Abstract
This study was aimed at determining the effect of duloxetine (a serotonin-norepinephrine reuptake inhibitor) on pudendal inhibition of bladder overactivity. Cystometrograms were performed on 15 cats under α-chloralose anesthesia by infusing saline and then 0.25% acetic acid (AA) to induce bladder overactivity. To inhibit bladder overactivity, pudendal nerve stimulation (PNS) at 5 Hz was applied to the right pudendal nerve at two and four times the threshold (T) intensity for inducing anal twitch. Duloxetine (0.03–3 mg/kg) was administered intravenously to determine the effect on PNS inhibition. AA irritation significantly (P < 0.01) reduced bladder capacity to 27.9 ± 4.6% of saline control capacity. PNS alone at both 2T and 4T significantly (P < 0.01) inhibited bladder overactivity and increased bladder capacity to 83.6 ± 7.6% and 87.5 ± 7.7% of saline control, respectively. Duloxetine at low doses (0.03–0.3 mg/kg) caused a significant reduction in PNS inhibition without changing bladder capacity. However, at high doses (1–3 mg/kg) duloxetine significantly increased bladder capacity but still failed to enhance PNS inhibition. WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide; a 5-HT1A receptor antagonist, 0.5–1 mg/kg i.v.) reversed the suppressive effect of duloxetine on PNS inhibition and significantly (P < 0.05) increased the inhibitory effect of duloxetine on bladder overactivity but did not enhance the effect of PNS. These results indicate that activation of 5-HT1A autoreceptors on the serotonergic neurons in the raphe nucleus may suppress duloxetine and PNS inhibition, suggesting that the coadministration of a 5-HT1A antagonist drug might be useful in enhancing the efficacy of duloxetine alone and/or the additive effect of PNS-duloxetine combination for the treatment of overactive bladder symptoms.
Introduction
Overactive bladder (OAB) is a syndrome characterized by urinary urgency, frequency, and often incontinence that is estimated to affect approximately 30 million adults in the United States (Coyne et al., 2011). Antimuscarinic drugs are the first-line therapy for OAB, but bothersome side effects and moderate efficacy have resulted in low long-term compliance (Chapple et al., 2008). A search for more effective alternative therapies has led to the development of several neuromodulation devices (van Kerrebroeck et al., 2007; Peters et al., 2009) and the recent approvals of mirabegron (a β3-adrenergic receptor agonist) (Chapple et al., 2013) and onabotulinum toxin A (Nitti et al., 2013) for the treatment of OAB, as well as to the clinical testing of a number of experimental drugs that act at sites in the central nervous system or in the lower urinary tract (Andersson, 2004). Duloxetine [a serotonin-norepinephrine reuptake inhibitor (SNRI)], which is currently approved in Europe for the treatment of stress urinary incontinence (Jost and Marsalek, 2005), has also exhibited efficacy in clinical trials in treating OAB (Steers et al., 2007; Di Rezze et al., 2012). However, its use is plagued with high numbers of reported side effects such as nausea (31%), dry mouth (16%), dizziness (14%), and constipation (14%) (Steers et al., 2007; Di Rezze et al., 2012).
Sacral and tibial neuromodulation are US Food and Drug Administration (FDA)–approved OAB treatments that are only provided to patients after pharmacotherapies have failed (van Kerrebroeck et al., 2007; Peters et al., 2009). In addition, pudendal neuromodulation, which is not FDA-approved, has been shown in preliminary studies to be superior to sacral neuromodulation in OAB treatment (Peters et al., 2005, 2010). Our recent pharmacological studies in cats have revealed that modulation of acetic acid (AA)–induced bladder overactivity by tibial and pudendal nerve stimulation (PNS) may be mediated by different mechanisms. Naloxone (an opioid receptor antagonist) reduced the inhibitory effect of tibial nerve stimulation (Tai et al., 2012) but did not alter the response to PNS (Mally et al., 2013). Furthermore, tramadol (an opioid agonist) at a low dose significantly enhanced the inhibition of bladder overactivity induced by tibial nerve stimulation (Zhang et al., 2012). On the other hand, methysergide (a 5-HT2 receptor antagonist) or ondansetron (a 5-HT3 receptor antagonist) can reduce the PNS inhibitory effect (Matsuta et al., 2013; Schwen et al., 2013). These data suggest that activation of opioid receptors plays an important role in tibial (Tai et al., 2012; Zhang et al., 2012) but not pudendal neuromodulation (Mally et al., 2013) and that 5-HT2/3 receptors are involved in PNS inhibition of bladder overactivity (Matsuta et al., 2013; Schwen et al., 2013).
In the present experiments, we explored further the role of 5-HT mechanisms in pudendal neuromodulation in anesthetized cats by studying the effect of duloxetine on PNS inhibition of bladder overactivity induced by intravesical infusion of dilute AA (0.25%). We hypothesized that a low dose of duloxetine might upregulate 5-HT inhibitory mechanisms in the brain or spinal cord and significantly enhance the PNS inhibition of bladder overactivity. When used clinically, a low dose of duloxetine might cause fewer side effects, whereas its efficacy in the treatment of OAB might still be maintained when combined with PNS. Because the facilitatory effect of duloxetine on 5-HT mechanisms can be suppressed by activation of the 5-HT1A autoreceptors on serotonergic neurons in the raphe nucleus (Gartside et al., 1997; Bjorvatn et al., 2000), WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide; a 5-HT1A receptor antagonist) was also used to investigate the role of 5-HT1A receptors in duloxetine and PNS inhibition of bladder overactivity.
Materials and Methods
All protocols involving the use of animals in the present study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental Setup.
Experiments were conducted in a total of 15 cats (9 male and 6 female, weight 2.9–3.9 kg) anesthetized with 2–5% isoflurane for surgery and maintained with α-chloralose (65 mg/kg i.v. with supplemental doses as needed) during data collection. Heart rate and blood oxygen saturation levels were monitored by a pulse oximeter (9847 V; NONIN Medical, Inc., Plymouth, MN) attached to the tongue. Arterial blood pressure was monitored via a catheter in the right carotid artery. Drugs and fluid were given via the cephalic vein and airway access was secured using a tracheostomy tube.
The ureters were isolated via a midline abdominal incision, cut and drained externally. The bladder was cannulated through the urethra with a double lumen catheter that was secured in place with a ligature to prevent urethral leakage. One lumen was used to instill saline or 0.25% AA at a rate of 1–2 ml/min; the other lumen was attached to a pressure transducer to measure bladder pressure. The pudendal nerve was dissected in the region of the right sciatic notch and a tripolar cuff electrode (NC223pt; MicroProbe, Inc., Gaithersburg, MD) was applied around the nerve and connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA).
Simulation Protocol.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-millisecond pulse width) were delivered to the pudendal nerve. Stimulation intensity threshold (T) was defined as the minimal intensity to induce anal twitch. Multiples (2T and 4T) of the threshold intensity were used in the pharmacological experiments.
Initial cystometrograms (CMG) were performed using saline infusion to determine bladder capacity, which was defined as the minimal bladder volume necessary to induce a large amplitude (>30 cm H2O) and long duration (>20 seconds) bladder contraction. Multiple saline CMGs were repeated to ensure reproducibility. Repeated CMGs then were performed with instillation of 0.25% AA to irritate the bladder, activate nociceptive bladder C-fiber afferents, and induce bladder overactivity. In this article, bladder overactivity is defined as an overactive micturition reflex, evidenced by a marked reduction in bladder capacity (Fig. 1). The change in bladder capacity was measured by normalizing the AA irritation–induced small bladder capacity to the normal capacity during saline distention. Small bladder contractions (<30 cm H2O or <20 seconds duration) occurring before the large micturition reflex contraction (see Fig. 2) were not used as a measure of bladder overactivity, because they could be caused by intrinsic bladder muscle contractions instead of reflexes. In addition they occurred inconsistently under the same conditions (duloxetine treatment or during PNS); and therefore it was difficult to use these small contractions to study the effects of drugs or PNS (see Supplemental Fig. 1).
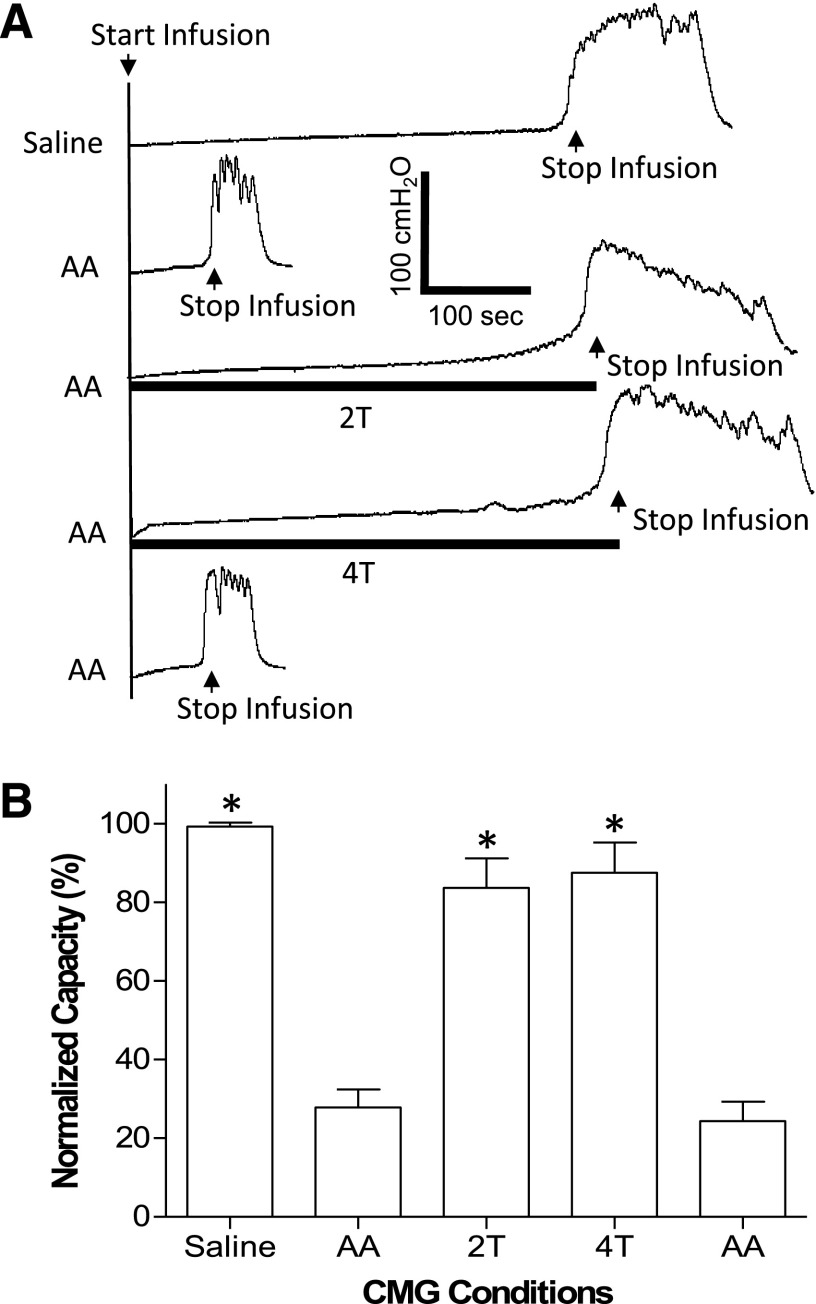
Fig. 1.
Pudendal nerve stimulation inhibits bladder overactivity (i.e., smaller bladder capacity) caused by 0.25% AA irritation. (A) CMG traces during saline or AA infusion with or without PNS. The stimulation duration is indicated by the black bars under the bladder pressure traces. T, PNS threshold intensity for inducing anal sphincter twitch. T = 1 V. Infusion rate = 2 ml/min. (B) Bladder capacity measured during CMGs under different conditions (N = 5 cats). PNS, 5 Hz, 0.2 milliseconds. T = 0.3–1.8 V. *Significantly (P < 0.01) different from the AA control before PNS (one-way ANOVA).
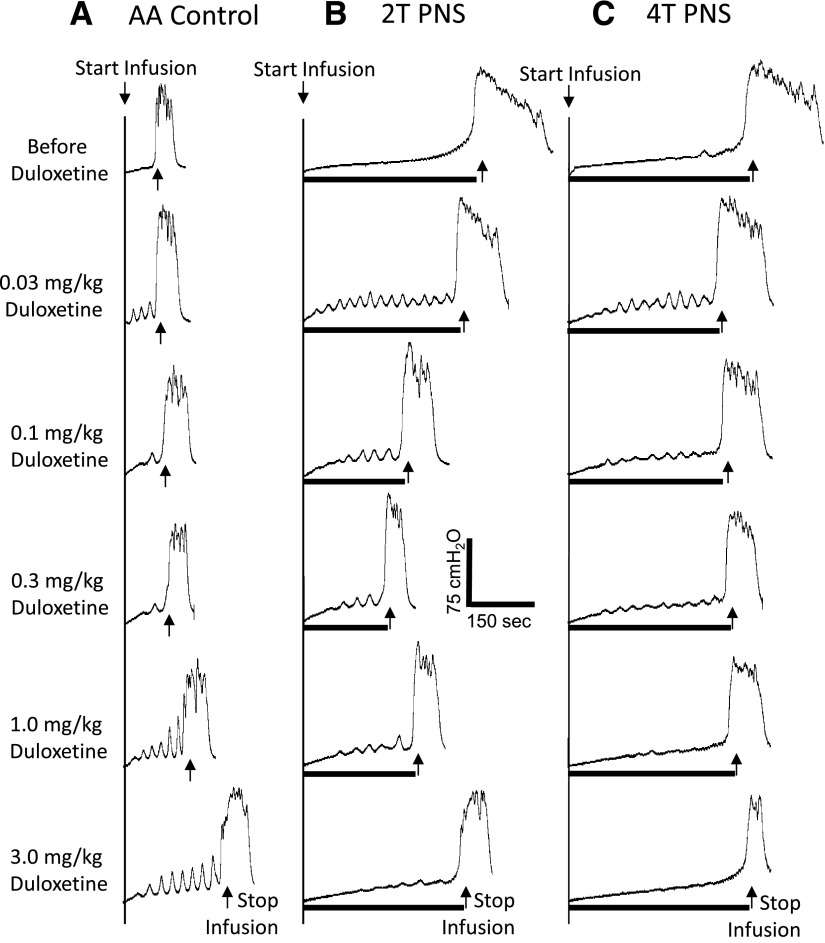
Fig. 2.
Dose-dependent effect of duloxetine on PNS-induced inhibition of bladder overactivity caused by AA irritation. The CMGs were performed in sequence from left to right (A–C) and from top to bottom in each figure. PNS duration is indicated by black bar under bladder pressure trace. T, PNS threshold intensity for inducing anal sphincter twitch. (A) Control CMGs without PNS. (B) CMGs during 2T PNS. (C) CMGs during 4T PNS. PNS, 5 Hz, 0.2 milliseconds. T = 1 V. Infusion rate = 2 ml/min.
Once bladder capacity stabilized and prior to administration of any drug, four CMGs were performed with AA infusion: (1) control without PNS, (2) during 2T PNS, (3) during 4T PNS, and (4) control without PNS to determine any poststimulation effect. The bladder was emptied at the end of each CMG and a 3- to 5-minute rest period was inserted between CMGs. After the predrug CMGs were performed, the animals were divided into two groups. In the first group (N = 5 cats), increasing cumulative doses (0.03, 0.1, 0.3, 1, and 3 mg/kg i.v.) of duloxetine (Selleck Chemicals, Houston, TX) were given to determine the drug effect on bladder capacity and PNS inhibition. Ten minutes after administration of each dose of duloxetine, the four CMGs were repeated with AA infusion under the four conditions (control, 2T PNS, 4T PNS, and post-PNS control). In the second group (N = 10 cats), a low dose of duloxetine (0.03 mg/kg i.v.) was given followed by 0.5 mg/kg i.v. WAY100635. In seven of these cats, an additional dose of duloxetine (total cumulative dose, 0.3 mg/kg i.v.) was given followed by another dose of WAY100635 (total cumulative dose, 1.0 mg/kg i.v.). Ten minutes after administration of each dose of duloxetine and 5 minutes after administration of each dose of WAY100635, the set of four CMGs was repeated with AA infusion. The dosage of WAY100635 was chosen based on our previous study (Tai et al., 2006). The purpose of the second group experiment was to investigate the possible influence of 5-HT1A autoreceptors in the raphe nucleus on duloxetine and PNS inhibition of bladder overactivity.
Data Analysis.
For each CMG, bladder capacity was normalized to the initial saline control in the same animal, which allowed for comparisons between animals. The bladder capacities were averaged for each condition and reported with standard error of the mean. Analysis of variance (ANOVA) followed by Dunnett (one-way) or Bonferroni (two-way) post-tests were used to determine the statistical significance (P < 0.05).
Results
PNS Inhibition of Bladder Overactivity.
Acetic acid irritated the bladder, induced bladder overactivity, and significantly (P < 0.01) reduced bladder capacity to 27.9 ± 4.6% (3.2 ± 0.7 ml) of saline control capacity (11.4 ± 2.3 ml, N = 15 cats) (Fig. 1). PNS at 2T and 4T applied during AA CMGs significantly (P < 0.01) increased bladder capacity to 83.6 ± 7.6% and 87.5 ± 7.7% of saline control capacity, respectively (Fig. 1). After the stimulation, bladder capacity returned to the prestimulation volume, indicating that there was no poststimulation inhibition.
Dose-Dependent Effect of Duloxetine on Bladder Overactivity and PNS Inhibition.
In the absence of PNS, duloxetine dose dependently increased bladder capacity during AA infusion (Fig. 2A). This increase was significant (P < 0.05) at doses of 1–3 mg/kg, returning the bladder capacity to about 80% of saline control capacity at the highest dose (3 mg/kg) (see AA Control in Fig. 3).
Fig. 3.
Summarized results of dose-dependent effect of duloxetine on the following: (1) the capacity of the AA-irritated bladder and (2) PNS-induced increase in bladder capacity (N = 5 cats). Bladder capacity was normalized to saline control capacity before any treatment. PNS, 5 Hz, 0.2 milliseconds. T = 0.3–1.2 V. #Significantly (P < 0.05) different from AA control (two-way ANOVA). @Significantly (P < 0.05) different from the bladder capacity measured before duloxetine treatment (i.e., at 0 mg/kg of duloxetine); *significantly (P < 0.05) different from bladder capacity measured during PNS before duloxetine treatment (one-way ANOVA).
After administration of low doses (0.03–0.3 mg/kg) of duloxetine (Figs. 2 and 3), which alone did not significantly alter bladder capacity, PNS still increased bladder capacity, but the increase elicited by 2T was significantly (P < 0.05) reduced at doses of 0.03–0.3 mg/kg and the increase by 4T was reduced at doses of 0.03–0.1 mg/kg (Fig. 3). After higher doses of duloxetine (1–3 mg/kg), which alone partially or completely reversed the irritant effect of AA and significantly increased bladder capacity, neither low (2T) or high (4T) intensity PNS produced a further significant increase in bladder capacity (Fig. 3), although the combined effect of duloxetine and PNS restored bladder capacity to the control level before AA irritation.
Effect of WAY100635 on Duloxetine and PNS Inhibition of Bladder Overactivity.
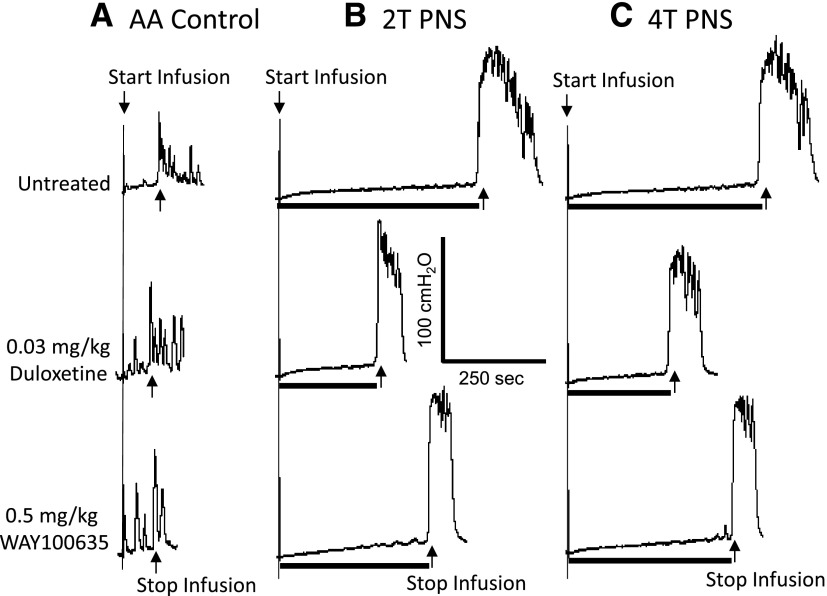
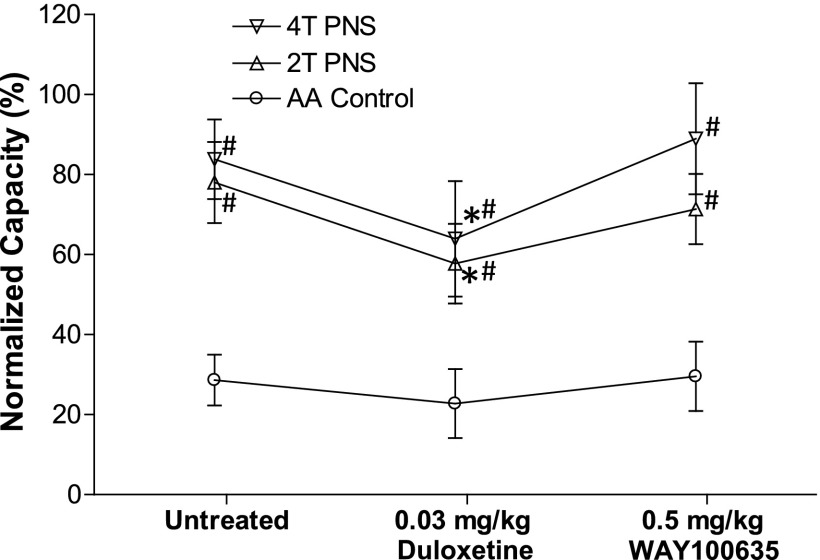
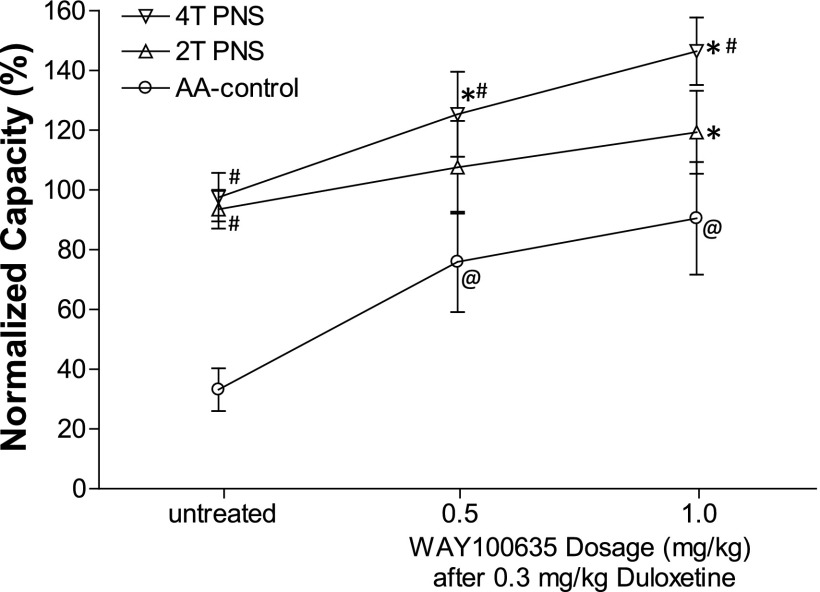
WAY100635 (0.5 mg/kg i.v., N = 10 cats) reversed the suppressive effect of duloxetine (0.03 mg/kg i.v.) on PNS inhibition without changing the bladder overactivity (Figs. 4 and 5). In 7 of these 10 cats, after treatment with WAY100635, a higher dose of duloxetine (0.3 mg/kg) significantly (P < 0.05) increased bladder capacity to 75.9 ± 16.8% of saline control, after which 4T PNS significantly (P < 0.05) increased bladder capacity by an additional 50% to 125.4 ± 14.2% of saline control (Fig. 6). Under the same conditions 2T PNS elicited a smaller and not statistically significant increase in bladder capacity. An additional higher dose of WAY100635 (1 mg/kg) did not significantly change the baseline bladder capacity or the bladder capacities during PNS (Fig. 6). However, after treatment with the combination of duloxetine (0.3 mg/kg) and WAY100635 the bladder capacities during 2T and 4T PNS were significantly larger than the capacities during PNS before treatment (Fig. 6).
Fig. 4.
WAY100635 reverses the suppressive effect of duloxetine on pudendal inhibition of bladder overactivity caused by AA irritation. The CMGs were performed in sequence from left to right (A–C) and from top to bottom in each figure. PNS duration is indicated by black bar under bladder pressure trace. T, PNS threshold intensity for inducing anal sphincter twitch. (A) Control CMGs without PNS. (B) CMGs during 2T PNS. (C) CMGs during 4T PNS. PNS, 5 Hz, 0.2 milliseconds. T = 1.6 V. Infusion rate = 1 ml/min.
Fig. 5.
Summarized results showing that WAY100635 reverses the suppressive effect of duloxetine on pudendal inhibition of bladder overactivity caused by 0.25% AA irritation (N = 10 cats). Bladder capacity was normalized to saline control capacity before any treatment. PNS, 5 Hz, 0.2 milliseconds. T = 0.4–1.8 V. *Significantly (P < 0.05) different from the bladder capacity measured during PNS before any drug treatment (one-way ANOVA). #Significantly (P < 0.05) different from AA control (two-way ANOVA).
Fig. 6.
WAY100635 significantly enhanced both duloxetine and pudendal inhibition of bladder overactivity caused by 0.25% AA irritation (N = 7 cats). Bladder capacity was normalized to saline control capacity before any treatment. PNS, 5 Hz, 0.2 milliseconds. T = 0.4–1.8 V. @Significantly (P < 0.05) different from the bladder capacity measured before any drug treatment; *significantly (P < 0.05) different from the bladder capacity measured during PNS before any drug treatment (one-way ANOVA); #significantly (P < 0.05) different from the AA control (two-way ANOVA).
Discussion
This study showed in anesthetized cats that PNS significantly inhibited bladder overactivity caused by AA irritation (Fig. 1). Duloxetine also dose dependently inhibited the bladder overactivity (Figs. 2 and 3), but it unexpectedly suppressed the PNS inhibition (Figs. 2 and 3). WAY100635 reversed the suppressive effect of duloxetine on PNS inhibition (Figs. 4 and 5) and increased the inhibitory effect of duloxetine (Fig. 6), indicating a role of 5-HT1A receptors in modulating duloxetine inhibition and the interaction between duloxetine and PNS inhibition. After treatment with WAY100635, the effects of PNS and duloxetine were additive (Fig. 6).
We confirmed reports from other investigators (Thor and Katofiasc, 1995; Katofiasc et al., 2002) that duloxetine alone increases bladder capacity and suppresses bladder overactivity induced by intravesical administration of a chemical irritant. In previous studies (Thor and Katofiasc, 1995; Katofiasc et al., 2002) this effect of duloxetine was mimicked by venlafaxine (another serotonin-norepinephrine reuptake inhibitor), whereas a selective serotonin reuptake inhibitor [(S)-norfluoxetine] had a much weaker effect, and a selective norepinephrine (NE) reuptake inhibitor (thionisoxetine) was ineffective. The effects of duloxetine were suppressed by methiothepin (a nonselective 5-HT receptor antagonist). These findings led to the conclusion that the inhibitory effect of duloxetine on bladder overactivity was due to the combined inhibition of 5-HT and NE reuptake. Although it seems likely that a similar mechanism contributes to the suppression of PNS inhibition, it is noteworthy that this effect occurred at doses below those necessary to directly increase bladder capacity (Fig. 3). Therefore, this action of duloxetine on PNS inhibition is considerably more sensitive to the drug than its inhibitory effect on the reflex pathway mediating bladder overactivity. When used clinically, differences in duloxetine dosing have also been noted for treatment of different conditions. For example the drug can be effective in treating depression and pain in lower doses than those for treating stress urinary incontinence (Jost and Marsalek, 2005; Robinson et al., 2013). The reason for this difference in sensitivity is not known.
A more prominent difference in sensitivity to duloxetine of bladder reflex pathways was reported in the chloralose-anesthetized cat where normal bladder activity induced by saline distension of the bladder was completely resistant to high doses of the drug, whereas bladder overactivity induced by AA irritation was reduced by the drug (Thor and Katofiasc, 1995). In the former condition, bladder activity was mediated by a supraspinal micturition reflex triggered by bladder Aδ afferent fibers, whereas in the irritated bladder, the overactivity was mediated at least in part by a spinal reflex pathway activated by nociceptive bladder C-fiber afferents (de Groat, 1997; Fowler et al., 2008). This difference raises the possibility that the role of 5-HT/NE mechanisms in the control of these two distinct bladder reflex circuits is markedly different, i.e., important in the control of the spinal circuitry and not important in the control of the supraspinal pathway. Alternatively, it might be argued that bladder irritation with AA unmasks a 5-HT/NE regulation of bladder reflexes that can be further enhanced by duloxetine.
Because duloxetine inhibits the reuptake of both 5-HT and NE, it is possible that multiple monoaminergic receptors may play a role in the effects of the drug in our experiments and that the effects on PNS inhibition may be mediated by different monoaminergic mechanisms/receptors than those mediating the effects on bladder capacity. Seven major families of serotonergic receptors (5-HT1–7) with multiple subtypes (total of 14) have been identified (Nichols and Nichols, 2008). Our previous studies (Matsuta et al., 2013; Schwen et al., 2013) in cats revealed that administration of drugs that block 5-HT2 and 5-HT3 receptors reduced PNS inhibition of bladder overactivity, suggesting that these receptors are tonically activated by endogenous 5-HT and facilitate the inhibitory pathway. Thus, it was logical to expect that enhancing 5-HT transmission by blocking 5-HT reuptake with duloxetine would enhance PNS inhibition. However, duloxetine suppressed PNS inhibition (Fig. 3), indicating that the putative tonic facilitatory mechanism in PNS inhibition mediated by 5-HT2 and 5-HT3 receptors was suppressed rather than enhanced by duloxetine. The reversal by WAY100635 of the duloxetine effect on PNS inhibition (Figs. 4–6) suggests that this may occur by modulation of bulbospinal 5-HT pathways that originate in medullary raphe nucleus and generate the release of 5-HT at various sites in the spinal cord, including the dorsal horn and autonomic nuclei (Gartside et al., 1997; Bjorvatn et al., 2000).
Administration of SNRIs such as duloxetine and venlafaxine as well as selective SNRIs is known to increase 5-HT concentrations in brain stem raphe nucleus, which in turn leads to activation of 5-HT1A autoreceptors expressed on the serotonergic raphe neurons, resulting in a decline in raphe neuron firing (Gartside et al., 1997; Bjorvatn et al., 2000) that could decrease tonic 5-HT release in the spinal cord. This negative feedback mechanism in the raphe nucleus would counteract a possible downstream duloxetine effect on 5-HT2/3 mechanisms in the spinal cord (Gobert et al., 1995; Fornal et al., 1996). Thus, PNS inhibition of bladder overactivity seems to depend on either a tonic descending 5-HT facilitation of the spinal inhibitory pathway or a direct activation of 5-HT raphe neuronal input to the spinal cord. This inhibition could be suppressed by a 5-HT1A autoreceptor mechanism in the raphe nucleus and thereby account for our finding that duloxetine suppresses PNS inhibition. This 5-HT1A autoreceptor mechanism is supported by the results that WAY100635 can reverse the suppressive effect of duloxetine on PNS inhibition (Fig. 5) and further enhance the inhibitory effects of duloxetine on bladder overactivity (Fig. 6).
In addition to the reuptake inhibition of 5-HT, duloxetine also inhibits the reuptake of NE. Although the inhibitory effect of duloxetine on bladder overactivity is mainly caused by reuptake inhibition of 5-HT instead of NE (Thor and Katofiasc, 1995; Katofiasc et al., 2002), we cannot exclude the possibility that duloxetine might also reduce the PNS inhibition by enhancing noradrenergic receptor mechanisms. NE-containing axons that originate from neurons in the brain stem are located in lumbosacral spinal autonomic and somatic motor nuclei as well as in the spinal dorsal horn (Mizukawa, 1980; Marks et al., 1990; Danuser et al., 1995) in proximity to the terminals of bladder and pudendal nerve primary afferent axons (Morgan et al., 1981; Thor et al., 1989), where they are well positioned to modulate lower urinary tract reflex mechanisms. After administration of duloxetine (0.3 mg/kg) and the largest dose of WAY100635, 2T PNS did not significantly increase bladder capacity even though it significantly increased bladder capacity before treatment (Fig. 6). This change in the effect of PNS might indicate that neurotransmitter mechanisms other than those related to activation of 5-HT1A receptors also suppress the duloxetine facilitatory effect on 5-HT pathways. On the other hand, duloxetine in combination with WAY100635 markedly increased baseline bladder capacity (Fig. 6). This increase in capacity could occur by the same mechanism as the increase elicited by PNS, and therefore, the combined drug treatment may have simply occluded the response to PNS. Clearly, more studies are warranted to further investigate the interaction between PNS and duloxetine as well as the role of different serotonergic or adrenergic receptors in PNS inhibition of bladder overactivity.
In conclusion, this study confirms previous reports about the inhibitory effect of duloxetine on bladder overactivity, reveals an unexpected suppressive effect of duloxetine on PNS inhibition of bladder overactivity, and provides evidence for a role of 5-HT1A receptors in a mechanism that modulates the effects of duloxetine. These results suggest that combining duloxetine with a 5-HT1A antagonist might be useful clinically to reduce the effective dose of duloxetine and/or to enhance the effects of a new combined PNS-duloxetine therapy. Identification of neurotransmitter receptors involved in neuromodulation of bladder overactivity will not only provide insight into the possible mechanisms of action for bladder neuromodulation but may also promote the development of new treatments for OAB using both drugs and neuromodulation.
Supplementary Material
Abbreviations
- AA
acetic acid
- ANOVA
analysis of variance
- CMG
cystometrogram
- 5-HT
5-hydroxytryptamine
- NE
norepinephrine
- OAB
overactive bladder
- PNS
pudendal nerve stimulation
- SNRI
serotonin-norepinephrine reuptake inhibitor
- T
threshold
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide
Authorship Contributions
Participated in research design: Reese, Xiao, Schwen, Matsuta, Shen, Wang, Roppolo, de Groat, Tai.
Conducted experiments: Reese, Xiao, Schwen, Matsuta, Shen, Wang, Roppolo, de Groat, Tai.
Contributed new reagents or analytic tools: Reese, Xiao, Schwen, Matsuta, Shen, Wang, Roppolo, de Groat, Tai.
Performed data analysis: Reese, Xiao, Schwen, Matsuta, Shen, Wang, Roppolo, de Groat, Tai.
Wrote or contributed to the writing of the manuscript: Reese, Xiao, Schwen, Matsuta, Shen, Wang, Roppolo, de Groat, Tai.
Footnotes
This study is supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK094905, R01-DK090006, R01-DK068566, and R01-DK091253].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Andersson KE. (2004) New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63(3, Suppl 1)32–41 [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Fornal CA, Martín FJ, Metzler CW, Jacobs BL. (2000) Venlafaxine and its interaction with WAY 100635: effects on serotonergic unit activity and behavior in cats. Eur J Pharmacol 404:121–132 [DOI] [PubMed] [Google Scholar]
- Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. (2008) The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54:543–562 [DOI] [PubMed] [Google Scholar]
- Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, Dorrepaal C, Martin N. (2013) Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 63:296–305 [DOI] [PubMed] [Google Scholar]
- Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. (2011) National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77:1081–1087 [DOI] [PubMed] [Google Scholar]
- Danuser H, Bemis K, Thor KB. (1995) Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther 274:820–825 [PubMed] [Google Scholar]
- de Groat WC. (1997) A neurologic basis for the overactive bladder. Urology 50(6A, Suppl) 36–52, discussion 53–56 [DOI] [PubMed] [Google Scholar]
- Di Rezze S, Frasca V, Inghilleri M, Durastanti V, Cortese A, Giacomelli E, Millefiorini E. (2012) Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin Neuropharmacol 35:231–234 [DOI] [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, Jacobs BL. (1996) WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther 278:752–762 [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. (2008) The neural control of micturition. Nat Rev Neurosci 9:453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Sharp T. (1997) Inhibition of 5-HT cell firing in the DRN by non-selective 5-HT reuptake inhibitors: studies on the role of 5-HT1A autoreceptors and noradrenergic mechanisms. Psychopharmacology (Berl) 130:261–268 [DOI] [PubMed] [Google Scholar]
- Gobert A, Lejeune F, Rivet JM, Audinot V, Newman-Tancredi A, Millan MJ. (1995) Modulation of the activity of central serotoninergic neurons by novel serotonin1A receptor agonists and antagonists: a comparison to adrenergic and dopaminergic neurons in rats. J Pharmacol Exp Ther 273:1032–1046 [PubMed] [Google Scholar]
- Jost WH, Marsalek P. (2005) Duloxetine in the treatment of stress urinary incontinence. Ther Clin Risk Manag 1:259–264 [PMC free article] [PubMed] [Google Scholar]
- Katofiasc MA, Nissen J, Audia JE, Thor KB. (2002) Comparison of the effects of serotonin selective, norepinephrine selective, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci 71:1227–1236 [DOI] [PubMed] [Google Scholar]
- Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. (2013) Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189:1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SA, Stein RD, Dashwood MR, Gilbey MP. (1990) [3H]prazosin binding in the intermediolateral cell column and the effects of iontophoresed methoxamine on sympathetic preganglionic neuronal activity in the anaesthetized cat and rat. Brain Res 530:321–324 [DOI] [PubMed] [Google Scholar]
- Matsuta Y, Schwen Z, Mally AD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. (2013) Effect of methysergide on pudendal inhibition of micturition reflex in cats. Exp Neurol 247:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukawa K. (1980) The segmental detailed topographical distribution of monoaminergic terminals and their pathways in the spinal cord of the cat. Anat Anz 147:125–144 [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de Groat WC. (1981) The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol 201:415–440 [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. (2008) Serotonin receptors. Chem Rev 108:1614–1641 [DOI] [PubMed] [Google Scholar]
- Nitti VW, Dmochowski R, Herschorn S, Sand P, Thompson C, Nardo C, Yan X, Haag-Molkenteller C, EMBARK Study Group (2013) OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 189:2186–2193 [DOI] [PubMed] [Google Scholar]
- Peters KM, Feber KM, Bennett RC. (2005) Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24:643–647 [DOI] [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Boguslawski BM, Boura JA. (2010) Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29:1267–1271 [DOI] [PubMed] [Google Scholar]
- Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, et al. (2009) Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182:1055–1061 [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Sheehan D, Gaynor PJ, Marangell LB, Tanaka Y, Lipsius S, Ohara F, Namiki C. (2013) Relationship between major depressive disorder and associated painful physical symptoms: analysis of data from two pooled placebo-controlled, randomized studies of duloxetine. Int Clin Psychopharmacol 28:330–338 [DOI] [PubMed] [Google Scholar]
- Schwen Z, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. (2013) Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am J Physiol Renal Physiol 305:F663–F671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Herschorn S, Kreder KJ, Moore K, Strohbehn K, Yalcin I, Bump RC, Duloxetine OAB Study Group (2007) Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int 100:337–345 [DOI] [PubMed] [Google Scholar]
- Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. (2012) Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302:F1090–F1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. (2006) Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol 199:427–437 [DOI] [PubMed] [Google Scholar]
- Thor KB, Katofiasc MA. (1995) Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther 274:1014–1024 [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, De Groat WC. (1989) Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol 288:263–279 [DOI] [PubMed] [Google Scholar]
- van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, et al. (2007) Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178:2029–2034 [DOI] [PubMed] [Google Scholar]
- Zhang F, Mally AD, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. (2012) Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol 302:F1576–F1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.