Abstract
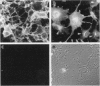
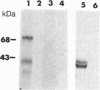
Monoclonal antibody MAb K1 recognizes a 40-kDa glycoprotein present on the surface of mesothelial cells, mesotheliomas, and ovarian cancers. We have used MAb K1 to isolate a 2138-bp cDNA that encodes this antigen. The cDNA has an 1884-bp open reading frame encoding a 69-kDa protein. When the cDNA was transfected into COS and NIH 3T3 cells, the antigen was found on the cell surface and could be released by treatment with phosphatidylinositol-specific phospholipase C. The 69-kDa precursor is processed to the 40-kDa form. The protein has been named mesothelin because it is made by mesothelial cells. Mesothelin may play a role in cellular adhesion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Klug T. L., St John E., Jenison E., Niloff J. M., Lazarus H., Berkowitz R. S., Leavitt T., Griffiths C. T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Chang K., Pai L. H., Batra J. K., Pastan I., Willingham M. C. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992 Jan 1;52(1):181–186. [PubMed] [Google Scholar]
- Chang K., Pai L. H., Pass H., Pogrebniak H. W., Tsao M. S., Pastan I., Willingham M. C. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992 Mar;16(3):259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Chang K., Pastan I. Molecular cloning and expression of a cDNA encoding a protein detected by the K1 antibody from an ovarian carcinoma (OVCAR-3) cell line. Int J Cancer. 1994 Apr 1;57(1):90–97. doi: 10.1002/ijc.2910570117. [DOI] [PubMed] [Google Scholar]
- Chang K., Pastan I., Willingham M. C. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992 Jun 19;51(4):548–554. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- Chang K., Pastan I., Willingham M. C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992 Feb 1;50(3):373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron M. F., Fryling C. M., FitzGerald D. J. Cleavage of pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J Biol Chem. 1994 Jul 8;269(27):18167–18176. [PubMed] [Google Scholar]
- Dustin M. L., Selvaraj P., Mattaliano R. J., Springer T. A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. 1987 Oct 29-Nov 4Nature. 329(6142):846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- Engert A., Burrows F., Jung W., Tazzari P. L., Stein H., Pfreundschuh M., Diehl V., Thorpe P. Evaluation of ricin A chain-containing immunotoxins directed against the CD30 antigen as potential reagents for the treatment of Hodgkin's disease. Cancer Res. 1990 Jan 1;50(1):84–88. [PubMed] [Google Scholar]
- Grossbard M. L., Press O. W., Appelbaum F. R., Bernstein I. D., Nadler L. M. Monoclonal antibody-based therapies of leukemia and lymphoma. Blood. 1992 Aug 15;80(4):863–878. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach J. P., Pèlegrin A., Buchegger F. Imaging and therapy with monoclonal antibodies in non-hematopoietic tumors. Curr Opin Immunol. 1991 Oct;3(5):685–693. doi: 10.1016/0952-7915(91)90097-k. [DOI] [PubMed] [Google Scholar]
- Pandey A., Shao H., Marks R. M., Polverini P. J., Dixit V. M. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995 Apr 28;268(5210):567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stiernberg J., Low M. G., Flaherty L., Kincade P. W. Removal of lymphocyte surface molecules with phosphatidylinositol-specific phospholipase C: effects on mitogen responses and evidence that ThB and certain Qa antigens are membrane-anchored via phosphatidylinositol. J Immunol. 1987 Jun 1;138(11):3877–3884. [PubMed] [Google Scholar]