Abstract
Glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are the two primary incretin hormones secreted from the intestine on ingestion of various nutrients to stimulate insulin secretion from pancreatic β‐cells glucose‐dependently. GIP and GLP‐1 undergo degradation by dipeptidyl peptidase‐4 (DPP‐4), and rapidly lose their biological activities. The actions of GIP and GLP‐1 are mediated by their specific receptors, the GIP receptor (GIPR) and the GLP‐1 receptor (GLP‐1R), which are expressed in pancreatic β‐cells, as well as in various tissues and organs. A series of investigations using mice lacking GIPR and/or GLP‐1R, as well as mice lacking DPP‐4, showed involvement of GIP and GLP‐1 in divergent biological activities, some of which could have implications for preventing diabetes‐related microvascular complications (e.g., retinopathy, nephropathy and neuropathy) and macrovascular complications (e.g., coronary artery disease, peripheral artery disease and cerebrovascular disease), as well as diabetes‐related comorbidity (e.g., obesity, non‐alcoholic fatty liver disease, bone fracture and cognitive dysfunction). Furthermore, recent studies using incretin‐based drugs, such as GLP‐1 receptor agonists, which stably activate GLP‐1R signaling, and DPP‐4 inhibitors, which enhance both GLP‐1R and GIPR signaling, showed that GLP‐1 and GIP exert effects possibly linked to prevention or treatment of diabetes‐related complications and comorbidities independently of hyperglycemia. We review recent findings on the extrapancreatic effects of GIP and GLP‐1 on the heart, brain, kidney, eye and nerves, as well as in the liver, fat and several organs from the perspective of diabetes‐related complications and comorbidities.
Keywords: Diabetic complication, Glucose‐dependent insulinotropic polypeptide, Glucagon‐like peptide‐1
Introduction
The history of incretins dates back as early as the year 1904, when Moore, who was inspired by the discovery of secretin by Bayliss and Starling1, hypothesized that gut extracts contain a hormone that regulates the endocrine pancreas. Based on his hypothesis, Moore showed that administration of gut extracts reduced urine sugars in diabetic patients, presumably through stimulation of the endocrine pancreas2. In 1929, La Barre purified the glucose‐lowering element from gut extracts, and named it incretin3. To date, gastric inhibitory polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are recognized as the two primary incretin hormones secreted from the gut after ingestion of glucose or various nutrients to stimulate insulin secretion from pancreatic β‐cells glucose‐dependently4. GIP and GLP‐1 undergo rapid inactivation catalyzed by dipeptidyl peptidase‐4 (DPP‐4), and their biological activity is drastically reduced. GIP and GLP‐1 exert their effects by binding to their specific receptors, the GIP receptor (GIPR) and the GLP‐1 receptor (GLP‐1R), which belong to the G‐protein coupled receptor family. Receptor binding activates and increases the level of intracellular cyclic adenosine monophosphate in pancreatic β‐cells, thereby stimulating insulin secretion glucose‐dependently. Genetic ablation of GIPR and GLP‐1R separately or simultaneously in mice showed their critical roles in the entero‐insular axis, and confirmed that GIP and GLP‐1 act as incretins8. In addition to their insulinotropic actions, a series of investigations using mice lacking GIPR and/or GLP‐1R, as well as mice lacking DPP‐4, showed the involvement of GIP and GLP‐1 in various biological effects (Figure 1), some of which could have implications for preventing or treating diabetic complications independently of hyperglycemia.
Figure 1.
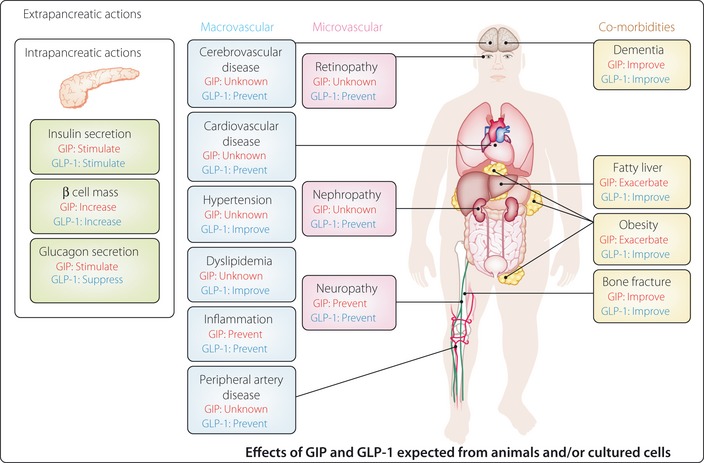
Extrapancreatic actions of glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1), and implications for diabetes‐related complications and comorbidities. GIP and GLP‐1 exert various intra‐ and extrapancreatic actions suggested from animal studies, some of which might be beneficial for prevention and treatment of diabetes‐related complications and comorbidities shown independently of glycemic control. Note that beneficial and adverse effects of GIP and GLP‐1 listed largely await confirmation in patients with diabetes.
Incretin‐based drugs are now being used to achieve better glycemic control in patients with type 2 diabetes worldwide11. To date, two incretin‐based drugs are clinically available: (i) GLP‐1 receptor agonists (GLP‐1RA) that increase resistance to DPP‐4 degradation to enable strong and steady activation of GLP‐1R; and (ii) DPP‐4 inhibitors (DPP‐4i) that inhibit DPP‐4‐dependent inactivation of GIP and GLP‐1, thereby enhancing their various biological actions. A series of clinical trials of GLP‐1RA and DPP‐4i showed that they significantly lower glycated hemoglobin without serious hypoglycemia and bodyweight gain15. It was noticed that incretin‐based drugs are more effective in Asians, likely a result of amelioration of defective early phase insulin secretion, which is a characteristic feature of type 2 diabetes in Asians4. Recent meta‐analyses of DPP‐4i therapy support this notion20. Large prospective clinical trials are ongoing to ascertain the effects of incretin‐based drugs on diabetes‐related complications of patients with type 2 diabetes, but the results are not yet available (Table 1). Thus, our knowledge today is still largely restricted to results obtained from experimental animals and cultured cells.
Table 1. Cardiovascular outcomes trials with selected incretin‐based drugs.
| Trial name (ClinicalTrials.gov ID) | Agent | Description |
|---|---|---|
| CAROLINA (NCT01243424) | Linagliptin |
Comparator: Glimepiride (superiority if non‐inferiority met) Period: October 2010–September 2018 Time to first occurrence of major CV events (Approximately 6,000 high risk type 2 diabetes; >5 years), multinational |
| EXAMINE (NCT00968708) | Alogliptin |
Comparator: Placebo (non‐inferiority design) Period: September 2009–December 2013 Time to first occurrence of primary major adverse cardiac events (Approximately 5,400 type 2 diabetes with ACS; 5 years), multinational |
| SAVOR‐TIMI53 (NCT01107886) | Saxagliptin |
Comparator: Placebo (superiority design) Period: May 2010–April 2013 To evaluate the effect on major CV events (Approximately 16,500 high‐CV‐risk type 2 diabetes; 4 years), multinational |
| TECOS (NCT00790205) | Sitagliptin |
Comparator: Placebo (non‐inferiority design) Period: December 2008–December 2014 Time to first occurrence of composite CV outcome (>14,000 type 2 diabetes; >4 years), multinational |
| ELIXA (NCT01147250) | Lixisenatide |
Comparator: Placebo (superiority design) Period: June 2010–May 2013 To evaluate the effect of lixisenatide on CV morbidity and mortality (Apporximately 6,000 type 2 diabetes with ACS; until occurrence of Apporximately 840 primary events), multinational |
| EXSCEL (NCT01144338) | Exenatide Once weekly |
Comparator: Placebo (superiority design) Period: June 2010–March 2017 To evaluate the effect of exenatide once weekly on major CV events (>9,000 type 2 diabetes; >5 years), multinational |
| LEADER (NCT01179048) | Liraglutide |
Comparator: Placebo (superiority design) Period: August 2010–January 2016 To evaluate the effect on CV outcomes (Apporximately 9,000 high‐CV‐risk type 2 diabetes; 5 years), multinational |
| REWIND (NCT01394952) | Dulaglutide |
Comparator: Placebo (superiority design) Period: July 2011–April 2019 Time to first occurrence of major CV events (Approximately 9,600 type 2 diabetes with established CV risk; approximately 6.5 years), multinational |
ACS, acute coronary syndrome; CV, cardiovascular.
Diabetes‐related vascular complications are the major causes of morbidity and mortality in patients with diabetes. Microvascular complications include retinopathy, nephropathy and neuropathy, which are the leading causes of blindness, renal failure and nerve injuries associated with foot ulcers and amputations, respectively. Macrovascular complications involve atherosclerosis‐related diseases, such as coronary artery disease (CAD), peripheral artery disease (PAD), cerebrovascular disease (CVD) and possible cognitive dysfunction. Classical clinical trials, such as the Diabetic Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS), clearly showed that intensive antidiabetic treatment can ameliorate hyperglycemia and reduce the progression of microvascular complications22; the follow up of DCCT and UKPDS also showed that such intensive treatment can suppress the incidence of macrovascular complications24. These findings show that hyperglycemia is a critical cause of diabetes‐related vascular complications, and provide a basis to search for strategies to obtain better glycemic control. In addition, various studies have clarified mechanisms underlying the pathogenesis of diabetic vascular complications including: (i) the polyol pathway; (ii) the protein kinase C (PKC) pathway; (iii) oxidative stress; (iv) advanced glycation end‐products (AGEs) pathway; and (v) the hexosamine pathway26. All of these findings are suggestive for the development of agents to prevent diabetes‐related vascular complications independently of glycemic conditions.
In the present review, we highlight recent findings on the extrapancreatic effects of GIP and GLP‐1 in various tissues and organs that might be relevant to the prevention and management of diabetes‐related complications.
Diabetes‐related Macrovascular Complications
Several studies showing the effects of GLP‐1 and GIP on macrovascular function have recently been published. We summarize the effects of GLP‐1 and GIP that might be of significance for treatment of macrovascular complications, such as CAD, PAD and CVD, in patients with diabetes.
Effects on Atherosclerosis
Atherosclerosis constitutes the underlying pathological lesion in the clinical entities CAD, CVD and PAD. The inflammatory responses induced by adhesion of monocytes to the vascular wall have been known to play important roles in the early stages of the development of atherosclerosis. GLP‐1 was shown to significantly inhibit macrophage infiltration and atherosclerosis development in normal and diabetic apolipoprotein E‐deficient mice (Figure 2)27, whereas a recent study using the long‐acting GLP‐1RA taspoglutide failed to reproduce attenuation of atherosclerosis by GLP‐1R activation30. Exendin‐4 significantly inhibited monocyte adhesion to the vascular endothelial cells by reducing production of intercellular adhesion molecule (ICAM)‐129. Liraglutide also attenuated the expressions of vascular adhesion molecules (VAM) and ICAM‐1 in human vascular endothelial cells, thereby suppressing development of atherosclerosis31. In patients with diabetes, it has been shown that exenatide suppressed markers for inflammation, high sensitive C‐reactive protein and monocyte chemo‐attractant protein‐1 (MCP‐1), oxidative stress, and prostaglandin F2α33, and that exenatide improved induced endothelial dysfunction34.
Figure 2.
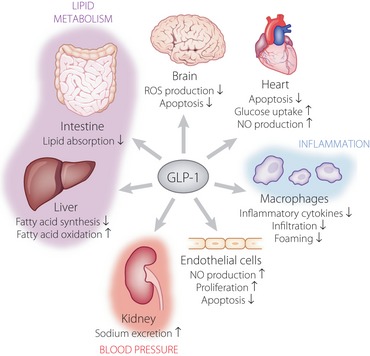
Glucagon‐like peptide‐1 (GLP‐1) prevents macrovascular complications through multiple mechanisms. GLP‐1 prevents macrovascular complications, such as coronary artery disease, cerebrovascular disease and peripheral artery disease, through direct actions on the brain, heart and vascular endothelial cells. GLP‐1 also indirectly exerts beneficial effects through regulation of lipid metabolism, blood pressure and inflammation. NO, nitric oxide; ROS, reactive oxygen species.
Although GLP‐1R expression in mouse macrophages is still controversial30, it has been shown that exendin‐4 somehow inhibits lipopolysaccharide (LPS)‐induced production of the inflammatory cytokine tumor necrosis factor (TNF)‐α and MCP‐1 from isolated mouse macrophages29. Exendin‐4 suppressed the nuclear translocation of p65, a component of nuclear factor (NF)‐κB, and this effect was reversed by both MDL‐12330A, a cyclic adenosine monophosphate (cAMP) inhibitor, and PKI14‐22, a protein kinase A (PKA)‐specific inhibitor. Therefore, it was suggested that these actions of exendin‐4 are likely to be mediated through a GLP‐1R/PKA pathway in mouse macrophages29. It also has been shown that GLP‐1 activates human macrophages through signal transducers and activator of transcription (STAT3) activation36.
In addition to its effects on macrophages, it has been shown that GLP‐1 exerts direct effects on vascular endothelial cells (Figure 2). GLP‐1R activation directly activated endothelial nitric oxide synthase (eNOS) in human umbilical vein endothelial cells (HUVECs)37 and the aortic endothelium31. GLP‐1 increased production of nitric oxide (NO), resulting in an increase of the microvascular blood flow in the blood38. It was reported that NO production was mediated by the poly(adenosine diphosphate‐ribose) polymerase pathway39. GLP‐1 also promoted proliferation and differentiation of endothelial progenitor cells by upregulating vascular endothelial growth factor (VEGF) expression40. Incubation of human coronary artery endothelial cells (HCAECs) with exendin‐4 resulted in a dose‐dependent increase in DNA synthesis and an increased cell number associated with enhanced eNOS and v‐akt murine thymoma viral oncogene homolog 1 (Akt1) activation that were abolished by a GLP‐1R antagonist41; Exendin‐4 directly improves endothelial dysfunction in isolated aortas42. Liraglutide also inhibited the NF‐κB pathway and suppressed apoptosis of HUVECs43. There is a report that GLP‐1 also inhibited AGE‐induced apoptosis in HUVECs44. Taken together, it appears that GLP‐1 can exert anti‐atherogenic effects through various mechanisms.
GIP was also shown to significantly inhibit macrophage infiltration and atherosclerosis development in normal and diabetic apolipoprotein E‐deficient mice27. It was recently found that GIP inhibits AGEs‐enhanced production of reactive oxygen species (ROS), and expression of VAM‐1 and PAI‐1 through the GIPR/Epac pathway45. These findings show that GLP‐1 and GIP can exert anti‐atherosclerosis effects, and might have implications in preventing macrovascular complications.
Consistent with the anti‐atherosclerotic effects of GLP‐1 and GIP, DPP‐4i des‐fluor‐sitagliptin was also shown to inhibit the production of inflammatory cytokines, such as TNF‐α, interleukin (IL)‐6, IL‐1β and MCP‐1, as well as that of VACM‐1 and ICAM‐1, and to increase endothelial NO production and suppress the NF‐κB pathway in experimental animals46. Furthermore, they showed that DPP‐4 inhibition increases circulating endothelial precursor cells (EPCs), which exert vasoprotective effects46. Similar observations were made with various DPP‐4i including sitagliptin47, alogliptin47 and vildagliptin49. Such anti‐atherosclerotic effects were also observed in type 2 diabetic patients treated with sitagliptin51.
Effect on Cardiac Function
Studies in experimental animals have shown beneficial roles of GLP‐1 or GLP‐1RA in CAD (Figure 2). In pigs, exendin‐4 administration significantly reduced infarct size and improved wall motion54. In mice, liraglutide administration showed similar cardioprotective effects with activation of cardioprotective genes55. In rabbits, administration of GLP‐1 fused to human transferrin significantly reduced infarct size and improved the wall motion and ejection fraction after myocardial ischemia/reperfusion injury56. Like GLP‐1RA, native GLP‐1 also showed a cardioprotective function in rats with myocardial ischemia/reperfusion injury, partly by suppressing activation of peripheral neutrophil57. Another study on dogs with myocardial ischemia suggested that GLP‐1 exerted a cardioprotective role by augmenting glucose uptake58. This is consistent with a recent study showing that the long‐acting GLP‐1RA albiglutide enhanced myocardial glucose uptake and promoted a shift toward a more energetically favorable substrate metabolism by increasing both glucose and lactate oxidation, thereby reducing infarct size and improving cardiac function59. Despite recent debates on GLP‐1R expression in mouse hearts, the earlier study showed that GLP‐1R activation increases cAMP production and suppresses caspase‐3 activation in cardiomyocytes, thereby preventing apoptosis of cardiomyocytes55. Another study showed that GLP‐1R activation increases cAMP and phosphorylation of Akt and extracellular signal‐regulated kinase (ERK), regulators of growth and glucose metabolism in cardiomyocytes60. Importantly, it was shown that although GLP‐1 increased cAMP levels in cardiomyocytes, the increased cAMP did not correlate with the intracellular calcium concentration and subsequent cardiomyocyte contractility61. It is interesting to note that GLP‐1 was previously shown to expert cardioprotective effects mediated through both GLP‐1R‐dependent and GLP‐1R‐independent mechanisms62. It was suggested that GLP‐1R‐independent cardioprotective actions of GLP‐1 are mediated by GLP‐1(9‐36)amide, the primary GLP‐1 metabolite in vivo, because GLP‐1(9‐36)amide exerts cardioprotective actions in GLP‐1R‐deficient mice60. However, the molecular mechanisms underlying the GLP‐1R‐independent cardioprotective actions of GLP‐1(9‐36)amide are largely unknown. Little is known of the effects of GIP on the heart.
In a clinical study, it was reported that GLP‐1 significantly improved left ventricle ejection fraction, global wall motion indices, and regional wall motion indices in patients with acute myocardial infarction and severe systolic dysfunction63. More recently, it was reported that GLP‐1 infusion protects the heart from ischemic left ventricle dysfunction induced by dobutamine stress in CAD patients64. Furthermore, it was shown that exenatide reduces reperfusion injury and final infarct size in patients with ST‐segment elevated myocardial infarction65.
Studies in experimental animals showed that DPP‐4 inhibition also results in similar cardioprotective effects67. It has been shown that the DPP‐4i sitagliptin increased the intracellular cAMP and PKA activity, and that H‐89, a potent selective PKA inhibitor, completely blocked the effect of sitagliptin in reducing the size of myocardial infarct, suggesting involvement of the GLP‐1R/cAMP/PKA pathway70. However, a recent study also suggested that the cardioprotective effects of DPP‐4i are mediated not only by GLP‐1, but also by other bioactive peptides, such as stromal‐derived factor‐1α51.
It was also suggested that GLP‐1R activation might exert beneficial effects in heart failure. In a dog model of pacing‐induced heart failure, GLP‐1 increased glucose uptake, thereby increasing left ventricular function75. In the spontaneously hypertensive and heart failure‐prone rat, GLP‐1 infusion improved survival and preserved left ventricular function with reduced apoptosis of cardiomyocytes76. In a rat model of chronic heart failure with permanent occlusion of the left anterior descending artery, infusion of GLP‐1 or GLP‐1RA enhanced left ventricular function, reducing left ventricular remodeling and improving survival77. In humans, infusion of GLP‐1 or GLP‐1RA in patients with heart failure improved left ventricular ejection faction78. Furthermore, it has been shown that DPP‐4i improved left ventricular function in experimental models of heart failure71. Although further studies of the underlying mechanisms are required, these results together strongly suggest clinical implications for GLP‐1RA in treatment of heart failure.
Effect on Cerebrovascular Function
Neuroprotective effects of GLP‐1RA after cerebral ischemia in non‐diabetic and diabetic animals have been described (Figure 2)81. Intravenous injection of exendin‐4 after cerebral ischemia reduced the infarct size and neurological deficits induced by reperfusion after occlusion of the middle cerebral artery in a mouse model of acute cerebral infarction81. Exendin‐4 also attenuated the oxidative stress and reduced neuronal cell death after reperfusion in this focal ischemia model. As exendin‐4 injection has been associated with increased intracellular cAMP levels81, this compound likely exerts its neuroprotective effect through a cAMP activation pathway. It therefore appears that GLP‐1RA might be potentially useful in the treatment of cerebral infarction. No study has been carried out on the neuroprotective effects of GIP after cerebral ischemia, although it has been shown that GIPR‐deficient mice showed impaired learning, synaptic plasticity and neurogenesis84, and that GIP and GIPR agonist enhance long‐term potentiation (LTP) and neurogenesis85. Consistent with the neuroprotective effects of GLP‐1RA after cerebral ischemia, the DPP‐4i linagliptin was found to reduce ischemic brain damage in diabetic mice87. In the same study, sulfonylurea glimepiride lowered glucose levels, but did not show similar neuroprotective effects, suggesting that the neuroptotective effects are independent of glycemic control and presumably mediated by GLP‐187.
Effect on Blood Pressure
Hypertension plays a critical role in the development of macrovascular complications. Chronic infusion of GLP‐1 reduced the incidence of hypertension, and prevented cardiac hypertrophy and fibrosis in salt‐sensitive Dahl rats88. In this model of hypertension, GLP‐1 also reduced urinary albumin excretion, increased urinary sodium excretion and improved histopathological abnormalities, such as glomerulosclerosis and tubular necrosis (Figure 2)88. In salt‐sensitive obese db/db mice and angiotensin II‐infused C57BL/6J mice, exendin‐4 prevented the onset of hypertension and increased the urinary sodium excretion89. Similarly, infusion of GLP‐1RA AC3174 also attenuated hypertension and the histopathological changes associated with the renal dysfunction in the Dahl rats90. Administration of DPP‐4i sitagliptin also reduced blood pressure in spontaneously hypertensive rats by decreasing expression of Na+/H+ exchanger isoform 3 in microvilli membranes of the proximal renal tubule, thereby increasing the urinary sodium excretion and the urinary volume, and reducing blood pressure91. Furthermore, GLP‐1RA and DPP‐4i also ameliorates endothelial dysfunction92.
Antihypertensive effects of GLP‐1 have consistently been shown in several clinical trials. In the trials to evaluate efficacy and safety of GLP‐1RA liraglutide carried out in type 2 diabetic patients, liraglutide administration was found to decrease systolic blood pressure by 2–6 mmHg from baseline in 26 weeks93. In addition, measurement of flow‐mediated vasodilatation (FMD) of the brachial artery as a measure of endothelial function in patients with type 2 diabetes after 16‐week exenatide treatment showed a significantly higher value of FMD in the exenatide‐treated group compared with that in the glimepiride‐treated group94. This result shows that exenatide exerts vasodilatory action and might reduce blood pressure.
Increase of urinary sodium excretion and urinary volume are well‐known effects of GLP‐1 and GLP‐1RA in rodents. In rats, intracerebroventricular injection of GLP‐1 was found to exert marked natriuretic and diuretic effects mediated by GLP‐1R that were blocked by treatment with GLP‐1R antagonist exendin(9‐39)95. GLP‐1 was expressed in porcine proximal tubular cells isolated from kidneys, and inhibited sodium reabsorption96. It is therefore likely that the marked diuretic effect of GLP‐1 is mediated by direct regulation of sodium reabsorption in kidney proximal tubules, as well as through hypothalamic GLP‐1R. Clinically, the effects of GLP‐1 infusion on urinary sodium excretion, urinary output and the glomerular filtration rate after an intravenous administration of salt load were investigated in obese men; GLP‐1 was found to significantly enhance the urinary sodium excretion, H+ secretion and glomerular hyperfiltration in obese men97.
Although no report has shown an association of GIP with blood pressure, DPP‐4i sitagliptin attenuated elevation of blood pressure in spontaneously hypertensive rats91. It also has been reported that DPP‐4i reduces blood pressure98, which supports the notion that GLP‐1 regulates blood pressure in humans.
Effect on Dyslipidemia
Dyslipidemia plays a critical role in the development of macrovascular complications. It has been shown that GLP‐1 ameliorates dyslipidemia in experimental animals, as well as in humans, whereas little is known on the effects of GIP on lipid metabolisms.
GLP‐1 infusion reduced apolipoprotein B‐48 production and triglycerides absorption (Figure 2)100. These effects were reproduced in mice and hamsters infused with exendin‐4, which acutely decreased postprandial serum triacylglycerol and apolipoprotein B‐48 GLP‐1R‐dependently101. These effects were observed even if exendin‐4 was given 1 h after fat ingestion, showing that the effects on postprandial lipid metabolism were not related to delayed gastric emptying101. Secretion of apolipoprotein B‐48 was significantly reduced from hamster primary enterocytes treated by enendin‐4101, suggesting that GLP‐1R activation expressed on enterocytes controls secretion of chylomicron. GLP‐1 controls hepatic lipid metabolism. GLP‐1RA markedly reduced hepatic lipid content by suppressing genes involved in fatty acid synthesis (e.g., sterol‐regulatory element binding protein‐1c, fatty acid synthase and steroyl CoA desaturase‐1) and enhancing expression genes regulating fatty acid oxidation (e.g., acyl‐coenzyme A oxidase and carnitine palmitoyltransferase 1a)102. Mechanisms regulating expression of genes involved in lipid metabolism by GLP‐1RA are largely unknown, inasmuch as the presence of hepatic GLP‐1R expression is still controversial. Nevertheless, both GLP‐1 and GLP‐1RA clearly ameliorate dyslipidemia in experimental animals, suggesting clinical implications in patients with dyslipidemia.
GLP‐1 infusion inhibited the postprandial elevation of triglycerides and free fatty acids in healthy human subjects104. A single subcutaneous injection of exenatide in patients with newly diagnosed type 2 diabetes also showed marked reduction in postprandial triacylglycerol, as well as in apolipoprotein B‐48105. Although these effects of GLP‐1 or GLP‐1RA on triglycerides and free fatty acids could be partly a result of delayed gastric emptying, these results clearly show that GLP‐1R activation ameliorates postprandial lipidemia. It has also been shown that DPP‐4i vildagliptin and sitagliptin suppressed postprandial elevation of triglycerides and apolipoprotein B‐48 in patients with type 2 diabetes106. As DPP‐4i shows little effect on gastric emptying108, the effects of DPP‐4i on postprandial lipidemia might be largely mediated by inhibition of intestinal lipid absorption. It is noteworthy that DPP‐4i ameliorates dyslipidemia in patients with type 2 diabetes109, which, although small, could also contribute to the prevention of macrovascular complications.
Diabetes‐related Microvascular Complications
GIPR and GLP‐1R are expressed in the peripheral and central nervous system, the eyes, and the kidney, suggesting the possibility of some effects in these organs. We summarize the effects of GIP and GLP‐1 in those organs that are likely to be of significance in the treatment of the microvascular complications (i.e., retinopathy, nephropathy and neuropathy) of diabetes.
Effect on the Peripheral Nervous System
Most recognized diabetes‐related neurological complications involve the peripheral nervous system110. Several studies have shown beneficial effects of GLP‐1 and GIP on the peripheral nervous system that might have therapeutic implications for the treatment of diabetic neuropathy.
GLP‐1R has been found to be expressed in the lumbar dorsal root ganglion neurons (DRG) of C57BL6/J mice111. GLP‐1 and GLP‐1RA exendin‐4 significantly promoted the neurite outgrowth of DRG neurons (Figure 3). Exendin‐4 attenuated the hypoalgesia, and delayed motor and sensory nerve conduction velocity in diabetic mice112. In the same study, exendin‐4 also ameliorated the decrease in intra‐epidermal nerve fiber densities in the sole skins of the diabetic mice. These findings suggest that exendin‐4 might ameliorate the severity of diabetic polyneuropathy through exerting direct actions on the DRG neurons and their axons. GLP‐1R was also found to be expressed in the sciatic nerve112. GLP‐1RA significantly increased the phosphorylated ERK 1/2 levels in the sciatic nerves of diabetic rats, showing GLP‐1R activity in this diabetic tissue. Exendin‐4 exerted no effect on the blood sugar or insulin levels in diabetic mice, and also had no effect on paw thermal response latencies in these mice, but attenuated the reductions of motor nerve conduction velocity and paw intra‐epidermal fiber density seen in diabetic mice. Thus, GLP‐1R‐mediated ERK‐signaling in the sciatic nerve of diabetic rodents might protect the large‐motor fiber functions and small C fiber structure by a mechanism independent of glycemic control113. GLP‐1R has been found to be expressed in the skin. Exendin‐4 treatment reduced the increase in the current perception threshold seen in diabetic rats. The decrease in the size of myelinated fibers or in the axon/fiber area ratio in the sciatic nerve and loss of the intra‐epidermal nerve fibers in the skin of diabetic rats were also ameliorated by exendin‐4 treatment. Thus, exendin‐4 might prevent the peripheral nerve degeneration seen in diabetic rats, suggesting that GLP‐1RA might be useful in the treatment of peripheral neuropathy112. Pyridoxine‐induced peripheral neuropathy is characterized by sensory nerve conduction deficits associated with disturbances of the nerve fiber geometry and axonal atrophy. In an evaluation carried out using behavioral and morphometric techniques, GLP‐1 and GLP‐1RA exendin‐4 were found to improve pyridoxine‐induced sensory peripheral neuropathy in rats114. Based on these findings, it has been suggested that GLP‐1RA might be useful in the treatment of diabetic neuropathy.
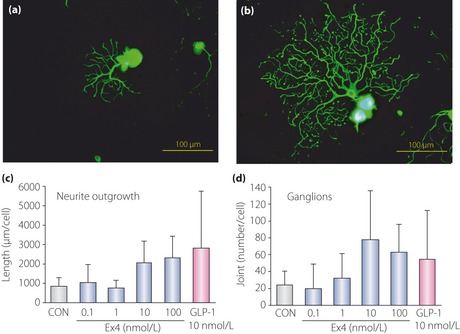
Figure 3.
Glucagon‐like peptide‐1 (GLP‐1) receptor activation promotes neurite outgrowth of the dorsal root ganglion. Representative fluorescence micrograph of dorsal root ganglion neurons cultured in the (a) absence or (b) presence of GLP‐1. GLP‐1 or GLP‐1 receptor agonist exendin‐4 (Ex4) increased the total neurite length (c) and joint number (d) of neurites. CON, control. Reproduced from Himeno et al.111, with permission from the American Diabetes Association © 2011.
Expressions of GIP and GIPR were enhanced after sciatic nerve crush injury in DRG, spinal cord and nerve fragments of rats, suggesting involvement of GIP/GIPR in axonal regeneration. Indeed, GIPR‐deficient mice show impaired axonal regeneration115. Thus, GIPR activation might have therapeutic implications for the treatment of diabetic neuropathy.
Consistent with the beneficial effects of GIP and GLP‐1 on the peripheral nervous system, DPP‐4i vildagliptin analog PKF275‐055 partially counteracted the nerve conduction velocity deficit observed in diabetic rats116. Diabetic rats developed mechanical hyperalgesia and showed significantly longer thermal response latencies117. PKF275‐055 induced recovery of the mechanical sensitivity thresholds by approximately 50% and progressively improved the alterations in the thermal responsiveness. DPP‐4i is therefore likely to have a potential therapeutic effect in the treatment of diabetic neuropathy. Vildagliptin was found to protect against nerve fiber loss in diabetic animals117. The decrease in intra‐epidermal nerve fiber density in diabetic rats was significantly inhibited by vildagliptin treatment117. Based on these results, it is suggested that DPP‐4i might prevent peripheral nerve degeneration in diabetic animals and might be useful in treatment of peripheral neuropathy.
Effect on the Kidney
Diabetic nephropathy is a critical diabetes‐related microvascular complication that is a major cause of renal failure118. Recently, many studies have been carried out to investigate the effects of GLP‐1, GLP‐1RA and DPP‐4i on kidney dysfunctions, but little is known about the effect of GIP on kidney. We review the effects of GLP‐1, GLP‐1RA and DPP‐4i on the kidney functions that might be of significance in the treatment of diabetic nephropathy.
GLP‐1R messenger ribonucleic acid (mRNA) was detected in rat glomeruli and glomerular endothelial cells, as well as in human monocytes and macrophages120. In diabetic db/db mice, exendin‐4 was found to reduce the urinary albumin excretion and histological parameters of glomerular injury characterized by mesangial extracellular matrix expansion and glomerulosclerosis (Figure 4)121. Exendin‐4 also significantly reduced the expression of transforming growth factor (TGF)‐β1, deposition of type‐IV collagen, macrophage infiltration and apoptosis in the glomeruli120. Similarly, it also reduced the glomerular hypertrophy and mesangial matrix expansion noted in diabetic rats120. Exendin‐4 also reduced the marker levels of macrophage infiltration, plasma levels of ICAM‐1, deposition of type‐IV collagen, oxidative stress and NF‐κB activation in the kidney tissue120. Furthermore, exendin‐4 attenuated the production of high glucose‐stimulated inflammatory cytokines, such as TNF‐α and IL‐1β, in human acute monocytic leukemia cell line THP‐1 cells and human glomerular endothelial cells120. These effects were significantly blocked by the GLP‐1 antagonist, exendin(9‐39)120. GLP‐1RA liraglutide normalized the levels of the oxidative stress markers, 8‐hydroxydeoxyguanosine and malondialdehyde, and expressions of the nicotinamide adenine dinucleotide phosphate oxidase components TGF‐β and fibronectin in the renal tissues, and also decreased the urinary albumin excretion that was significantly increased in diabetic rats122. Native GLP‐1 has been shown to suppress the AGE receptor and reduce MCP‐1 expression in mesangial cells123. Furthermore, recent studies suggested involvement of PKA and PKCβ activation in the renoprotective effects of GLP‐1124. Consistent with the renoprotective effects of GLP‐1, DPP‐4i vildagliptin significantly reduced proteinuria, albuminuria and the urinary albumin/creatinine ratio in diabetic rats126. Vildagliptin also improved the creatinine clearance, and inhibited the histological changes of interstitial expansion, glomerulosclerosis and thickening of the glomerular basement membrane in a dose‐dependent manner in diabetic rats. These renoprotective effects of vildagliptin were related to a reduction in the production of the inflammatory TGF‐β1. Vildagliptin also exerted renoprotective effects in rats with renal ischemia‐reperfusion injury127. Sitagliptin ameliorated renal lesions in the diabetic rats, including glomerular, tubulointerstitial and vascular lesions, accompanied by reduced lipid peroxidation128.
Figure 4.
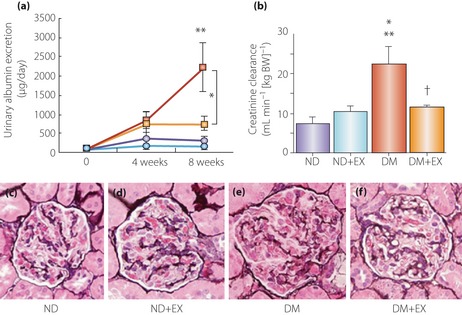
Glucagon‐like peptide‐1 prevents kidney lesions in streptozotocin‐induced diabetic rats. (a) Changes in 24‐h urinary albumin excretion. Urinary albumin excretion increased gradually over 8 weeks in the diabetic group. Exendin‐4 resulted in a significantly lower level of urinary albumin excretion at 8 weeks than in the untreated diabetes group. *P < 0.05; **P < 0.01 versus non‐diabetic and non‐diabetic + exendin‐4 groups. Blue circles, non‐diabetic group (ND); light blue circles, non‐diabetic + exendin‐4 (ND+EX); red squares, streptozotocin‐induced diabetes group (DM); yellow squares, diabetes + exendin‐4 group (DM+EX). (b) Creatinine clearance. Hyperfiltration in DM was significantly decreased by DM+EX at 8 weeks. *P < 0.05 versus ND+EX; **P < 0.01 versus ND; †P < 0.05 versus DM. Periodic acid–methenamine‐silver (PAM) staining in (c) glomeruli of ND, (d) ND+EX, (e) DM and (f) DM+EX (magnification: ×200). Mesangial matrix index, calculated by the PAM‐positive area in the tuft area, was significantly increased in DM, whereas mesangial matrix expansion was significantly reduced in DM+EX. Reproduced from Kodera et al.120, with permission from Diabetologia © 2011.
Taking these results together, it is likely that GLP‐1RA and DPP‐4i might have potential renoprotective effects in patients with diabetic nephropathy. Indeed, it has been recently shown that exenatide reduced urinary TGF‐β1 and type‐IV collagen excretion in patients with type 2 diabetes129, and that sitagliptin reduced albuminuria in patients with type 2 diabetes130. However, it remains to be seen if the renoprotective effects in patients depend on amelioration of glycemic control or if GLP‐1 can exert direct effects on the kidney.
Effect on the Eye
Diabetic retinopathy is one of critical diabetes‐related microvascular complications that occurs in approximately 60% of patients with duration of diabetes more than 20 years, and is one of the major causes of blindness131. Some studies regarding the effects of GLP‐1RA and DPP‐4i on the retina have been available for several years132. In contrast, the effects of GIP on the retina are scarcely known, although it has been shown that GIP and GIPR are expressed in the rat retina136. We summarize the effects of GLP‐1, GLP‐1RA and DPP‐4i on the retina that have potential clinical significance in the treatment of retinopathy.
Both the mRNA and protein expressions of GLP‐1R have been detected in the inner layer of the retina in rats132. In diabetic rats, subcutaneous injection of exendin‐4 prevented the loss of the b‐wave amplitude and oscillatory potentials in the retina132. The retinal thickness, which depends on the duration of diabetes, is reduced, and the cell counts of both the outer and inner nuclear layers are also reduced in diabetic rats; however, subcutaneous administration of exendin‐4 prevented the loss of the retinal cells and maintained the normal retinal thickness. In addition, intravitreal injection of exendin‐4, independent of its pancreatic effect, also prevented both the loss of the b‐wave amplitude and the oscillatory potentials observed in diabetes, and also the cell loss in the outer and inner nuclear layers in the retina133. The excessive retinal glutamate was significantly reduced by exendin‐4 treatment in diabetic rats. Rapid removal or inactivation of glutamate is required to maintain the normal functions of the retina through the prevention of glutamate‐induced injury of the neurons. Consistent with such changes, retinal GLP‐1R and glutamate aspartate transporter expressions were also reduced in the diabetic retina, but were upregulated in the exendin‐4‐treated rats133. The effect of exendin‐4 in improving glucose‐induced retinal ganglion cell impairment has also been investigated. Exendin‐4 protected retinal ganglion cells by affecting expression in pro‐apoptotic Bax and anti‐apoptotic Bcl‐2, and the protective effects of exenatide were inhibited by GLP‐1R antagonist, exendin(9‐39)134. Taking these results together, GLP‐1R activation might well protect retinal functions in diabetic retinopathy.
In diabetic rats, DPP‐4i sitagliptin reduced the intracellular accumulation of tight junction proteins, such as occludin and claudin‐5, in the blood retinal barrier observed in diabetes135. Sitagliptin also decreased the nitrosative stress, inflammatory IL‐1β production and cell death by apoptosis in diabetic retinas135. Sitagliptin recovered the number of circulating CD34+ cells to the control level in diabetic rats and increased the adhesion ability of endothelial progenitor cells to the retinal vessels135. These results suggest that DPP‐4i might exert beneficial effects on the integrity of the blood–retinal barrier in diabetic rat retinas through prevention of nitrosative stress, inflammatory responses and apoptosis of the retinal cells, although it needs to be tested whether these beneficial effects are mediated by GLP‐1.
Diabetes‐related Comorbidities
GIP and GLP‐1 might exert biological effects in various organs, such as the brain, bone, fat and liver, the normal functions of which have been affected by diabetes. We summarize the effects of GIP and GLP‐1 in the organs that are likely to be of significance in the treatment of diabetes.
Effects on Adipose Tissues
Obesity and overweight are closely associated with the incidence of diabetes138 and glycemic control in diabetic patients142. In addition, obesity has been linked to chronic inflammation that leads to vascular complications143. It has been shown that GIP plays physiological roles in nutrient uptake directly into adipose tissues, thereby linking overnutrition to obesity (Figure 5)145. In contrast, although GLP‐1RA is known to reduce bodyweight and body fat146, GLP‐1 seems to have no direct effect on adipocytes. We summarize the effects of GLP‐1 and GIP on the adipose tissues and obesity that might have potential clinical significance in the control of bodyweight.
Figure 5.
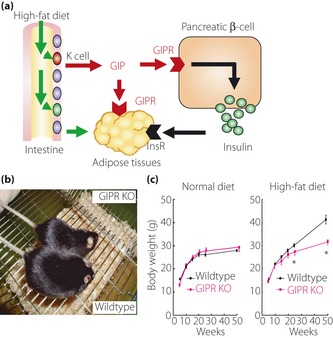
Effect of glucose‐dependent insulinotropic polypeptide (GIP) on fat accumulation. GIP plays physiological roles in nutrient uptake directly into adipose tissues, thereby linking overnutrition to obesity. (a) GIP, in collaboration with insulin whose secretion is also enhanced by GIP, promotes fat accumulation. A recent study showed that GIP receptor (GIPR) in adipose tissues alone is not sufficient for fat accumulation156. InsR, insulin receptor. (b) Gross appearance of GIPR‐deficient mice (GIPR KO) and wild‐type mice (Wildtype) fed on a high‐fat diet149. (c) Comparison of bodyweight changes in GIPR KO and Wildtype on either a normal diet or high‐fat diet. Note that GIPR KO and Wildtype showed a significant difference in bodyweight changes when they were on a high‐fat diet, but not on a normal diet149. Reproduced from Miyawaki et al.149.
Regarding effects of GIP on adipocytes, an initial clue came in the early 1980s from an experiment showing that GIP, in the presence of insulin, induces fatty acid incorporation into rat epididymal fat pads147. Later, GIPR was shown to be expressed in adipose tissues148, and genetic disruption of GIPR further shows the critical role of GIP in fat accumulation149. Although mice chronically fed on high‐fat diets show an increase in bodyweight as well as visceral and subcutaneous fat mass, such bodyweight gain and fat accumulation was not observed in GIPR‐deficient mice fed the same high‐fat diet149. GIPR‐deficient mice on high‐fat diets showed higher energy expenditure with a reduction of oxygen consumption and respiratory quotient during the light phase, the latter indicating that fat is utilized as the preferred energy substrate149. In addition, GIPR‐deficient mice show increased adiponection secretion, which promotes fat oxidation in muscle and increases the respiratory quotient150. Genetic ablation of GIPR in diabetic ob/ob mice prevents obesity by increasing energy expenditure, and improves insulin sensitivity and glucose tolerance without seriously affecting insulin secretion145. These observations were reproduced in high‐fat fed mice and obese ob/ob mice treated with a GIPR antagonist, (Pro3)GIP152, and in mice lacking GIP‐secreting K cells155, establishing the critical role of GIP in fat accumulation. Transgenic rescue of GIPR in GIPR‐deficient mice suggested that GIP, in collaboration with insulin, facilitates fat accumulation156. Although a recent report that GIP‐overexpressing mice resulted in reduced diet‐induced obesity while they showed enhanced β‐cell function and improved glucose tolerance157 appeared to be inconsistent with these lines of evidence, it was speculated that overexpression of hypothalamic GIP decreased energy intake, resulting in reduced adiposity.
GIP increases the activity of lipoprotein lipase (LPL), an enzyme bound to the cell surface of adipocytes that hydrolyzes lipoprotein‐associated triglycerides to produce free fatty acids available for local uptake. It was shown that activation of GIPR in 3T3‐L1 cells and rat epididymal fats results in enhanced resistin secretion through a pathway involving p38 mitogen‐activated protein kinases (MAPK) and Jun amino‐terminal kinase (JNK)158. GIP activates phosphoinositide 3‐kinase and Akt1 through secreted resistin, thereby suppressing adenosine monophosphate‐activated protein kinase (AMPK) and increasing LPL activity in adipocytes158. It has been also shown that GIP reduces expression and activity of 11β‐hydroxysteroid dehydrogenase type 1, and inhibits the release of free fatty acids in 3T3‐L1 cells and healthy humans160, providing another mechanism underlying GIP‐dependent fat accumulation in adipose tissues. Furthermore, it has been shown that GIP activates expression of GIPR through upregulation of peroxisome proliferator‐activated receptor (PPAR)‐γ161, suggesting feed‐forward action of GIP signaling in adipose tissues. In addition, a recent study has shown that GIP potentiates adipocyte insulin sensitivity of adipocytes through the activation of cAMP/PKA/cAMP response element binding protein signaling module and p110 phosphoinositol‐3 kinase, suggesting a feed‐forward interaction of GIP with insulin signaling. Furthermore, it has been shown that GIP also enhances glucocorticoid secretion GIPR‐dependently, thereby promoting adipocity162. It is of note that GIP might directly enhance secretion of inflammatory cytokines (e.g., IL‐6, TNF‐α and MCP‐1)163, which could worsen vascular complications.
GLP‐1 does not show any role in fat accumulation. Although GLP‐1R is expressed in adipocytes164, activation of GLP‐1R does not increase LPL activity in adipocytes158. Recently, it has been shown that GLP‐1 directly activates adipogenesis through activation of PKC, ERK and Akt1, which leads to altered proliferation, apoptosis and differentiation165. However, the physiological relevance of this finding is unknown and needs to be determined in future.
Effects on Food Intake and Gastric Emptying
It has been shown that GLP‐1RA reduces bodyweight likely by inhibiting food intake and slowing gastric emptying146. We summarize the effects of GLP‐1 and GIP on food intake and gastric emptying that might have effects on overweight.
GLP‐1 has been shown to be expressed in the arcuate nucleus and other hypothalamic regions involved in the regulation of food intake168. Subcutaneous and intracerebroventricular GLP‐1 injections were shown to significantly inhibit food intake and bodyweight in non‐diabetic rats, and this inhibitory effect on the food intake was blocked by GLP‐1R antagonist exendin(9‐39)169. Intraperitoneal injection of GLP‐1RA liraglutide and exendin‐4 also reduced food intake in non‐diabetic rats170. In addition, subcutaneous injections of liraglutide significantly reduced the food intake and bodyweight in diabetic ob/ob and db/db mice171, as well as in obese candy‐fed rats172, obese mini‐pigs173 and obese rats carrying hypothalamic lesions174. It was also shown that GLP‐1 or GLP‐1RA liraglutide infusion reduced food intake and reduced gastric emptying in healthy volunteers175, patients with type 1 diabetes176 and patients with type 2 diabetes177.
The doses of exendin‐4 required for significant reduction of food intake were higher in subdiaphragmatically vagotomized rats than in non‐vagotomized rats179. This result suggests that the reduction of the food intake noted after administration of exendin‐4 might be mediated by activation of the GLP‐1 expressed on the vagal afferents, as well as by direct activation of GLP‐1R in the central nervous system. In contrast, direct injection of exendin(9‐39) into the nucleus tractus solitarius in rats significantly increased the food intake180. Blockage of hindbrain GLP‐1R by exendin(9‐39) inhibited the suppression of the food intake induced by gastric distention, but did not affect the suppression of the food intake induced by intraduodenal nutrient infusion. These results suggest that nucleus tractus solitarius activation by GLP‐1 might also contribute to the control of food intake through the satiating effects of gastric distension. A recent study showed that GLP‐1R activation in the nucleus tractus solitarius neurons increased PKA activity concomitant with an increase in phosphorylation of p44/42‐MAPK and a decrease in phosphorylation of AMPK181. The combined increase in PKA and p44/42‐MAPK activity, together with decreased AMPK activity, is hypothesized to increase cAMP‐response element binding protein‐mediated nuclear transcription and protein synthesis, resulting in reduced food intake181. Furthermore, it has been reported recently that GLP‐1 neurons in the nucleus tractus solitaries also projects directly to the ventral tegmental area and nucleus accumbens, the site in which GLP‐1 is thought to regulate food intake182. Regarding gastric emptying, intracisternal GLP‐1 injection induced delayed gastric emptying, an effect that was partially antagonized by celiac ganglion‐ectomy, but not by atropine or NOS inhibitor, L‐NG‐nitroarginine methyl ester (L‐NAME)183. Corticotropin‐releasing hormone antagonist, astressin, also partially antagonized the GLP‐1‐induced delay of gastric emptying183. These observations show that central corticotropin releasing hormone and peripheral sympathetic pathways might mediate the delay of gastric emptying induced by centrally administered GLP‐1. It is clinically of interest that chronic infusion of GLP‐1 or the relatively long‐acting GLP‐1RA, liraglutide, but not the short acting GLP‐1RA, exenatide, loses its effects on gastric emptying rapidly as a result of tachyphylaxis in patients with diabetes and in rats184. Although GLP‐1 reduces the food intake and delays gastric emptying in experimental animals and humans, GIP does not have such effects in humans, but rather facilitates gastric emptying175. It is noteworthy that although DPP‐4i sitagliptin has no effects on gastric emptying, the GIP response negatively correlates with gastric emptying and the GLP‐1 response positively correlates with gastric emptying108. A recent report showed that exogenous GIP inhibits intestinal motility through a somatostatin‐mediated pathway rather than through a GLP‐1‐mediated pathway, thereby inhibiting glucose absorption187.
Effect on the Liver
Diabetes is closely associated with non‐alcoholic fatty liver disease (NAFLD), the prevalence of which is 10–30% depending on geographical region. NAFLD is caused by impaired hepatic lipid homeostasis that is usually maintained through a balance between the influx or production of fatty acids and their use for oxidation or secretion as very low‐density lipoprotein triglycerides188. Approximately 10–25% of individuals with NAFLD progress to non‐alcoholic steatohepatitis (NASH), a silent disease with few or no symptoms, and eventually develops liver cirrhosis and hepatocellular carcinoma. Several clinical trials showed that agents, such as pioglitazone, vitamin E, ursodeoxycholic acid and L‐carnitine, exert beneficial effects against NAFLD. However, reducing bodyweight by appropriate diet and exercise therapies is still considered superior to any pharmacological therapies today. It has been shown that GLP‐1R activation results in amelioration of hepatic lipid accumulation102. In contrast, GIP has been associated with fat accumulation in the liver of high‐fat fed animals, but GIPR expression in the liver is controversial149. We summarize the effects of GLP‐1 and GIP on the liver that might have potential clinical significance in the treatment of NASH and NAFLD.
Although GLP‐1R expression in the mouse liver or hepatocytes is controversial, exendin‐4 reversed high‐fat induced hepatic lipid accumulation and inflammation in hepatocytes and C57BL/6J mice191. Exendin‐4 also reversed hepatic steatosis in ob/ob mice102. In the same study, GLP‐1‐treated mouse hepatocytes resulted in a significant increase in cAMP production along with reduced expression of stearoyl‐CoA desaturase (SCD)‐1 and genes associated with fatty acid synthesis, and increased expression of genes involved in fatty acid oxidization102. It was also shown that incubation of rat hepatocytes with exendin‐4 increased expression of PPAR‐α along with their downstream genes, acyl‐coenzyme A oxidase and carnitine palmitoyltransferase 1A, thereby reducing intracellular fatty acids192. Similarly, it was shown that another GLP‐1RA liraglutide significantly ameliorates lipid accumulation by increasing expression of genes related to fatty acid transport and beta‐oxidation in mice on high‐fat and high‐fructose diets103. In addition to improvement of lipid metabolisms, exendin‐4 has been shown to improve hepatic insulin sensitivity. Administration of exendin‐4 increased expression of Sirt1 along with AMPK in livers of high‐fat fed C57BL/6J mice, as well as in cultured hepatic cell lines, HepG2 and Huh7 cells, suggesting involvement of GLP‐1 signaling not only in lipid metabolism, but also in glucose metabolism in the liver. Another study showed that incubation of rat hepatocytes with exendin‐4 increased expression of PPAR‐γ, which exerted its insulin‐sensitizing action by reducing JNK phosphorylation, and increased phosphorylation of Akt1 and AMPK192. Similarly, it was shown that exendin‐4 increased the phosphorylation of Akt1 and PKC‐ζ in HepG2 and Huh7 cells195. A recent study also showed that exendin‐4 activates glucokinase and increases hepatic glycogen contents in streptozotocin (STZ)‐treated C57BL/6J mice independently of insulin196. Collectively, GLP‐1RA is supposed to ameliorate insulin sensitivity and regulate glucose metabolism in the liver. Another effect of GLP‐1RA in the liver includes protection against hepatocellular injuries. It was shown that exendin‐4 exerts a protective role in the steatoic liver by inhibiting cell death through upregulation of genes associated with autophagy, thereby reducing endoplasmic reticulum (ER) stress‐related apoptosis in human hepatocytes treated with fatty acids, as well as in mice fed a high‐fat diet193. Furthermore, exendin‐4 administration in STZ‐treated diabetic mice showed that exendin‐4 suppresses hepatocyte injury by decreasing proliferation of hepatocytes197, which might have implications in treatment of NAFLD. Indeed, it was shown that exenatide treatment ameliorates hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years198. Whether the liver or hepatocytes in humans express GLP‐1R needs to be addressed cautiously, as it is possible that effects of GLP‐1 in the liver are partly mediated through the nervous system, as evidenced in mice.
In contrast to GLP‐1, GIP was associated with fat accumulation in the livers of high‐fat fed animals, but GIPR expression in the liver is still controversial. It was shown that GIP anta‐gonist (Pro3)GIP not only reduces bodyweight, but also ameliorates fat accumulation in the livers of high‐fat fed mice152 and ob/ob mice153. These findings were further established by recent a investigation in high‐fat fed mice showing that active immunization against (Pro3)GIP resulted in GIP antibody production and significant reduction of liver triglyceride along with reduction in blood glucose levels194. These lines of evidence suggest that GIP antagonism might be beneficial as treatment for NASH and NAFLD. However, this was challenged by a recent report that GIP‐overexpressing mice resulted in reduced diet‐induced obesity and amelioration of liver steatosis, whereas they showed enhanced β‐cell function and improved glucose tolerance157. Although this finding by McIntosh et al.157 was unexpected from the results on GIPR‐deficient mice that also showed reduced diet‐induced obesity, they speculated that overexpression of hypothalamic GIP decreased energy intake, resulting in reduced adiposity157. Further studies are required to develop GIP‐related therapies for obesity and NAFLD.
Effects of DPP‐4 inhibition have been investigated in mice and humans. DPP‐4 inhibition in diet‐induced diabetic glucokinase+/− mice suppressed hepatic inflammation and reduced expression of sterol‐regulatory binding protein (SREBP)‐1c, SCD‐1 and fatty acid synthase (FAS), and upregulation of PPAR‐α, thereby ameliorating hepatic steatosis199. Similarly, DPP‐4 inhibition improves insulin sensitivity and hepatic steatosis in diet‐induced obese C57BL/6 mice with reduced hepatic expression of SREBP‐1c, SCD‐1 and FAS200. Furthermore, it was shown that DPP‐4i sitagliptin ameliorates hepatic biomarkers in type 2 diabetic patients with NAFLD treated for 4 months201. In addition, sitagliptin was shown to ameliorate liver enzymes and hepatocyte ballooning in type 2 diabetic patients with NASH202. Taken together, these results suggest clinical implications of DPP‐4i in treatment for NASH and NAFLD.
Effect on the Brain
Dementia has been postulated as one of the diabetic comorbidities203. GIPR and GLP‐1R have been found to be expressed in the hippocampus, which is the brain region most closely involved in memory formation169. GIPR‐deficient mice are impaired in learning, synaptic plasticity and hippocampal neurogenesis84, whereas GIP and GIPR agonist, N‐AcGIP, has been shown to strongly enhance hippocampal long‐term potentiation (LTP)85. GLP‐1R‐deficient mice were also found to be impaired in a memory‐related behavioral task, and hippocampal LTP was severely impaired205, whereas exendin‐4 administration improved cognitive function in adult mice206, and enhanced neurogenesis in the hippocampus of diabetic and non‐diabetic mice207.
Injection of β‐amyloid (Aβ), which is well‐known to accumulate in the brain of Alzheimer's disease (AD) patients, impaired LTP; N‐AcGIP fully reversed the impairment of LTP induced by the injection of Aβ85. GLP‐1 and GLP‐1RA also exert a protective role in a transgenic mouse model of AD208. GLP‐1 protected against the cellular apoptosis induced by Aβ210. GLP‐1 has the ability to reduce the levels of Aβ in the brain in vivo, and also reduces the levels of the amyloid precursor protein in cultured neuronal cells211. Furthermore, GLP‐1 and exendin‐4 protected cultured hippocampal neurons against apoptosis induced by Aβ and oxidative insults211. Furthermore, GLP‐1R activation allows physiological tyrosine phosphorylation of IRS (insulin receptor substrate)‐1 and stimulation of downstream insulin signaling by inhibition of Aβ oligomer‐activated JNK212. A recent study has shown that exendin‐4 restored dopamine and noradrenaline contents, and reversed neurological dysfunction in a mouse model of Parkinson's disease213. The neuroprotective effects of exendin‐4 on hyperglycemia‐ and LPS‐induced cognitive dysfunction also were shown214. Furthermore, GLP‐1RA (Val8)GLP‐1 prevents tau hyperphosphorylation, impairment of spatial learning and ultrastructural cellular damage in STZ‐treated rat brains215.
These findings suggest that GIP and GLP‐1 might also play important roles in the control of synaptic plasticity and memory formation, and that GIP and GLP‐1 analogs might well inhibit impairment of memory formation in patients with AD, as well as other cognitive deficits in neurodegenerative disorders.
Effect on the Bone
Bone fracture, which is directly related to decreased mobility and compromised quality of life (QOL), is associated with diabetes216. It has been reported that GIP promotes bone formation, whereas GLP‐1 inhibits bone resorption (Figure 6)217. Thus, both GIP and GLP‐1 could play important roles in the reduction of the bone fracture risk in diabetic patients.
Figure 6.
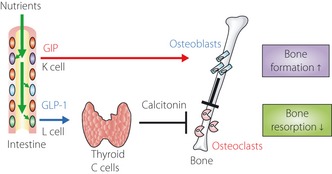
Regulation of bone metabolism by glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1). The effects of GIP and GLP‐1 on bone metabolism. GIP binds to GIP receptors expressed on osteoblasts, thereby activating new bone formation. GIP also acts on osteoclasts, presumably through osteoblasts, to suppress bone resorption. In contrast, GLP‐1 stimulates calcitonin secretion from the thyroid gland, which then suppresses bone resorption by osteoclasts. Notably, it has been reported that GLP‐1 receptor expression in humans is much lower than in rodents240. Reproduced from Yabe and Seino217.
GIPR has been shown to be present in osteoclasts. GIP inhibits the osteoclast resorptive activity in organ culture systems and the resorptive activity of mature osteoclasts219. The bone formation parameters in GIPR‐deficient mice were significantly lower than those in wild‐type mice, and the number of osteoclasts was significantly increased in GIPR‐deficient mice, indicative of high‐turnover osteoporosis220. In addition, GIP suppressed the apoptosis of osteoblasts in vitro, suggesting that GIP stimulates bone formation through inhibition of osteoblast apoptosis221. Furthermore, GIP transgenic mice showed a significant increase in the markers of bone formation and a decrease in the markers of bone resorption, and also a significant increase of the bone mass221. From these findings in GIPR‐deficient mice and GIP‐transgenic mice, it is suggested that GIP inhibits bone resorption and stimulates bone formation, and that excess signaling through the GIPR results in the gain of bone mass. In addition, the plasma calcium concentration after meal ingestion in GIPR‐deficient mice was increased, and GIP might play an important role in the stimulation of calcium deposition in the bone223.
GLP‐1R‐decifient mice showed cortical osteopenia, bone fragility and an increase in the markers of bone resorption and osteoclast numbers224. GLP‐1R‐decifient mice also showed reduced calcitonin gene expression in the thyroid, whereas exendin‐4 increased calcitonin expression. GLP‐1 did not show any direct effect on the osteoblasts or osteoclasts, although it appears to have an essential role in endogenous GLP‐1R signaling in bone resorption through activation of the calcitonin‐dependent pathway, which in turn, plays an important role in bone formation224. In the analysis of bone structure in STZ‐induced diabetic rats and fructose‐induced insulin resistance rats, GLP‐1 significantly reduced the trabecular separation225, whereas exendin‐4 induced a significant decrease in trabecular separation, and increase in trabecular thickness and trabecular spacing. It also has been also shown that GLP‐1 and exendin‐4 reversed hyprelipidic‐related osteopenia226. Exendin‐4 normalized the LDL‐receptor‐related protein 5 (an activator of the windless type [Wnt] pathway)/Dickkopf‐related protein 1 (a blocker of LDL‐receptor‐related protein 5) ratio in diabetic rats and insulin resistant rats227. This finding suggests that exendin‐4 might induce the bone formation in diabetic and insulin resistant rats through the Wnt signaling pathway227. Consistent with the effects of GIP and GLP‐1 on bone metabolism, DPP‐4i sitagliptin significantly improved vertebral volumetric bone mineral density and trabecular architecture in female mice228.
Although these lines of evidence suggest an association of GIP and GLP‐1 with bone metabolism in humans, the effects of GIP and GLP‐1 on human bone turnover are largely unknown. A meta‐analysis of 28 clinical trials of treatment with DPP‐4i (i.e., vildagliptin, sitagliptin, saxagliptin, alogliptin, linagliptin and dutogliptin for at least 24 weeks) in patients with type 2 diabetes showed reduced the risk of bone fractures in patients treated with DPP‐4i229. However, 44‐week exenatide treatment did not affect the bone mineral density or serum markers of bone homeostasis (i.e., serum alkaline phosphatase, calcium and phosphate), as compared with insulin glargine, in patients with type 2 diabetes230. As mentioned, GLP‐1 action on the bone is presumably mediated through calcitonin. A series of clinical trials on liraglutide showed few changes in serum calcitonin levels in patients with type 2 diabetes, suggesting that GLP‐1 might not play a role in human bone metabolism. Regarding GIP, it has been previously reported that postprandial reduction of bone resorption was not mediated by GIP, but by GLP‐2, another intestinal hormone secreted simultaneously with GLP‐1231. However, they investigated the effects of subcutaneous single injections of native GIP that should be rapidly inactivated by DPP‐4 before it reached the bones. Thus, further investigations are definitely required to understand GIP and GLP‐1 actions on bone metabolism in humans.
Effects on Thyroid and Tumorigenesis
Studies in experimental animals, including toxicology studies, did not show that incretins had an effect on tumorigenesis. However, it has been recently reported that GLP‐1RA liraglutide and exenatide were associated with benign and malignant thyroid tumors in rats and mice232. GLP‐1R was found to be expressed in normal rat thyroid, as well as medullary thyroid carcinoma cells233. GLP‐1 and GLP‐1RA stimulate expression and secretion of calcitonin, a clinical biomarker for C cell diseases, such as medullary thyroid carcinoma234, in a dose‐dependent manner along with production of cAMP in rodent C cell lines232 and rodents233. However, it was reported that GLP‐1R expression was low in a human C cell line, and that liraglutide enhances little calcitonin secretion or C cell proliferation in primates232. Furthermore, clinical trials of GLP‐1RA, such as liraglutide, did not report elevation of calcitonin237. Thus, although the preclinical studies in rodents show a link between GLP‐1 and thyroid tumors, the relevance of these findings for humans is still unclear. In addition to the marked differences in the way that rodent and human C cells respond to GLP‐1, spontaneous C cell tumors are frequently seen in rats, whereas medullary thyroid cancer in humans is rare. Nevertheless, a recent study indicates expression of GLP‐1R in neoplastic and hyperplastic lesions of thyroid C cells239. Thus, long‐term observational studies are required to monitor the effects of sustained GLP‐1R signaling over the long term on the human thyroid.
Conclusion
Biological processes regulated by the incretin hormones, GIP and GLP‐1, are much broader than previously expected. Their insulinotropic actions are applied in the development of incretin‐based therapies, DPP‐4i and GLP‐1RA that exert glucose lowering effects, thereby suppressing diabetes‐related complications in patients with type 2 diabetes. In addition, it is conceivable that extrapancreatic function of GIP and GLP‐1 might be exploited to prevent onset and progression of diabetes‐related complications independently of glycemic control. A series of experimental results obtained so far suggest that diabetes‐related microvascular complications (e.g., retinopathy, nephropathy and neuropathy) and macrovascular complications (e.g., CAD, CVD and PAD) are directly affected by incretin‐based therapies. Furthermore, diabetes‐related comorbidities, such as cognitive dysfunction, obesity, fatty liver and bone fracture, might be also ameliorated by incretin‐based therapies. Clinical trials with adequately powered, prospective, controlled relevant end‐points will clarify, in future, the effects of incretin‐based drugs on diabetes‐related complications.
Acknowledgements
The authors thank current and former colleagues in the laboratory of Yutaka Seino, and apologize for citing only part of the relevant work in this field due to limited space, and are indebted to many authors for their contributions. YS reports receiving consulting and/or speaker fees from Eli Lilly, MSD, Novartis, Novo Nordisk, Sanofi‐Aventis and Takeda. DY reports receiving speaker fees from Eli Lilly, MSD, Sanofi‐Aventis, Novo Nordisk and Takeda.
(J Diabetes Invest, doi: 10.1111/jdi.12065, 2013)
References
- 1.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 1902; 28: 325–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore B. On the treatment of Diabetus mellitus by acid extract of Duodenal Mucous Membrane. Biochem J 1906; 1: 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zunz E, La Barre J. Contributiona a l'etude des variations physiologiques de la secretion interne du pancreas. relations entre les secretions externe et interne du pancreas. Arch Int Physiol Biochim 1929; 31: 20–44 [Google Scholar]
- 4.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Invest 2010; 1: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165 [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87: 1409–1439 [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon‐like peptide‐1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(Suppl 3): S190–S196 [DOI] [PubMed] [Google Scholar]
- 8.Hansotia T, Baggio LL, Delmeire D, et al Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP‐IV inhibitors. Diabetes 2004; 53: 1326–1335 [DOI] [PubMed] [Google Scholar]
- 9.Miyawaki K, Yamada Y, Yano H, et al Glucose intolerance caused by a defect in the entero‐insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 1999; 96: 14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scrocchi LA, Brown TJ, MaClusky N, et al Glucose intolerance but normal satiety in mice with a null mutation in the glucagon‐like peptide 1 receptor gene. Nat Med 1996; 2: 1254–1258 [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA. Incretin‐based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011; 124: S3–S18 [DOI] [PubMed] [Google Scholar]
- 12.Lovshin JA, Drucker DJ. Incretin‐based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009; 5: 262–269 [DOI] [PubMed] [Google Scholar]
- 13.Dicker D. DPP‐4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 2011; 34(Suppl 2): S276–S278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deacon CF. Dipeptidyl peptidase‐4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 2011; 13: 7–18 [DOI] [PubMed] [Google Scholar]
- 15.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta‐analysis. JAMA 2007; 298: 194–206 [DOI] [PubMed] [Google Scholar]
- 16.Monami M, Iacomelli I, Marchionni N, et al Dipeptydil peptidase‐4 inhibitors in type 2 diabetes: a meta‐analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2010; 20: 224–235 [DOI] [PubMed] [Google Scholar]
- 17.Monami M, Marchionni N, Mannucci E. Glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a meta‐analysis of randomized clinical trials. Eur J Endocrinol 2009; 160: 909–917 [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA, Vardarli I. Comparative evaluation of incretin‐based antidiabetic medications and alternative therapies to be added to metformin in the case of monotherapy failure. J Diabetes Invest 2010; 1: 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66S: S37–S44 [DOI] [PubMed] [Google Scholar]
- 20.Park H, Park C, Kim Y, et al Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: meta‐analysis. Ann Pharmacother 2012; 46: 1453–1469 [DOI] [PubMed] [Google Scholar]
- 21.Kim YG, Hahn S, Oh TJ, et al Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and Non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013; doi: 10.1007/s00125‐012‐2827‐3 [DOI] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352: 837–853 [PubMed] [Google Scholar]
- 23.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993;329: 977–986 [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Cleary PA, Backlund JY, et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 26.Kitada M, Zhang Z, Mima A, et al Molecular mechanisms of diabetic vascular complications. J Diabetes Invest 2010; 1: 77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagashima M, Watanabe T, Terasaki M, et al Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011; 54: 2649–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogi Y, Nagashima M, Terasaki M, et al Glucose‐dependent insulinotropic polypeptide prevents the progression of macrophage‐driven atherosclerosis in diabetic apolipoprotein E‐null mice. PLoS ONE 2012; 7: e35683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arakawa M, Mita T, Azuma K, et al Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon‐like peptide‐1 receptor agonist, exendin‐4. Diabetes 2010; 59: 1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panjwani N, Mulvihill EE, Longuet C, et al GLP‐1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE‐/‐ mice. Endocrinology 2013; 154: 127–139 [DOI] [PubMed] [Google Scholar]
- 31.Gaspari T, Liu H, Welungoda I, et al A GLP‐1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE‐/‐ mouse model. Diab Vasc Dis Res 2011; 8: 117–124 [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Dear AE, Knudsen LB, et al A long‐acting glucagon‐like peptide‐1 analogue attenuates induction of plasminogen activator inhibitor type‐1 and vascular adhesion molecules. J Endocrinol 2009; 201: 59–66 [DOI] [PubMed] [Google Scholar]
- 33.Wu JD, Xu XH, Zhu J, et al Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther 2011; 13: 143–148 [DOI] [PubMed] [Google Scholar]
- 34.Ha SJ, Kim W, Woo JS, et al Preventive effects of exenatide on endothelial dysfunction induced by ischemia‐reperfusion injury via KATP channels. Arterioscler Thromb Vasc Biol 2012; 32: 474–480 [DOI] [PubMed] [Google Scholar]
- 35.Koska J, Schwartz EA, Mullin MP, et al Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent‐onset type 2 diabetes. Diabetes Care 2010; 33: 1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiraishi D, Fujiwara Y, Komohara Y, et al Glucagon‐like peptide‐1 (GLP‐1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun 2012; 425: 304–308 [DOI] [PubMed] [Google Scholar]
- 37.Ding L, Zhang J. Glucagon‐like peptide‐1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 2012; 33: 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai W, Dong Z, Wang N, et al Glucagon‐like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide‐dependent mechanism. Diabetes 2012; 61: 888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu FQ, Zhang XL, Gong L, et al Glucagon‐like peptide 1 protects microvascular endothelial cells by inactivating the PARP‐1/iNOS/NO pathway. Mol Cell Endocrinol 2011; 339: 25–33 [DOI] [PubMed] [Google Scholar]
- 40.Xiao‐Yun X, Zhao‐Hui M, Ke C, et al Glucagon‐like peptide‐1 improves proliferation and differentiation of endothelial progenitor cells via upregulating VEGF generation. Med Sci Monit 2011;17: BR35–BR41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdogdu O, Nathanson D, Sjoholm A, et al Exendin‐4 stimulates proliferation of human coronary artery endothelial cells through eNOS‐, PKA‐ and PI3K/Akt‐dependent pathways and requires GLP‐1 receptor. Mol Cell Endocrinol 2010; 325: 26–35 [DOI] [PubMed] [Google Scholar]
- 42.Han L, Yu Y, Sun X, et al Exendin‐4 directly improves endothelial dysfunction in isolated aortas from obese rats through the cAMP or AMPK‐eNOS pathways. Diabetes Res Clin Pract 2012; 97: 453–460 [DOI] [PubMed] [Google Scholar]
- 43.Shiraki A, Oyama J, Komoda H, et al The glucagon‐like peptide 1 analog liraglutide reduces TNF‐alpha‐induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012; 221: 375–382 [DOI] [PubMed] [Google Scholar]
- 44.Zhan Y, Sun HL, Chen H, et al Glucagon‐like peptide‐1 (GLP‐1) protects vascular endothelial cells against advanced glycation end products (AGEs)‐induced apoptosis. Med Sci Monit 2012; 18: BR286–BR291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojima A, Matsui T, Maeda S, et al Glucose‐dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm Metab Res 2012; 44: 501–505 [DOI] [PubMed] [Google Scholar]
- 46.Matsubara J, Sugiyama S, Sugamura K, et al A dipeptidyl peptidase‐4 inhibitor, des‐fluoro‐sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E‐deficient mice. J Am Coll Cardiol 2012; 59: 265–276 [DOI] [PubMed] [Google Scholar]
- 47.Shah Z, Kampfrath T, Deiuliis JA, et al Long‐term dipeptidyl‐peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011; 124: 2338–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ta NN, Schuyler CA, Li Y, et al DPP‐4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E‐deficient mice. J Cardiovasc Pharmacol 2011; 58: 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terasaki M, Nagashima M, Watanabe T, et al Effects of PKF275‐055, a dipeptidyl peptidase‐4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E‐null mice. Metabolism 2012; 61: 974–977 [DOI] [PubMed] [Google Scholar]
- 50.Maeda S, Matsui T, Yamagishi S. Vildagliptin inhibits oxidative stress and vascular damage in streptozotocin‐induced diabetic rats. Int J Cardiol 2012; 158: 171–173 [DOI] [PubMed] [Google Scholar]
- 51.Fadini GP, Boscaro E, Albiero M, et al The oral dipeptidyl peptidase‐4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal‐derived factor‐1alpha. Diabetes Care 2010; 33: 1607–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makdissi A, Ghanim H, Vora M, et al Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 2012; 97: 3333–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satoh‐Asahara N, Sasaki Y, Wada H, et al A dipeptidyl peptidase‐4 inhibitor, sitagliptin, exerts anti‐inflammatory effects in type 2 diabetic patients. Metabolism 2012; 62: 347–351 [DOI] [PubMed] [Google Scholar]
- 54.Timmers L, Henriques JP, de Kleijn DP, et al Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 2009; 53: 501–510 [DOI] [PubMed] [Google Scholar]
- 55.Noyan‐Ashraf MH, Momen MA, Ban K, et al GLP‐1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009; 58: 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsubara M, Kanemoto S, Leshnower BG, et al Single dose GLP‐1‐Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res 2011; 165: 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dokken BB, La Bonte LR, Davis‐Gorman G, et al Glucagon‐like peptide‐1 (GLP‐1), immediately prior to reperfusion, decreases neutrophil activation and reduces myocardial infarct size in rodents. Horm Metab Res 2011; 43: 300–305 [DOI] [PubMed] [Google Scholar]
- 58.Moberly SP, Berwick ZC, Kohr M, et al Intracoronary glucagon‐like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Exp Biol Med (Maywood) 2012; 237: 334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao W, Aravindhan K, Alsaid H, et al Albiglutide, a long lasting glucagon‐like peptide‐1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS ONE 2011; 6: e23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ban K, Kim KH, Cho CK, et al Glucagon‐like peptide (GLP)‐1(9‐36)amide‐mediated cytoprotection is blocked by exendin(9‐39) yet does not require the known GLP‐1 receptor. Endocrinology 2010; 151: 1520–1531 [DOI] [PubMed] [Google Scholar]
- 61.Vila Petroff MG, Egan JM, Wang X, et al Glucagon‐like peptide‐1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res 2001; 89: 445–452 [DOI] [PubMed] [Google Scholar]
- 62.Ban K, Noyan‐Ashraf MH, Hoefer J, et al Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation 2008; 117: 2340–2350 [DOI] [PubMed] [Google Scholar]
- 63.Nikolaidis LA, Mankad S, Sokos GG, et al Effects of glucagon‐like peptide‐1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109: 962–965 [DOI] [PubMed] [Google Scholar]
- 64.Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon‐like peptide‐1 in patients with coronary artery disease. Heart 2011; 98: 408–413 [DOI] [PubMed] [Google Scholar]
- 65.Lonborg J, Kelbaek H, Vejlstrup N, et al Exenatide reduces final infarct size in patients with ST‐segment‐elevation myocardial infarction and short‐duration of ischemia. Circ Cardiovasc Interv 2012; 5: 288–295 [DOI] [PubMed] [Google Scholar]
- 66.Lonborg J, Vejlstrup N, Kelbaek H, et al Exenatide reduces reperfusion injury in patients with ST‐segment elevation myocardial infarction. Eur Heart J 2012; 33: 1491–1499 [DOI] [PubMed] [Google Scholar]
- 67.Huisamen B, Genis A, Marais E, et al Pre‐treatment with a DPP‐4 inhibitor is infarct sparing in hearts from obese, pre‐diabetic rats. Cardiovasc Drugs Ther 2011; 25: 13–20 [DOI] [PubMed] [Google Scholar]
- 68.Sauve M, Ban K, Momen MA, et al Genetic deletion or pharmacological inhibition of dipeptidyl peptidase‐4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010; 59: 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hocher B, Sharkovska Y, Mark M, et al The novel DPP‐4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol 2012; doi:10.1016/j.ijcard.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 70.Ye Y, Keyes KT, Zhang C, et al The myocardial infarct size‐limiting effect of sitagliptin is PKA‐dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol 2010; 298: H1454–H1465 [DOI] [PubMed] [Google Scholar]
- 71.Yin M, Sillje HH, Meissner M, et al Early and late effects of the DPP‐4 inhibitor vildagliptin in a rat model of post‐myocardial infarction heart failure. Cardiovasc Diabetol 2011; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lenski M, Kazakov A, Marx N, et al Effects of DPP‐4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol 2011; 51: 906–918 [DOI] [PubMed] [Google Scholar]
- 73.Huber BC, Brunner S, Segeth A, et al Parathyroid hormone is a DPP‐IV inhibitor and increases SDF‐1‐driven homing of CXCR4(+) stem cells into the ischaemic heart. Cardiovasc Res 2011; 90: 529–537 [DOI] [PubMed] [Google Scholar]
- 74.Connelly K, Zhang Y, Advani A, et al DPP‐4 inhibition attenuates cardiac dysfunction and adverse remodelling following myocardial infarction in rats with experimental diabetes. Cardiovasc Ther 2012; doi: 10.1111/1755‐5922.12005 [DOI] [PubMed] [Google Scholar]
- 75.Nikolaidis LA, Elahi D, Hentosz T, et al Recombinant glucagon‐like peptide‐1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing‐induced dilated cardiomyopathy. Circulation 2004; 110: 955–961 [DOI] [PubMed] [Google Scholar]
- 76.Poornima I, Brown SB, Bhashyam S, et al Chronic glucagon‐like peptide‐1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure‐prone rat. Circ Heart Fail 2008; 1: 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Q, Anderson C, Broyde A, et al Glucagon‐like peptide‐1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol 2010; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sokos GG, Nikolaidis LA, Mankad S, et al Glucagon‐like peptide‐1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006; 12: 694–699 [DOI] [PubMed] [Google Scholar]
- 79.Nathanson D, Ullman B, Lofstrom U, et al Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double‐blind, randomised controlled clinical trial of efficacy and safety. Diabetologia 2012; 55: 926–935 [DOI] [PubMed] [Google Scholar]
- 80.Gomez N, Touihri K, Matheeussen V, et al Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing‐induced heart failure. Eur J Heart Fail 2012; 14: 14–21 [DOI] [PubMed] [Google Scholar]
- 81.Teramoto S, Miyamoto N, Yatomi K, et al Exendin‐4, a glucagon‐like peptide‐1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 2011; 31: 1696–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee CH, Yan B, Yoo KY, et al Ischemia‐induced changes in glucagon‐like peptide‐1 receptor and neuroprotective effect of its agonist, exendin‐4, in experimental transient cerebral ischemia. J Neurosci Res 2011; 89: 1103–1113 [DOI] [PubMed] [Google Scholar]
- 83.Darsalia V, Mansouri S, Ortsater H, et al Glucagon‐like peptide‐1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond) 2012; 122: 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faivre E, Gault VA, Thorens B, et al Glucose‐dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol 2011; 105: 1574–1580 [DOI] [PubMed] [Google Scholar]
- 85.Gault VA, Holscher C. Protease‐resistant glucose‐dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta‐amyloid. J Neurophysiol 2008; 99: 1590–1595 [DOI] [PubMed] [Google Scholar]
- 86.Faivre E, Hamilton A, Holscher C. Effects of acute and chronic administration of GIP analogues on cognition, synaptic plasticity and neurogenesis in mice. Eur J Pharmacol 2012; 674: 294–306 [DOI] [PubMed] [Google Scholar]
- 87.Darsalia V, Ortsater H, Olverling A, et al The DPP‐4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes 2012; doi: 10.2337/db12‐0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu M, Moreno C, Hoagland KM, et al Antihypertensive effect of glucagon‐like peptide 1 in Dahl salt‐sensitive rats. J Hypertens 2003; 21: 1125–1135 [DOI] [PubMed] [Google Scholar]
- 89.Hirata K, Kume S, Araki S, et al Exendin‐4 has an anti‐hypertensive effect in salt‐sensitive mice model. Biochem Biophys Res Commun 2009; 380: 44–49 [DOI] [PubMed] [Google Scholar]
- 90.Liu Q, Adams L, Broyde A, et al The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt‐sensitive rats. Cardiovasc Diabetol 2010; 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pacheco BP, Crajoinas RO, Couto GK, et al Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens 2011; 29: 520–528 [DOI] [PubMed] [Google Scholar]
- 92.Liu L, Liu J, Wong WT, et al Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon‐like peptide 1‐dependent mechanism. Hypertension 2012; 60: 833–841 [DOI] [PubMed] [Google Scholar]
- 93.McGill JB. Liraglutide: effects beyond glycaemic control in diabetes treatment. Int J Clin Pract Suppl 2010; 167: 28–34 [DOI] [PubMed] [Google Scholar]
- 94.Irace C, De Luca S, Shehaj E, et al Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab Vasc Dis Res 2013; 10: 72–77 [DOI] [PubMed] [Google Scholar]
- 95.Tang‐Christensen M, Larsen PJ, Goke R, et al Central administration of GLP‐1‐(7–36) amide inhibits food and water intake in rats. Am J Physiol 1996; 271: R848–R856 [DOI] [PubMed] [Google Scholar]
- 96.Schafer SA, Tschritter O, Machicao F, et al Impaired glucagon‐like peptide‐1‐induced insulin secretion in carriers of transcription factor 7‐like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007; 50: 2443–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gutzwiller JP, Tschopp S, Bock A, et al Glucagon‐like peptide 1 induces natriuresis in healthy subjects and in insulin‐resistant obese men. J Clin Endocrinol Metab 2004; 89: 3055–3061 [DOI] [PubMed] [Google Scholar]
- 98.Kubota A, Maeda H, Kanamori A, et al Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res 2012; 4: 309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogawa S, Ishiki M, Nako K, et al Sitagliptin, a dipeptidyl peptidase‐4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med 2011; 223: 133–135 [DOI] [PubMed] [Google Scholar]
- 100.Qin X, Shen H, Liu M, et al GLP‐1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 2005; 288: G943–G949 [DOI] [PubMed] [Google Scholar]
- 101.Hsieh J, Longuet C, Baker CL, et al The glucagon‐like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010; 53: 552–561 [DOI] [PubMed] [Google Scholar]
- 102.Ding X, Saxena NK, Lin S, et al Exendin‐4, a glucagon‐like protein‐1 (GLP‐1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006; 43: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mells JE, Fu PP, Sharma S, et al Glp‐1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol 2012; 302: G225–G235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meier JJ, Gethmann A, Gotze O, et al Glucagon‐like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non‐esterified fatty acids in humans. Diabetologia 2006; 49: 452–458 [DOI] [PubMed] [Google Scholar]
- 105.Schwartz EA, Koska J, Mullin MP, et al Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 2010; 212: 217–222 [DOI] [PubMed] [Google Scholar]
- 106.Matikainen N, Manttari S, Schweizer A, et al Vildagliptin therapy reduces postprandial intestinal triglyceride‐rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 2006; 49: 2049–2057 [DOI] [PubMed] [Google Scholar]
- 107.Tremblay AJ, Lamarche B, Deacon CF, et al Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 366–373 [DOI] [PubMed] [Google Scholar]
- 108.Stevens JE, Horowitz M, Deacon CF, et al The effects of sitagliptin on gastric emptying in healthy humans – a randomised, controlled study. Aliment Pharmacol Ther 2012; 36: 379–390 [DOI] [PubMed] [Google Scholar]
- 109.Monami M, Lamanna C, Desideri CM, et al DPP‐4 inhibitors and lipids: systematic review and meta‐analysis. Adv Ther 2012; 29: 14–25 [DOI] [PubMed] [Google Scholar]
- 110.Freeman R. The Nervous System and Diabetes In: Kahn CR, Weir GC, King GL, et al (eds). Joslin's Diabetes Mellitus. 14th edn Lippincott Williams and Wilkins, Boston, 2005: 951–974 [Google Scholar]
- 111.Himeno T, Kamiya H, Naruse K, et al Beneficial effects of exendin‐4 on experimental polyneuropathy in diabetic mice. Diabetes 2011; 60: 2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu WJ, Jin HY, Lee KA, et al Neuroprotective effect of the glucagon‐like peptide‐1 receptor agonist, synthetic exendin‐4, in streptozotocin‐induced diabetic rats. Br J Pharmacol 2011; 164: 1410–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jolivalt CG, Fineman M, Deacon CF, et al GLP‐1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab 2011; 13: 990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perry T, Holloway HW, Weerasuriya A, et al Evidence of GLP‐1‐mediated neuroprotection in an animal model of pyridoxine‐induced peripheral sensory neuropathy. Exp Neurol 2007; 203: 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buhren BA, Gasis M, Thorens B, et al Glucose‐dependent insulinotropic polypeptide (GIP) and its receptor (GIPR): cellular localization, lesion‐affected expression, and impaired regenerative axonal growth. J Neurosci Res 2009; 87: 1858–1870 [DOI] [PubMed] [Google Scholar]
- 116.Bianchi R, Cervellini I, Porretta‐Serapiglia C, et al Beneficial effects of PKF275‐055, a novel, selective, orally bioavailable, long‐acting dipeptidyl peptidase IV inhibitor in streptozotocin‐induced diabetic peripheral neuropathy. J Pharmacol Exp Ther 2012; 340: 64–72 [DOI] [PubMed] [Google Scholar]
- 117.Jin HY, Liu WJ, Park JH, et al Effect of dipeptidyl peptidase‐IV (DPP‐IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin‐induced diabetic rats. Arch Med Res 2009; 40: 536–544 [DOI] [PubMed] [Google Scholar]
- 118.Koya D, Araki S, Haneda M. Therapeutic management of diabetic kidney disease. J Diabetes Invest 2011; 2: 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Buren PN, Toto R. Current update in the management of diabetic nephropathy. Curr Diabetes Rev 2013; 9: 62–77 [PubMed] [Google Scholar]
- 120.Kodera R, Shikata K, Kataoka HU, et al Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965–978 [DOI] [PubMed] [Google Scholar]
- 121.Park CW, Kim HW, Ko SH, et al Long‐term treatment of glucagon‐like peptide‐1 analog exendin‐4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 2007; 18: 1227–1238 [DOI] [PubMed] [Google Scholar]
- 122.Hendarto H, Inoguchi T, Maeda Y, et al GLP‐1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin‐induced diabetic rats via protein kinase A‐mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012; 61: 1422–1434 [DOI] [PubMed] [Google Scholar]
- 123.Ishibashi Y, Nishino Y, Matsui T, et al Glucagon‐like peptide‐1 suppresses advanced glycation end product‐induced monocyte chemoattractant protein‐1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 2011; 60: 1271–1277 [DOI] [PubMed] [Google Scholar]
- 124.Ishibashi Y, Matsui T, Ojima A, et al Glucagon‐like peptide‐1 inhibits angiotensin II‐induced mesangial cell damage via protein kinase A. Microvasc Res 2013; 182: 132–141 [DOI] [PubMed] [Google Scholar]
- 125.Mima A, Hiraoka‐Yamomoto J, Li Q, et al Protective effects of GLP‐1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes 2012; 61: 2967–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu WJ, Xie SH, Liu YN, et al Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin‐induced diabetic rats. J Pharmacol Exp Ther 2012; 340: 248–255 [DOI] [PubMed] [Google Scholar]
- 127.Glorie LL, Verhulst A, Matheeussen V, et al DPP4 inhibition improves functional outcome after renal ischemia‐reperfusion injury. Am J Physiol Renal Physiol 2012; 303: F681–F688 [DOI] [PubMed] [Google Scholar]
- 128.Mega C, de Lemos ET, Vala H, et al Diabetic nephropathy amelioration by a low‐dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res 2011; 2011: 162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H, Zhang X, Hu C, et al Exenatide reduces urinary transforming growth factor‐beta(1) and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press Res 2012; 35: 483–488 [DOI] [PubMed] [Google Scholar]
- 130.Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J 2011; 58: 69–73 [DOI] [PubMed] [Google Scholar]
- 131.Fong DS, Aiello LP, Ferris FL III, et al Diabetic retinopathy. Diabetes Care 2004; 27: 2540–2553 [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y, Wang Q, Zhang J, et al Protection of exendin‐4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2009; 247: 699–706 [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y, Zhang J, Wang Q, et al Intravitreal injection of exendin‐4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci 2011; 52: 278–285 [DOI] [PubMed] [Google Scholar]
- 134.Fu Z, Kuang H‐Y, Hao M, et al Protection of exenatide for retinal ganglion cells with different glucose concentrations. Peptides 2012; 37: 25–31 [DOI] [PubMed] [Google Scholar]
- 135.Goncalves A, Leal E, Paiva A, et al Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood‐retinal barrier in a type 2 diabetes animal model. Diabetes Obes Metab 2012; 14: 454–463 [DOI] [PubMed] [Google Scholar]
- 136.Cho GJ, Ryu S, Kim YH, et al Upregulation of glucose‐dependent insulinotropic polypeptide and its receptor in the retina of streptozotocin‐induced diabetic rats. Curr Eye Res 2002; 25: 381–388 [DOI] [PubMed] [Google Scholar]
- 137.Mathis U, Schaeffel F. Glucagon‐related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol 2007; 245: 267–275 [DOI] [PubMed] [Google Scholar]
- 138.Holbrook TL, Barrett‐Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes 1989; 13: 723–729 [PubMed] [Google Scholar]
- 139.Knowler WC, Pettitt DJ, Savage PJ, et al Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981; 113: 144–156 [DOI] [PubMed] [Google Scholar]
- 140.Knowler WC, Barrett‐Connor E, Fowler SE, et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan XR, Li GW, Hu YH, et al Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20: 537–544 [DOI] [PubMed] [Google Scholar]
- 142.Wing RR, Koeske R, Epstein LH, et al Long‐term effects of modest weight loss in type II diabetic patients. Arch Intern Med 1987; 147: 1749–1753 [PubMed] [Google Scholar]
- 143.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation–mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol 2012; 32: 1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taube A, Schlich R, Sell H, et al Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol 2012; 302: H2148–H2165 [DOI] [PubMed] [Google Scholar]
- 145.Yamada Y, Miyawaki K, Tsukiyama K, et al Pancreatic and extrapancreatic effects of gastric inhibitory polypeptide. Diabetes 2006; 55: S88–S91 [Google Scholar]
- 146.Vilsboll T, Christensen M, Junker AE, et al Effects of glucagon‐like peptide‐1 receptor agonists on weight loss: systematic review and meta‐analyses of randomised controlled trials. BMJ 2012; 344: d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beck B, Max JP. Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regul Pept 1983; 7: 3–8 [DOI] [PubMed] [Google Scholar]
- 148.Yip RG, Boylan MO, Kieffer TJ, et al Functional GIP receptors are present on adipocytes. Endocrinology 1998; 139: 4004–4007 [DOI] [PubMed] [Google Scholar]
- 149.Miyawaki K, Yamada Y, Ban N, et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742 [DOI] [PubMed] [Google Scholar]
- 150.Naitoh R, Miyawaki K, Harada N, et al Inhibition of GIP signaling modulates adiponectin levels under high‐fat diet in mice. Biochem Biophys Res Commun 2008; 376: 21–25 [DOI] [PubMed] [Google Scholar]
- 151.Zhou H, Yamada Y, Tsukiyama K, et al Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun 2005; 335: 937–942 [DOI] [PubMed] [Google Scholar]
- 152.Gault VA, McClean PL, Cassidy RS, et al Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high‐fat and cafeteria diets. Diabetologia 2007; 50: 1752–1762 [DOI] [PubMed] [Google Scholar]
- 153.Irwin N, McClean PL, O'Harte FP, et al Early administration of the glucose‐dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia 2007; 50: 1532–1540 [DOI] [PubMed] [Google Scholar]
- 154.McClean PL, Irwin N, Cassidy RS, et al GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high‐fat diet. Am J Physiol Endocrinol Metab 2007; 293: E1746–E1755 [DOI] [PubMed] [Google Scholar]
- 155.Althage MC, Ford EL, Wang S, et al Targeted ablation of glucose‐dependent insulinotropic polypeptide‐producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 2008; 283: 18365–18376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ugleholdt R, Pedersen J, Bassi MR, et al Transgenic rescue of adipocyte glucose‐dependent insulinotropic polypeptide receptor expression restores high fat diet‐induced body weight gain. J Biol Chem 2011; 286: 44632–44645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim SJ, Nian C, Karunakaran S, et al GIP‐overexpressing mice demonstrate reduced diet‐induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE 2012; 7: e40156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim SJ, Nian C, McIntosh CH. Resistin is a key mediator of glucose‐dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J Biol Chem 2007; 282: 34139–34147 [DOI] [PubMed] [Google Scholar]
- 159.Kim SJ, Nian C, McIntosh CH. Activation of lipoprotein lipase by glucose‐dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP‐activated protein kinase cascade. J Biol Chem 2007; 282: 8557–8567 [DOI] [PubMed] [Google Scholar]
- 160.Gogebakan O, Andres J, Biedasek K, et al Glucose‐dependent insulinotropic polypeptide reduces fat‐specific expression and activity of 11beta‐hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes 2012; 61: 292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim SJ, Nian C, McIntosh CH. Adipocyte expression of the glucose‐dependent insulinotropic polypeptide receptor involves gene regulation by PPARgamma and histone acetylation. J Lipid Res 2011; 52: 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bates HE, Campbell JE, Ussher JR, et al Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr(−/−) mice. Diabetes 2012; 61: 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nie Y, Ma RC, Chan JC, et al Glucose‐dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose‐dependent insulinotropic peptide receptor‐overexpressing adipocytes. FASEB J 2012; 26: 2383–2393 [DOI] [PubMed] [Google Scholar]
- 164.Montrose‐Rafizadeh C, Yang H, Wang Y, et al Novel signal transduction and peptide specificity of glucagon‐like peptide receptor in 3T3‐L1 adipocytes. J Cell Physiol 1997; 172: 275–283 [DOI] [PubMed] [Google Scholar]
- 165.Challa TD, Beaton N, Arnold M, et al Regulation of adipocyte formation by GLP‐1/GLP‐1R signaling. J Biol Chem 2012; 287: 6421–6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Halford JC, Boyland EJ, Blundell JE, et al Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol 2010; 6: 255–269 [DOI] [PubMed] [Google Scholar]
- 167.Hellstrom PM. Glucagon‐like peptide‐1 gastrointestinal regulatory role in metabolism and motility. Vitam Horm 2010; 84: 319–329 [DOI] [PubMed] [Google Scholar]
- 168.Goke R, Larsen PJ, Mikkelsen JD, et al Distribution of GLP‐1 binding sites in the rat brain: evidence that exendin‐4 is a ligand of brain GLP‐1 binding sites. Eur J Neurosci 1995; 7: 2294–2300 [DOI] [PubMed] [Google Scholar]
- 169.Turton MD, O'Shea D, Gunn I, et al A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 1996; 379: 69–72 [DOI] [PubMed] [Google Scholar]
- 170.McKay NJ, Kanoski SE, Hayes MR, et al Glucagon‐like peptide‐1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol 2011; 301: R1755–R1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rolin B, Larsen MO, Gotfredsen CF, et al The long‐acting GLP‐1 derivative NN2211 ameliorates glycemia and increases beta‐cell mass in diabetic mice. Am J Physiol Endocrinol Metab 2002; 283: E745–E752 [DOI] [PubMed] [Google Scholar]
- 172.Raun K, von Voss P, Gotfredsen CF, et al Liraglutide, a long‐acting glucagon‐like peptide‐1 analog, reduces body weight and food intake in obese candy‐fed rats, whereas a dipeptidyl peptidase‐IV inhibitor, vildagliptin, does not. Diabetes 2007; 56: 8–15 [DOI] [PubMed] [Google Scholar]
- 173.Raun K, von Voss P, Knudsen LB. Liraglutide, a once‐daily human glucagon‐like peptide‐1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 2007; 15: 1710–1716 [DOI] [PubMed] [Google Scholar]
- 174.Elfers CT, Simmons JH, Roth CL. Glucagon‐like peptide‐1 agonist exendin‐4 leads to reduction of weight and caloric intake in a rat model of hypothalamic obesity. Horm Res Paediatr 2012; 78: 47–53 [DOI] [PubMed] [Google Scholar]
- 175.Edholm T, Degerblad M, Gryback P, et al Differential incretin effects of GIP and GLP‐1 on gastric emptying, appetite, and insulin‐glucose homeostasis. Neurogastroenterol Motil 2010; 22: 1191–e315 [DOI] [PubMed] [Google Scholar]
- 176.Asmar M, Bache M, Knop FK, et al Do the actions of glucagon‐like peptide‐1 on gastric emptying, appetite, and food intake involve release of amylin in humans? J Clin Endocrinol Metab 2010; 95: 2367–2375 [DOI] [PubMed] [Google Scholar]
- 177.Horowitz M, Flint A, Jones KL, et al Effect of the once‐daily human GLP‐1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 2012; 97: 258–266 [DOI] [PubMed] [Google Scholar]
- 178.Willms B, Werner J, Holst JJ, et al Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon‐like peptide‐1 (GLP‐1)‐(7–36) amide in type 2 (noninsulin‐dependent) diabetic patients. J Clin Endocrinol Metab 1996; 81: 327–332 [DOI] [PubMed] [Google Scholar]
- 179.Kanoski SE, Fortin SM, Arnold M, et al Peripheral and central GLP‐1 receptor populations mediate the anorectic effects of peripherally administered GLP‐1 receptor agonists, liraglutide and exendin‐4. Endocrinology 2011; 152: 3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon‐like peptide‐1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009; 150: 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hayes MR, Leichner TM, Zhao S, et al Intracellular signals mediating the food intake‐suppressive effects of hindbrain glucagon‐like peptide‐1 receptor activation. Cell Metab 2011; 13: 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alhadeff AL, Rupprecht LE, Hayes MR. GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012; 153: 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nakade Y, Tsukamoto K, Pappas TN, et al Central glucagon like peptide‐1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res 2006; 1111: 117–121 [DOI] [PubMed] [Google Scholar]
- 184.Nauck MA, Kemmeries G, Holst JJ, et al Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes 2011; 60: 1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jelsing J, Vrang N, Hansen G, et al Liraglutide: short‐lived effect on gastric emptying – long lasting effects on body weight. Diabetes Obes Metab 2012; 14: 531–538 [DOI] [PubMed] [Google Scholar]
- 186.Meier JJ, Goetze O, Anstipp J, et al Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am J Physiol Endocrinol Metab 2004; 286: E621–E625 [DOI] [PubMed] [Google Scholar]
- 187.Ogawa E, Hosokawa M, Harada N, et al The effect of gastric inhibitory polypeptide on intestinal glucose absorption and intestinal motility in mice. Biochem Biophys Res Commun 2011; 404: 115–120 [DOI] [PubMed] [Google Scholar]
- 188.Milic S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis 2012; 30: 158–162 [DOI] [PubMed] [Google Scholar]
- 189.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 2012; 142: 711–725e6. [DOI] [PubMed] [Google Scholar]
- 190.Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Curr Opin Lipidol 2011; 22: 479–488 [DOI] [PubMed] [Google Scholar]
- 191.Lee J, Hong SW, Chae SW, et al Exendin‐4 improves steatohepatitis by increasing Sirt1 expression in high‐fat diet‐induced obese C57BL/6J mice. PLoS ONE 2012; 7: e31394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Svegliati‐Baroni G, Saccomanno S, Rychlicki C, et al Glucagon‐like peptide‐1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high‐fat diet in nonalcoholic steatohepatitis. Liver Int 2011; 31: 1285–1297 [DOI] [PubMed] [Google Scholar]
- 193.Sharma S, Mells JE, Fu PP, et al GLP‐1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS ONE 2011; 6: e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Montgomery IA, Irwin N, Flatt PR. Active immunization against (Pro(3))GIP improves metabolic status in high‐fat‐fed mice. Diabetes Obes Metab 2010; 12: 744–751 [DOI] [PubMed] [Google Scholar]
- 195.Gupta NA, Mells J, Dunham RM, et al Glucagon‐like peptide‐1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010; 51: 1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dhanesha N, Joharapurkar A, Shah G, et al Exendin‐4 reduces glycemia by increasing liver glucokinase activity: an insulin independent effect. Pharmacol Rep 2012; 64: 140–149 [DOI] [PubMed] [Google Scholar]
- 197.Gezginci‐Oktayoglu S, Sacan O, Yanardag R, et al Exendin‐4 improves hepatocyte injury by decreasing proliferation through blocking NGF/TrkA in diabetic mice. Peptides 2011; 32: 223–231 [DOI] [PubMed] [Google Scholar]
- 198.Klonoff DC, Buse JB, Nielsen LL, et al Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24: 275–286 [DOI] [PubMed] [Google Scholar]
- 199.Shirakawa J, Fujii H, Ohnuma K, et al Diet‐induced adipose tissue inflammation and liver steatosis are prevented by DPP‐4 inhibition in diabetic mice. Diabetes 2011; 60: 1246–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kern M, Kloting N, Niessen HG, et al Linagliptin improves insulin sensitivity and hepatic steatosis in diet‐induced obesity. PLoS ONE 2012; 7: e38744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iwasaki T, Yoneda M, Inamori M, et al Sitagliptin as a novel treatment agent for non‐alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 2011; 58: 2103–2105 [DOI] [PubMed] [Google Scholar]
- 202.Yilmaz Y, Yonal O, Deyneli O, et al Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 2012; 75: 240–244 [PubMed] [Google Scholar]
- 203.Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline? J Diabetes Invest 2012; 3: 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nyberg J, Anderson MF, Meister B, et al Glucose‐dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci 2005; 25: 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Abbas T, Faivre E, Holscher C. Impairment of synaptic plasticity and memory formation in GLP‐1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer's disease. Behav Brain Res 2009; 205: 265–271 [DOI] [PubMed] [Google Scholar]
- 206.Isacson R, Nielsen E, Dannaeus K, et al The glucagon‐like peptide 1 receptor agonist exendin‐4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol 2011; 650: 249–255 [DOI] [PubMed] [Google Scholar]
- 207.Li H, Lee CH, Yoo KY, et al Chronic treatment of exendin‐4 affects cell proliferation and neuroblast differentiation in the adult mouse hippocampal dentate gyrus. Neurosci Lett 2010; 486: 38–42 [DOI] [PubMed] [Google Scholar]
- 208.McClean PL, Parthsarathy V, Faivre E, et al The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci 2011; 31: 6587–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Klinge PM, Harmening K, Miller MC, et al Encapsulated native and glucagon‐like peptide‐1 transfected human mesenchymal stem cells in a transgenic mouse model of Alzheimer's disease. Neurosci Lett 2011; 497: 6–10 [DOI] [PubMed] [Google Scholar]
- 210.Qin Z, Sun Z, Huang J, et al Mutated recombinant human glucagon‐like peptide‐1 protects SH‐SY5Y cells from apoptosis induced by amyloid‐beta peptide (1–42). Neurosci Lett 2008; 444: 217–221 [DOI] [PubMed] [Google Scholar]
- 211.Perry T, Lahiri DK, Sambamurti K, et al Glucagon‐like peptide‐1 decreases endogenous amyloid‐beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 2003; 72: 603–612 [DOI] [PubMed] [Google Scholar]
- 212.Bomfim TR, Forny‐Germano L, Sathler LB, et al An anti‐diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease‐ associated Abeta oligomers. J Clin Invest 2012; 122: 1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rampersaud N, Harkavyi A, Giordano G, et al Exendin‐4 reverses biochemical and behavioral deficits in a pre‐motor rodent model of Parkinson's disease with combined noradrenergic and serotonergic lesions. Neuropeptides 2012; 46: 183–193 [DOI] [PubMed] [Google Scholar]
- 214.Huang HJ, Chen YH, Liang KC, et al Exendin‐4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS ONE 2012; 7: e39656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li L, Zhang ZF, Holscher C, et al (Val(8)) glucagon‐like peptide‐1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra‐structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol 2012; 674: 280–286 [DOI] [PubMed] [Google Scholar]
- 216.Hamann C, Kirschner S, Gunther KP, et al Bone, sweet bone‐osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol 2012; 8: 297–305 [DOI] [PubMed] [Google Scholar]
- 217.Yabe D, Seino Y. DPP‐4 inhibitors and prevention of bone fractures: effects beyond glyemic control. J Diabetes Invest 2012; 3: 347–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dicembrini I, Mannucci E, Rotella CM. Bone: incretin hormones perceiver or receiver? Exp Diabetes Res 2012; 2012: 519784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhong Q, Itokawa T, Sridhar S, et al Effects of glucose‐dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab 2007; 292: E543–E548 [DOI] [PubMed] [Google Scholar]
- 220.Tsukiyama K, Yamada Y, Yamada C, et al Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol 2006; 20: 1644–1651 [DOI] [PubMed] [Google Scholar]
- 221.Xie D, Zhong Q, Ding KH, et al Glucose‐dependent insulinotropic peptide‐overexpressing transgenic mice have increased bone mass. Bone 2007; 40: 1352–1360 [DOI] [PubMed] [Google Scholar]
- 222.Ding KH, Shi XM, Zhong Q, et al Impact of glucose‐dependent insulinotropic peptide on age‐induced bone loss. J Bone Miner Res 2008; 23: 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bollag RJ, Zhong Q, Ding KH, et al Glucose‐dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol 2001; 177: 35–41 [DOI] [PubMed] [Google Scholar]
- 224.Yamada C, Yamada Y, Tsukiyama K, et al The murine glucagon‐like peptide‐1 receptor is essential for control of bone resorption. Endocrinology 2008; 149: 574–579 [DOI] [PubMed] [Google Scholar]
- 225.Nuche‐Berenguer B, Moreno P, Esbrit P, et al Effect of GLP‐1 treatment on bone turnover in normal, type 2 diabetic, and insulin‐resistant states. Calcif Tissue Int 2009; 84: 453–461 [DOI] [PubMed] [Google Scholar]
- 226.Nuche‐Berenguer B, Lozano D, Gutierrez‐Rojas I, et al GLP‐1 and exendin‐4 can reverse hyperlipidic‐related osteopenia. J Endocrinol 2011; 209: 203–210 [DOI] [PubMed] [Google Scholar]
- 227.Nuche‐Berenguer B, Moreno P, Portal‐Nunez S, et al Exendin‐4 exerts osteogenic actions in insulin‐resistant and type 2 diabetic states. Regul Pept 2010; 159: 61–66 [DOI] [PubMed] [Google Scholar]
- 228.Kyle KA, Willett TL, Baggio LL, et al Differential effects of PPAR‐{gamma} activation versus chemical or genetic reduction of DPP‐4 activity on bone quality in mice. Endocrinology 2011; 152: 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Monami M, Dicembrini I, Antenore A, et al Dipeptidyl peptidase‐4 inhibitors and bone fractures: a meta‐analysis of randomized clinical trials. Diabetes Care 2011; 34: 2474–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bunck MC, Eliasson B, Corner A, et al Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 374–377 [DOI] [PubMed] [Google Scholar]
- 231.Henriksen DB, Alexandersen P, Bjarnason NH, et al Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res 2003; 18: 2180–2189 [DOI] [PubMed] [Google Scholar]
- 232.Bjerre Knudsen L, Madsen LW, Andersen S, et al Glucagon‐like Peptide‐1 receptor agonists activate rodent thyroid C‐cells causing calcitonin release and C‐cell proliferation. Endocrinology 2010; 151: 1473–1486 [DOI] [PubMed] [Google Scholar]
- 233.Crespel A, De Boisvilliers F, Gros L, et al Effects of glucagon and glucagon‐like peptide‐1‐(7–36) amide on C cells from rat thyroid and medullary thyroid carcinoma CA‐77 cell line. Endocrinology 1996; 137: 3674–3680 [DOI] [PubMed] [Google Scholar]
- 234.Costante G, Meringolo D, Durante C, et al Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab 2007; 92: 450–455 [DOI] [PubMed] [Google Scholar]
- 235.Lamari Y, Boissard C, Moukhtar MS, et al Expression of glucagon‐like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon‐like peptide 1. FEBS Lett 1996; 393: 248–252 [DOI] [PubMed] [Google Scholar]
- 236.Madsen LW, Knauf JA, Gotfredsen C, et al GLP‐1 receptor agonists and the thyroid: C‐cell effects in mice are mediated via the GLP‐1 receptor and not associated with RET activation. Endocrinology 2012; 153: 1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yabe D, Seino Y. Liraglutide in adults with type 2 diabetes: global perspective on safety, efficacy and patient preference. Clin Med Insights Endocrinol Diabetes 2011; 4: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hegedus L, Moses AC, Zdravkovic M, et al GLP‐1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP‐1 analog, liraglutide. J Clin Endocrinol Metab 2011; 96: 853–860 [DOI] [PubMed] [Google Scholar]
- 239.Gier B, Butler PC, Lai CK, et al Glucagon like peptide‐1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Waser B, Beetschen K, Pellegata NS, et al Incretin receptors in non‐neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin‐based diabetes therapy. Neuroendocrinology 2011; 94: 291–301 [DOI] [PubMed] [Google Scholar]


