stimulating factor {M-CSF}], 0.2 M l-Gln, 0.1 M sodium pyruvate, and 1% penicillin-streptomycin). After 7 days of ex vivo culture at 37°C in 5% CO2, the cells were visualized by light microscopy and analyzed by flow cytometry. As a positive control, bone marrow-derived macrophages were cultured from naive 129X1/SvJ mice, as described previously (12, 23).
T cell enrichment and T cell assays.
Splenocytes harvested from naive OT-I, OT-II, and 129X1/SvJ mice were used as a source of T cells. Following treatment of the splenocytes with ACK lysing buffer, MACS technology was used to enrich for CD90.2+ T cells. Enriched populations of T cells were suspended in RP-10 medium (RPMI 1640 medium [Invitrogen] supplemented with 10% fetal bovine serum, 0.2 M l-Gln, 0.1 M HEPES, 50 μM 2-mercaptoethanol [2-ME], and 1% penicillin-streptomycin), labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen), and used in T cell assays. CFSE is a cell-permeant fluorescent dye that, once taken up, is retained and distributed evenly among daughter cells with each round of cell division, resulting in a quantum reduction in cell fluorescence that can be measured by flow cytometry (24).
In assays aimed at measuring antigen-induced T cell proliferation, purified CD11b+ Ly6Chi Ly6G− cells were mock treated or coated with 5 nM OVA257–264 or 5 μM OVA323–339 peptide (Bio-Synthesis), suspended in RP-10, and seeded into round-bottom 96-well tissue culture plates at 5 × 104 cells per well. Where indicated, the mock-treated or peptide-coated CD11b+ Ly6Chi Ly6G− cells were fixed with 2% paraformaldehyde (Sigma) and treated with 0.2 M l-lysine (Sigma), as described previously (25). CFSE-labeled OT-I (Vα2+ Vβ5+ CD8β+) or OT-II (Vα2+ Vβ5+ CD4+) T cells were then added to the CD11b+ Ly6Chi Ly6G− cells at indicated ratios. After 4 days of incubation at 37°C in 5% CO2, cells were harvested, stained, and analyzed by flow cytometry.
In assays aimed at measuring polyclonal T cell proliferation, CFSE-labeled 129X1/SvJ T cells were suspended in RP-10, seeded into round-bottom 96-well tissue culture plates coated with 3 μg/ml anti-mouse CD3ε antibody (clone 145-2C11; BioLegend) at 5 × 104 cells per well, and cultured in the presence of 5 μg/ml anti-mouse CD28 antibody (clone E18; BioLegend). Purified CD11b+ Ly6Chi Ly6G− cells were then added to the T cells at a 10:1 or 1:1 ratio. Where indicated, the inducible nitric oxide synthase (iNOS) inhibitor 1400W (Sigma) was added to the cultures at a final concentration of 200 μM. After 4 days of incubation at 37°C in 5% CO2, cells were harvested, stained, and analyzed by flow cytometry.
In assays aimed at measuring antigen-induced T cell activation in vivo, the equivalent of 1 × 106 splenic OVA257–264-specific CD8+ T cells from naive OT-I mice were adoptively transferred into naive F1 (C57BL/6J × 129X1/SvJ) mice by intravenous injection. The number of OT-I splenocytes transferred was based on the frequency of Vα2+ Vβ5+ CD8β+ cells. One day later, recipient mice received 1 × 106 or 2 × 106 purified, OVA257–264 peptide-coated CD11b+ Ly6Chi Ly6G− cells by intravenous injection. As a positive control, recipient mice received 1 × 106 or 2 × 106 OVA257–264 peptide-coated bone marrow-derived macrophages. As a negative control, recipient mice received 200 μl of PBS. One day after the second adoptive transfer, splenocytes were harvested; stained with anti-mouse antibodies specific for Vα2, Vβ5, CD8β, and CD69; and analyzed by flow cytometry.
Statistical analysis.
Statistical analysis was performed using Prism 5.0b (GraphPad Software). Data were analyzed using two-tailed, paired Student's t test, one-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison posttest, or two-way ANOVA with Bonferroni's posttest; P values of <0.05 were considered to be statistically significant. Asterisks in the figures indicate statistically significant differences (***, P < 0.001; **, P < 0.01; *, P < 0.05).
RESULTS
Large numbers of CD11b+ Gr-1+ cells accumulate in tissues of mice infected with S. Typhimurium.
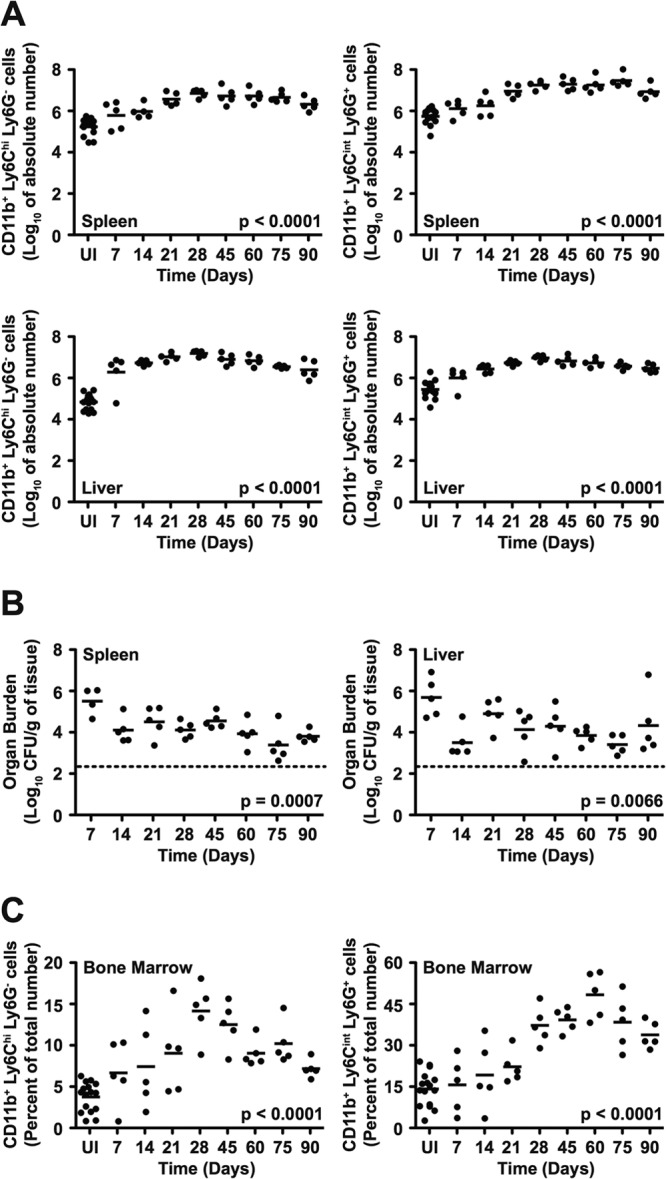
To dissect innate immune mechanisms that control spread and growth of S. Typhimurium, we characterized cellular responses and determined bacterial loads in spleens and livers of 129X1/SvJ mice inoculated intragastrically with 5 × 108 CFU of S. Typhimurium. After 7 days of infection, we found more CD11b+ Gr-1+ cells in spleens and livers of mice infected with S. Typhimurium than in spleens and livers of mice left uninfected (Fig. 1A; see also Fig. S1A in the supplemental material). After 60 days of infection, a long-term time point used here to assess bacterial persistence (Fig. 1B), absolute numbers and percentages of CD11b+ Gr-1+ cells in spleen and liver had increased even further (28-fold and 20-fold, respectively) (Fig. 1A; see also Fig. S1A). Similar results were obtained when we characterized cellular responses in C57BL/6J mice inoculated intragastrically with 5 × 107 CFU of S. Typhimurium (see Fig. S1B). Thus, large numbers of CD11b+ Gr-1+ cells accumulate in tissues of mice infected with S. Typhimurium.
FIG 1.

Large numbers of CD11b+ Gr-1+ cells accumulate in tissues of mice infected with S. Typhimurium. (A) Absolute numbers of CD11b+ Gr-1+ cells in spleens (left) and livers (right) of 129X1/SvJ mice (n = 4 to 5 per group per time point) left uninfected (UI) or infected for 7 or 60 days with S. Typhimurium. (B) Corresponding bacterial loads per gram of spleen (left) and liver (right) tissue. Dashed lines denote limits of detection. Data show mean with spread from a single experiment that is representative of two independent experiments. Data were analyzed using a one-way ANOVA with Bonferroni's multiple-comparison posttest; P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (***, P < 0.001; *, P < 0.05). See also Fig. S1 in the supplemental material.
CD11b+ Gr-1+ cells that accumulate in tissues of mice infected with S. Typhimurium exhibit phenotypic and morphological heterogeneity.
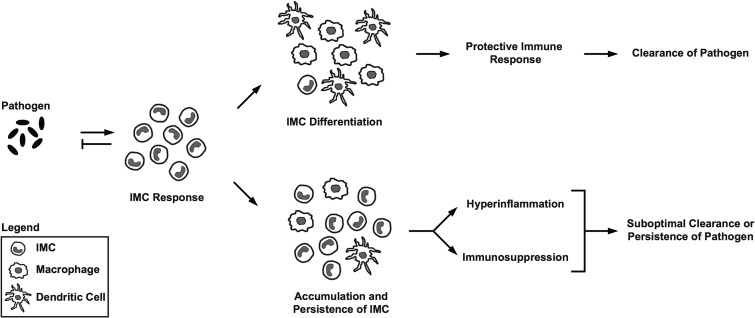
CD11b+ Gr-1+ cells are a heterogeneous population that can be further divided based on expression of Ly6C and Ly6G, two isoforms of Gr-1 that are differentially expressed on the surface of neutrophilic granulocytes, inflammatory monocytes, and some populations of dendritic cells (13, 14, 19–21). To define the phenotypic and morphological heterogeneity of CD11b+ Gr-1+ cells in the host response to S. Typhimurium, we further characterized the CD11b+ Gr-1+ cells present in spleens of 129X1/SvJ mice infected with S. Typhimurium for 21 to 28 days. We found the CD11b+ Gr-1+ cells to be a heterogeneous population that could be divided into CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells (Fig. 2A). Furthermore, we found that purified CD11b+ Ly6Chi Ly6G− cells exhibited a mononuclear morphology, whereas purified CD11b+ Ly6Cint Ly6G+ cells exhibited a polymorphonuclear or band-shaped nuclear morphology (Fig. 2B). Thus, CD11b+ Gr-1+ cells in the host response to S. Typhimurium are characterized by phenotypic and morphological heterogeneity.
FIG 2.
CD11b+ Gr-1+ cells that accumulate in tissues of mice infected with S. Typhimurium exhibit phenotypic and morphological heterogeneity. (A) Flow cytometric analysis of splenocytes harvested from 129X1/SvJ mice infected for 28 days with S. Typhimurium. Live splenocytes (left) that expressed CD11b (middle) could be divided into CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells (right). Red and blue colors indicate CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells, respectively. Numbers refer to CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells as percentages of live, CD11b+ cells. FSC, forward scatter; SSC, side scatter. (B) Morphological analysis of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells purified by fluorescence-activated cell sorting. The purity of the cells was consistently greater than 98% (left). Purified cells were stained with Reastain Quick-Diff and visualized by light microscopy at ×63 (middle) and ×100 (right) magnifications. Numbers refer to CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells as percentages of live cells. Data are representative of six independent experiments.
CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells accumulate and persist in tissues of mice infected with S. Typhimurium.
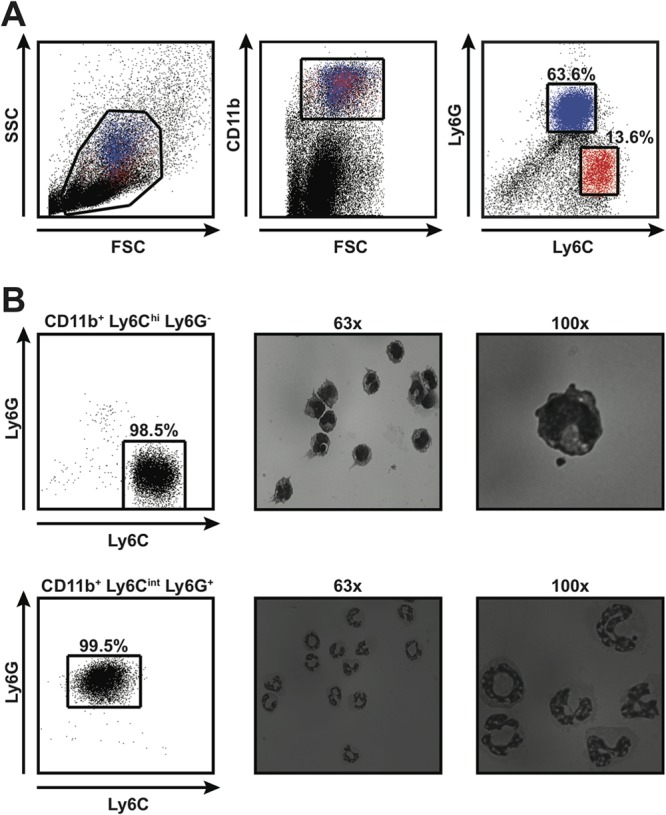
To determine the kinetics with which CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells respond during infection, we further characterized cellular responses in 129X1/SvJ mice inoculated with S. Typhimurium. After 7 days of infection, we found more CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells in spleens and livers of mice infected with S. Typhimurium than in spleens and livers of mice left uninfected (Fig. 3A; see also Fig. S2A in the supplemental material). Both absolute numbers and percentages of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells increased dramatically over time (34- and 28-fold increases in absolute numbers in spleen and 172- and 23-fold increases in absolute numbers in liver, respectively, after 28 days of infection), plateaued at around day 45 after inoculation, and persisted at peak or near peak levels over a period of 90 days (Fig. 3A; see also Fig. S2A). Similar results were obtained when we characterized CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cell responses in mesenteric lymph nodes (see Fig. S2B). We recovered substantial numbers of S. Typhimurium bacteria from spleens and livers of infected mice at every time point (Fig. 3B), indicating that the response of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells correlated with bacterial persistence. The kinetics with which CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells responded in bone marrow were similar to those in spleen and liver (Fig. 3C). Thus, accumulation and persistence of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells in peripheral tissues of mice infected with S. Typhimurium could be the result of increased output from this generative site.
FIG 3.
CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells accumulate and persist in tissues of mice infected with S. Typhimurium. (A) Absolute numbers of CD11b+ Ly6Chi Ly6G− cells (left) and CD11b+ Ly6Cint Ly6G+ cells (right) in spleens (top) and livers (bottom) of 129X1/SvJ mice (n = 5 per group per time point) left uninfected (UI) or infected for up to 90 days with S. Typhimurium. (B) Bacterial loads per gram of spleen (left) and liver (right) tissue. (C) Percentages of CD11b+ Ly6Chi Ly6G− cells (left) and CD11b+ Ly6Cint Ly6G+ cells (right) in bone marrow. Dashed lines denote limits of detection. Data show mean with spread from a single experiment that is representative of two independent experiments. Data were analyzed using a one-way ANOVA; P values of <0.05 were considered to be statistically significant. See also Fig. S2 in the supplemental material.
CCR2 is required for the recruitment of CD11b+ Ly6Chi Ly6G− cells from the bone marrow to systemic sites of S. Typhimurium infection.
CCR2 is critical for emigration of monocyte precursors from the bone marrow (14, 15). To define the role of CCR2 in innate immune defense against S. Typhimurium, we characterized cellular responses in wild-type (WT) and Ccr2−/− C57BL/6J mice inoculated intragastrically with 5 × 107 CFU of S. Typhimurium. After 7 days of infection, we observed significant accumulation of CD11b+ Ly6Chi Ly6G− cells in spleens of WT but not Ccr2−/− mice (Fig. 4A, left panel). As expected for granulocyte precursors, which do not require CCR2 for emigration from the bone marrow (15), we observed significant and comparable accumulation of CD11b+ Ly6Cint Ly6G+ cells in spleens of both WT and Ccr2−/− mice (Fig. 4A, right panel). We recovered similar numbers of S. Typhimurium bacteria from spleens of infected WT and Ccr2−/− mice (Fig. 4B), indicating that the lack of accumulation of CD11b+ Ly6Chi Ly6G− cells in spleens of infected Ccr2−/− mice was not due to a difference in bacterial load. Consistent with a role for CCR2 in emigration of monocyte precursors from the bone marrow (14), we observed significant accumulation of CD11b+ Ly6Chi Ly6G− cells in bone marrow of infected Ccr2−/− but not WT mice (Fig. 4C). Thus, CCR2 is required for the recruitment of CD11b+ Ly6Chi Ly6G− cells from the bone marrow to systemic sites of S. Typhimurium infection.
FIG 4.
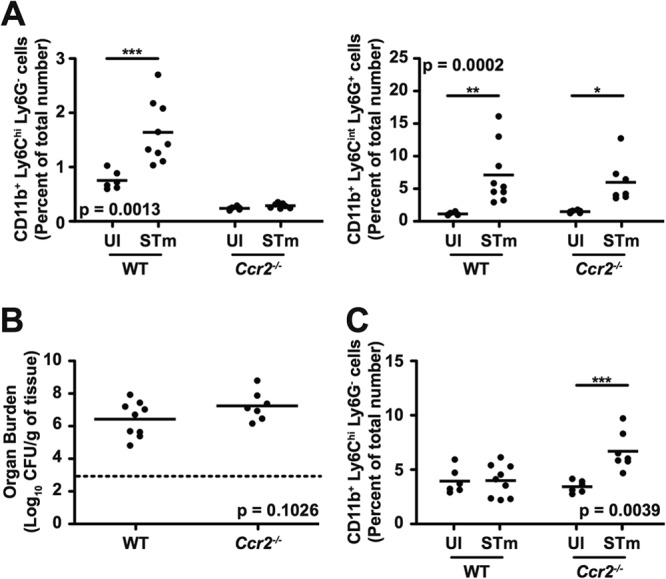
CCR2 is required for the recruitment of CD11b+ Ly6Chi Ly6G− cells from the bone marrow to systemic sites of S. Typhimurium infection. (A) Percentages of CD11b+ Ly6Chi Ly6G− cells (left) and CD11b+ Ly6Cint Ly6G+ cells (right) in spleens of WT and Ccr2−/− mice (n = 6 to 9 per group) left uninfected (UI) or infected for 7 days with S. Typhimurium (STm). (B) Bacterial loads per gram of spleen tissue. (C) Percentages of CD11b+ Ly6Chi Ly6G− cells in bone marrow. The dashed line denotes the limit of detection. Data show mean with spread and are cumulative of two independent experiments. Data were analyzed using a two-way ANOVA with Bonferroni's posttest (A and C) or using two-tailed, paired Student's t test (B); P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (***, P < 0.001; **, P < 0.01; *, P < 0.05).
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can differentiate into macrophages following ex vivo culture.
Next, we examined the ability of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells purified from spleens of 129X1/SvJ mice infected with S. Typhimurium to differentiate ex vivo. After 7 days of ex vivo culture in the presence of L-cell conditioned medium, a source of M-CSF, we found that the CD11b+ Ly6Chi Ly6G− but not CD11b+ Ly6Cint Ly6G+ cells had differentiated into adherent cells that exhibited a macrophage-like morphology (Fig. 5A and data not shown). The differentiated cells expressed increased levels of surface CD11b and F4/80 but had lost expression of surface Ly6C (Fig. 5B). The differentiated cells also expressed increased levels of surface class II MHC (I-Ab), but the observed difference did not reach statistical significance (Fig. 5B). Collectively, these results indicate that the CD11b+ Ly6Chi Ly6G− cells that accumulate in spleens of mice infected with S. Typhimurium are immature myeloid cells capable of differentiating into macrophages.
FIG 5.
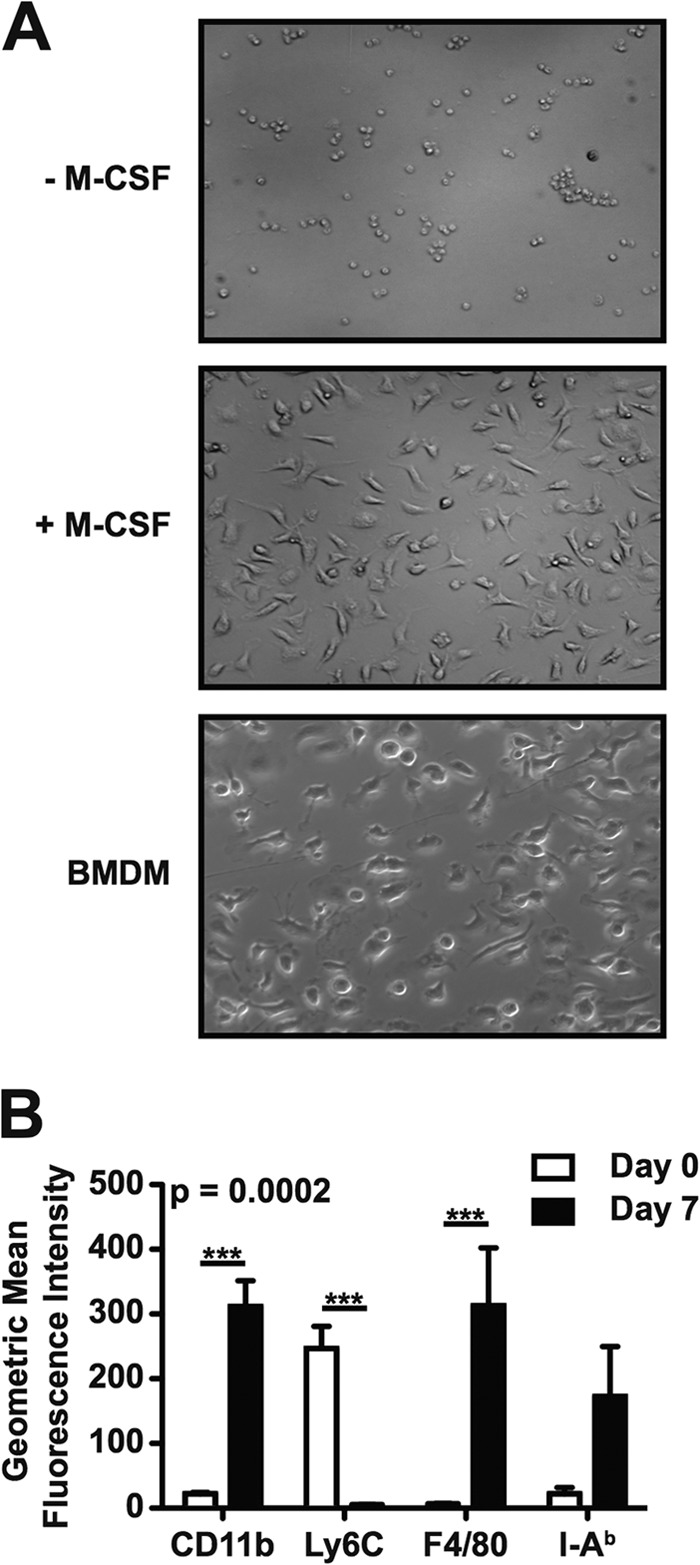
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can differentiate into macrophage-like cells following ex vivo culture. (A) Light microscopy images of purified CD11b+ Ly6Chi Ly6G− cells cultured ex vivo for 7 days in the absence (top) or presence (middle) of M-CSF. Cultures of bone marrow-derived macrophages (BMDM; bottom) were used as a control. (B) Flow cytometric analysis of purified CD11b+ Ly6Chi Ly6G− cells (white bars; day 0) or purified CD11b+ Ly6Chi Ly6G− cells cultured ex vivo for 7 days in the presence of M-CSF (black bars; day 7). Analysis was performed to detect expression of surface CD11b, Ly6C, F4/80, and class II MHC (I-Ab). Data are representative of (A), or show the mean with the standard error of the mean from (B), three independent experiments. Data were analyzed using a two-way ANOVA with Bonferroni's posttest; P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (***, P < 0.001).
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can present antigen to both CD4+ and CD8+ T cells.
Given that CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium could differentiate into macrophage-like cells (Fig. 5), we characterized the ability of these immature myeloid cells to present antigen to T cells in vitro. T cells enriched from spleens of naive OT-I mice, which produce CD8+ T cells specific for amino acid residues 257 to 264 of chicken egg ovalbumin (OVA257–264) bound to class I MHC (H-2Kb), or OT-II mice, which produce CD4+ T cells specific for amino acid residues 323 to 339 of chicken egg ovalbumin (OVA323–339) bound to class II MHC (I-Ab), were labeled with CFSE and used as responder cells. CD11b+ Ly6Chi Ly6G− cells purified from spleens of 129X1/SvJ mice infected with S. Typhimurium were mock treated or coated with OVA257–264 or OVA323–339 peptide, fixed with paraformaldehyde, and used as antigen-presenting cells. Responder T cells and antigen-presenting cells were mixed at a 5:1 ratio and seeded into tissue culture plates. After 4 days of incubation, we found that T cells cultured with peptide-coated CD11b+ Ly6Chi Ly6G− cells had proliferated extensively compared to T cells cultured with mock-treated CD11b+ Ly6Chi Ly6G− cells (Fig. 6A). However, significant proliferation of the T cells was not observed when live antigen-presenting cells were used (Fig. 6B). Thus, the CD11b+ Ly6Chi Ly6G− immature myeloid cells that accumulate and persist in spleens of mice infected with S. Typhimurium can present antigen to T cells when fixed but produce an inhibitor of T cell proliferation when alive.
FIG 6.
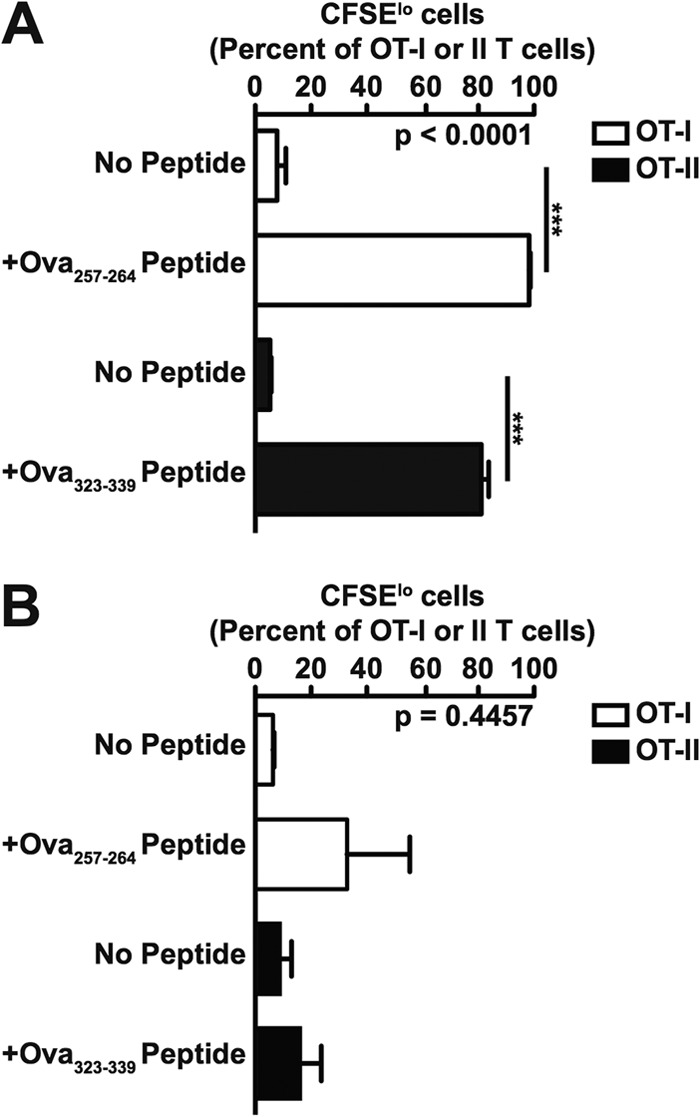
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can present antigen to both CD4+ and CD8+ T cells. Proliferation of CFSE-labeled OT-I T cells (white bars) or OT-II T cells (black bars) cultured with paraformaldehyde-fixed (A) or live (B), purified CD11b+ Ly6Chi Ly6G− cells mock treated (No Peptide) or coated with OVA257–264 or OVA323–339 peptide. The ratio of antigen-presenting cells to responder cells was 5:1. Data show means with standard errors of the means from three independent experiments. Data were analyzed using one-way ANOVA with Bonferroni's multiple-comparison posttest; P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (***, P < 0.001).
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can inhibit T cell proliferation via a NO-dependent mechanism.
Early studies linked NO-producing macrophage precursors to immunosuppression in salmonellosis (16). Therefore, we examined the ability of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells purified from spleens of mice infected with S. Typhimurium to produce NO. After 16 h of ex vivo culture, we found high levels of nitrite in the supernatants of the CD11b+ Ly6Chi Ly6G− but not CD11b+ Ly6Cint Ly6G+ cells (Fig. 7A), indicating that large amounts of NO were produced by the former but not the latter population of cells.
FIG 7.
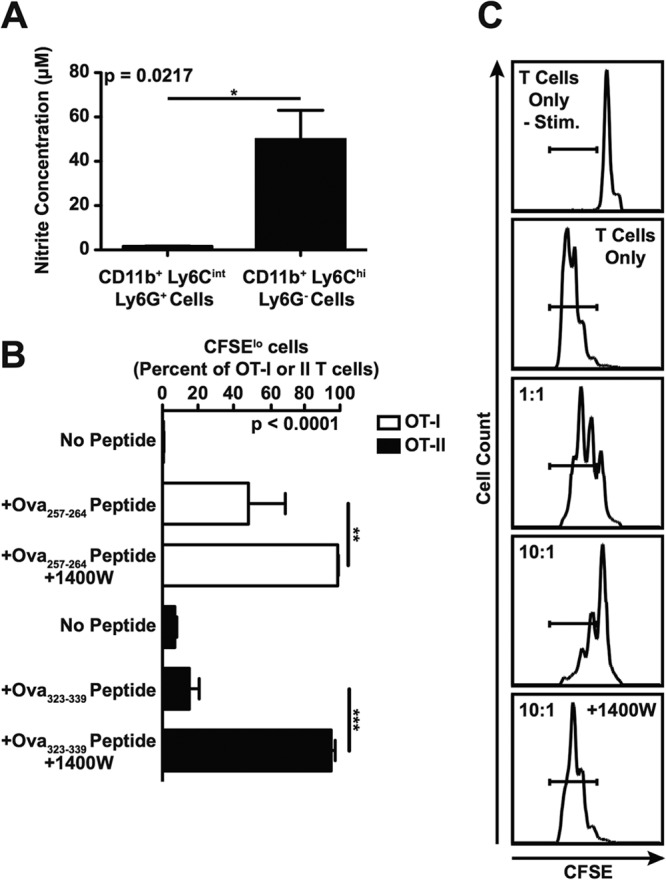
CD11b+ Ly6Chi Ly6G− cells purified from spleens of mice infected with S. Typhimurium can inhibit T cell proliferation via a NO-dependent mechanism. (A) Production of nitrite by purified CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells as measured using the Griess reagent system. (B) Proliferation of CFSE-labeled OT-I T cells (white bars) or OT-II T cells (black bars) cultured with purified CD11b+ Ly6Chi Ly6G− cells mock treated (No Peptide) or coated with OVA257–264 or OVA323–339 peptide. The ratio of antigen-presenting cells to responder cells was 10:1. Where indicated, the iNOS inhibitor 1400W was added to the cultures. (C) Proliferation of CFSE-labeled 129X1/SvJ T cells cultured in the absence or presence of purified CD11b+ Ly6Chi Ly6G− bystander cells. Proliferation of the T cells was induced using anti-mouse CD3ε and anti-mouse CD28 antibody, except where noted (−Stim.). The ratio of bystander cells to responder cells was 1:1 or 10:1. Where indicated, the iNOS inhibitor 1400W was added to the cultures. Data show means with standard errors of the means from (A and B), or are representative of (C), at least three independent experiments. Data were analyzed using two-tailed, paired Student's t test (A) or using a one-way ANOVA with Bonferroni's multiple-comparison posttest (B); P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (***, P < 0.001; **, P < 0.01; *, P < 0.05). See also Fig. S3 in the supplemental material.
To define the impact of NO production by the CD11b+ Ly6Chi Ly6G− cells on the ability of T cells to proliferate, we added the selective iNOS inhibitor 1400W to cultures of responder T cells mixed with OVA peptide-coated antigen-presenting cells, as described above. After 4 days of incubation, we found that T cells cultured with peptide-coated CD11b+ Ly6Chi Ly6G− cells and the selective iNOS inhibitor 1400W had proliferated significantly more than had T cells cultured with peptide-coated or mock-treated CD11b+ Ly6Chi Ly6G− cells only (Fig. 7B). Similar results were obtained when we examined the ability of purified CD11b+ Ly6Chi Ly6G− cells, most of which are uninfected, to suppress polyclonal T cell proliferation through a bystander effect (Fig. 7C; see also Fig. S3 in the supplemental material). Thus, the CD11b+ Ly6Chi Ly6G− immature myeloid cells that accumulate and persist in tissues of mice infected with S. Typhimurium can inhibit T cell proliferation via a NO-dependent mechanism. Furthermore, these results indicate that the accumulation and persistence of NO-producing CD11b+ Ly6Chi Ly6G− immature myeloid cells in peripheral tissues of mice infected with S. Typhimurium could lead to a bystander effect responsible for the delay in onset of protective immune responses.
CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection can modulate T cell function in vivo.
Next, we used an adoptive transfer approach to determine the ability of CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection to modulate T cell function in vivo. Splenocytes from naive OT-I mice were used as a source of T cells. We adoptively transferred 1 × 106 OT-I T cells into naive F1 (C57BL/6J × 129X1/SvJ) mice. One day later, recipient mice received 1 × 106 or 2 × 106 OVA257–264 peptide-coated CD11b+ Ly6Chi Ly6G− cells purified from spleens of 129X1/SvJ mice infected with S. Typhimurium. Recipient mice that received PBS or OVA257–264 peptide-coated bone marrow-derived macrophages were used as controls. One day after the second adoptive transfer, splenocytes were harvested, stained, and analyzed to determine the level of OT-I T cell activation. We found that the percentages of activated OT-I T cells in spleens of mice that received OVA257–264 peptide-coated CD11b+ Ly6Chi Ly6G− cells were significantly higher than the percentage of activated OT-I T cells in spleens of mice that received PBS (Fig. 8). However, no significant increase in the percentage of activated OT-I T cells was observed in spleens of mice that received 2 × 106 instead of 1 × 106 OVA257–264 peptide-coated CD11b+ Ly6Chi Ly6G− cells (Fig. 8). In contrast, we found that a 2-fold increase in the number of OVA257–264 peptide-coated bone marrow-derived macrophages transferred resulted in a corresponding 2-fold increase in the percentage of activated OT-I T cells in spleen (Fig. 8). Thus, CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection can present antigen to T cells in vivo, but as their numbers in the periphery increase, there does not appear to be a corresponding increase in the response of T cells. We interpret these results to suggest that accumulation and persistence of CD11b+ Ly6Chi Ly6G− cells beyond a certain threshold level may cause immunosuppression.
FIG 8.
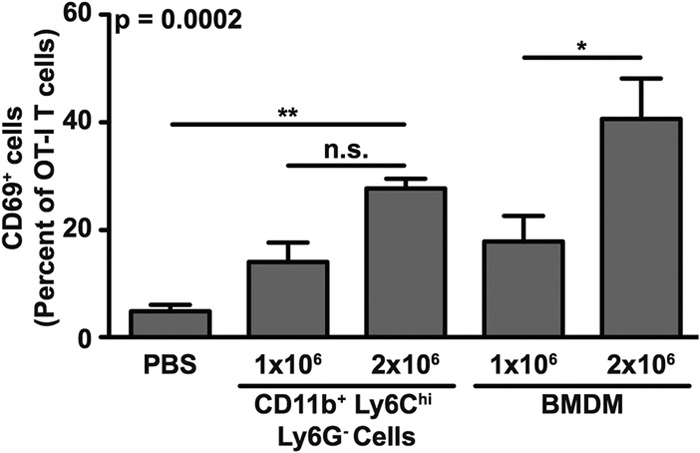
CD11b+ Ly6Chi Ly6G− cells recruited in response to S. Typhimurium infection can modulate T cell function in vivo. Activation of adoptively transferred OT-I T cells in F1 (C57BL/6J × 129X1/SvJ) mice (cumulative total of n = 4 per group) that received OVA257–264 peptide-coated CD11b+ Ly6Chi Ly6G− cells purified from spleens of 129X1/SvJ mice infected with S. Typhimurium. Activation of OT-I T cells was measured by analyzing expression of surface CD69. Recipient mice that received PBS or OVA257–264 peptide-coated bone marrow-derived macrophages were used as controls. Cumulative data from three independent experiments show means with standard errors of the means and were analyzed using a one-way ANOVA with Bonferroni's multiple-comparison posttest; P values of <0.05 were considered to be statistically significant. Asterisks indicate statistically significant differences (**, P < 0.01; *, P < 0.05; n.s., not significant).
DISCUSSION
We have found that large numbers of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells accumulate and persist in tissues of mice infected with S. Typhimurium. Furthermore, we have found that CCR2 is critical for the recruitment of CD11b+ Ly6Chi Ly6G− cells from the bone marrow to systemic sites of S. Typhimurium infection, where these cells may regulate CD4+ and CD8+ T cell responses via antigen presentation and NO production. Our results provide an understanding of a mechanism by which S. Typhimurium may induce acute immunosuppression and delay onset of protective immune responses. An immediate implication of our results is that, while phagocytes are essential for control of S. Typhimurium, accumulation and persistence of CD11b+ Ly6Chi Ly6G− phagocyte precursors in infected tissues could lead to a buildup of NO, a potent antimicrobial effector molecule that can also cause suppression of T cell function (26–28). We propose a model in which NO-producing CD11b+ Ly6Chi Ly6G− immature myeloid cells provide protective functions in the host response to infection, where accumulation and persistence of these cells beyond a certain threshold level may cause collateral effects that prolong or exacerbate disease (Fig. 9).
FIG 9.
Model of the role of CD11b+ Ly6Chi Ly6G− immature myeloid cells (IMC) in the host response to infection. We propose a model in which NO-producing CD11b+ Ly6Chi Ly6G− IMC provide protective functions in the host response to infection (top), where accumulation and persistence of these cells beyond a certain threshold level may cause collateral effects that prolong or exacerbate disease (bottom). For further explanation, see the text.
Consistent with a role for CD11b+ Ly6Chi Ly6G− cells in S. Typhimurium-induced inhibition of T cell responses, immature myeloid cells that have differentiated into inflammatory monocytes have been implicated recently in cytomegalovirus-induced suppression of antiviral CD8+ T cell responses (29). Furthermore, sustained expansion of immature myeloid cells in chronic but not acute infection with lymphocytic choriomeningitis virus has been linked to inhibition of antiviral CD8+ T cell responses (30). Thus, immature myeloid cells provide a balance of protective and immunosuppressive functions in the host response to infection.
The accumulation and persistence of CD11b+ Ly6Chi Ly6G− and CD11b+ Ly6Cint Ly6G+ cells in tissues of mice infected with S. Typhimurium could be the result of sustained inflammation, hyperinflammation, or failure to differentiate into protective cell populations such as macrophages, dendritic cells, and granulocytes. CD11b+ Gr-1+ cells are the predominant population of cells targeted by S. Typhimurium type III secretion in vivo and contain most of the total number of intracellular bacteria recovered from spleen (18). However, the majority of splenic immature myeloid cells are not directly infected with S. Typhimurium (see Fig. S3 in the supplemental material), and therefore, it is unknown if S. Typhimurium can directly inhibit differentiation of immature myeloid cells through a mechanism that would require targeting of these cells by type III secretion.
Suppression of T cell function by CD11b+ Ly6Chi Ly6G− cells occurs through the iNOS metabolic pathway and may be exploited by S. Typhimurium to avoid clearance. Consistent with this notion, the kinetics of CD11b+ Ly6Chi Ly6G− cell accumulation and persistence correspond to the delay in onset and dampening of T cell responses to S. Typhimurium, which peak in weeks 3 to 4 after infection (9, 12, 31). Furthermore, early studies linked NO-producing macrophage precursors to S. Typhimurium-induced immunosuppression and protective immunity against S. Typhimurium to diminished expansion of CD11b+ Gr-1+ cells (16, 17). Several recent studies have associated CD11b+ Gr-1+ cells with functional immunosuppression in the host response to infection (29, 30, 32–36), and some have implicated NO production by these cells in the suppression of T cell function. Thus, a role for NO-producing immature myeloid cells in mediating immunosuppression may be a common feature of many types of infections, particularly persistent or chronic infections.
The CD11b+ Ly6Chi Ly6G− cells that accumulate and persist in tissues of mice infected with S. Typhimurium resemble mononuclear myeloid-derived suppressor cells (MDSC), which have been associated with immunosuppression in cancer and, more recently, infection (37, 38). Direct roles of iNOS and arginase-1 in the suppressive activity of mononuclear MDSC are well established, whereas reactive oxygen species and peroxynitrite have emerged more recently as factors that contribute to the suppressive activity of MDSC (37, 38). Thus, production of NO, which can freely diffuse across membranes, may be only one of the immunomodulatory factors produced by CD11b+ Ly6Chi Ly6G− cells in the host response to S. Typhimurium.
In conclusion, our work provides an immunologic basis for a host-directed component that can exacerbate S. Typhimurium infection by inhibiting T cell responses. Historically, immature myeloid cells are considered essential for the protective host response to infection (15). Here, we establish that CD11b+ Ly6Chi Ly6G− immature myeloid cells provide a balance of protective and immunosuppressive functions in the host response to S. Typhimurium. The tipping of this balance may be an important factor influencing the outcome of infection. Thus, CD11b+ Ly6Chi Ly6G− immature myeloid cells could prove to be a promising target for the development of innovative, broad-spectrum therapeutic approaches to overcome infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jorge Benach, Martha Furie, Laurie Krug, Michael Starnbach, and David Thanassi for constructive feedback on the manuscript and Eric Pamer and members of the van der Velden laboratory for helpful discussions. We also thank Todd Rueb and Rebecca Connor in the Stony Brook University Flow Cytometry Core Facility for technical support.
This work was supported by NIH grants awarded to A.W.M.V.D.V. (R21AI092165, R01AI101221, and P01AI055621-Benach). J.W.T. was supported by the NIH under award number T32AI007539.
Footnotes
Published ahead of print 7 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01590-13.
REFERENCES
- 1.Andrews-Polymenis HL, Baumler AJ, McCormick BA, Fang FC. 2010. Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect. Immun. 78:2356–2369. 10.1128/IAI.00096-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pegues DA, Miller SI. 2010. Salmonella species, including Salmonella Typhi, p 2887–2903 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas and Bennett's principles and practice of infectious diseases, 7th ed, vol 2 Churchill Livingstone Elsevier, Philadelphia, PA [Google Scholar]
- 3.Dougan G, John V, Palmer S, Mastroeni P. 2011. Immunity to salmonellosis. Immunol. Rev. 240:196–210. 10.1111/j.1600-065X.2010.00999.x [DOI] [PubMed] [Google Scholar]
- 4.Tsolis RM, Xavier MN, Santos RL, Baumler AJ. 2011. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect. Immun. 79:1806–1814. 10.1128/IAI.01369-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luu RA, Gurnani K, Dudani R, Kammara R, van Faassen H, Sirard JC, Krishnan L, Sad S. 2006. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J. Immunol. 177:1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan A, Foley J, Ravindran R, McSorley SJ. 2004. Low-dose Salmonella infection evades activation of flagellin-specific CD4 T cells. J. Immunol. 173:4091–4099 [DOI] [PubMed] [Google Scholar]
- 7.Bueno SM, Gonzalez PA, Carreno LJ, Tobar JA, Mora GC, Pereda CJ, Salazar-Onfray F, Kalergis AM. 2008. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology 124:522–533. 10.1111/j.1365-2567.2008.02805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertelt JM, Johanns TM, Mysz MA, Nanton MR, Rowe JH, Aguilera MN, Way SS. 2011. Selective culling of high avidity antigen-specific CD4+ T cells after virulent Salmonella infection. Immunology 134:487–497. 10.1111/j.1365-2567.2011.03510.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanns TM, Ertelt JM, Rowe JH, Way SS. 2010. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 6:e1001043. 10.1371/journal.ppat.1001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sad S, Dudani R, Gurnani K, Russell M, van Faassen H, Finlay B, Krishnan L. 2008. Pathogen proliferation governs the magnitude but compromises the function of CD8 T cells. J. Immunol. 180:5853–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan A, McSorley SJ. 2007. Pivotal advance: exposure to LPS suppresses CD4+ T cell cytokine production in Salmonella-infected mice and exacerbates murine typhoid. J. Leukoc. Biol. 81:403–411. 10.1189/jlb.0306194 [DOI] [PubMed] [Google Scholar]
- 12.Kullas AL, McClelland M, Yang HJ, Tam JW, Torres A, Porwollik S, Mena P, McPhee JB, Bogomolnaya L, Andrews-Polymenis H, van der Velden AW. 2012. L-asparaginase II produced by Salmonella Typhimurium inhibits T cell responses and mediates virulence. Cell Host Microbe 12:791–798. 10.1016/j.chom.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science 327:656–661. 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serbina NV, Jia T, Hohl TM, Pamer EG. 2008. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26:421–452. 10.1146/annurev.immunol.26.021607.090326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi C, Pamer EG. 2011. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11:762–774. 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenstein TK. 2001. Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 3:1223–1231. 10.1016/S1286-4579(01)01482-4 [DOI] [PubMed] [Google Scholar]
- 17.Heithoff DM, Enioutina EY, Bareyan D, Daynes RA, Mahan MJ. 2008. Conditions that diminish myeloid-derived suppressor cell activities stimulate cross-protective immunity. Infect. Immun. 76:5191–5199. 10.1128/IAI.00759-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geddes K, Cruz F, Heffron F. 2007. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 3:e196. 10.1371/journal.ppat.0030196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- 20.Fleming TJ, Fleming ML, Malek TR. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399–2408 [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. 10.1016/S1074-7613(03)00174-2 [DOI] [PubMed] [Google Scholar]
- 22.Stojiljkovic I, Baumler AJ, Heffron F. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujol C, Bliska JB. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892–5899. 10.1128/IAI.71.10.5892-5899.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parish CR, Glidden MH, Quah BJ, Warren HS. 2009. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr. Protoc. Immunol. Chapter 4:Unit 4.9. 10.1002/0471142735.im0409s84 [DOI] [PubMed] [Google Scholar]
- 25.Harding CV, Ramachandra L. 2010. Presenting exogenous antigen to T cells. Curr. Protoc. Immunol. Chapter 16:Unit 16.2. 10.1002/0471142735.im1602s88 [DOI] [PubMed] [Google Scholar]
- 26.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. 2011. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 89:873–891. 10.1189/jlb.1010550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916. 10.1038/ni1001-907 [DOI] [PubMed] [Google Scholar]
- 28.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820–832. 10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- 29.Daley-Bauer LP, Wynn GM, Mocarski ES. 2012. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity 37:122–133. 10.1016/j.immuni.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. 2013. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity 38:309–321. 10.1016/j.immuni.2012.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittrucker HW, Kohler A, Kaufmann SH. 2002. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 70:199–203. 10.1128/IAI.70.1.199-203.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463–1474. 10.1084/jem.20062602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goni O, Alcaide P, Fresno M. 2002. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int. Immunol. 14:1125–1134. 10.1093/intimm/dxf076 [DOI] [PubMed] [Google Scholar]
- 34.Mencacci A, Montagnoli C, Bacci A, Cenci E, Pitzurra L, Spreca A, Kopf M, Sharpe AH, Romani L. 2002. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J. Immunol. 169:3180–3190 [DOI] [PubMed] [Google Scholar]
- 35.Van Ginderachter JA, Beschin A, De Baetselier P, Raes G. 2010. Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 40:2976–2985. 10.1002/eji.201040911 [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen JW, Tam JW, Okan NA, Mena P, Furie MB, Thanassi DG, Benach JL, van der Velden AW. 2012. Phenotypic, morphological, and functional heterogeneity of splenic immature myeloid cells in the host response to tularemia. Infect. Immun. 80:2371–2381. 10.1128/IAI.00365-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9:162–174. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. 2012. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12:253–268. 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.