Abstract
The peptide hormone ghrelin is important for both homeostatic and hedonic eating behaviors, and its orexigenic actions occur mainly via binding to the only known ghrelin receptor, the growth hormone secretagogue receptor (GHSR). GHSRs are located in several distinct regions of the central nervous system. This review discusses those central nervous system sites that have been found to play critical roles in the orexigenic actions of ghrelin, including hypothalamic nuclei, the hippocampus, the amygdala, the caudal brain stem, and midbrain dopaminergic neurons. Hopefully, this review can be used as a stepping stone for the reader wanting to gain a clearer understanding of the central nervous system sites of direct ghrelin action on feeding behavior, and as inspiration for future studies to provide an even-more-detailed map of the neurocircuitry controlling eating and body weight.
Keywords: GHSR, orexigenic, homeostatic, hedonic
INTRODUCTION
Ghrelin is a peptide hormone synthesized predominantly by specialized endocrine cells of the stomach (1). Ghrelin undergoes a GOAT (ghrelin O-acyltransferase)-catalyzed posttranslational addition of an octanoyl group to bind to its receptor, the growth hormone secretagogue receptor (GHSR; also known as the ghrelin receptor), and thus gains much of its bioactivity (2, 3). In addition to roles in regulating growth hormone release, gastrointestinal motility, gastric acid secretion, blood pressure, mood, and blood glucose, ghrelin has potent effects on eating (1, 4–12). These effects are perhaps most evident during states of energy insufficiency, which ghrelin signals and to which ghrelin helps respond. Circulating ghrelin increases before meals to levels that stimulate food intake when generated by peripheral administration of the hormone (8). Ghrelin levels also rise following food deprivation and after many forms of weight loss (9–11, 14–17). Infusions of ghrelin or GHSR agonists increase body weight via pro-orexigenic actions and/or decreases in energy expenditure (18–22). Ghrelin also influences body weight by engaging several food-reward behaviors, by shifting fuel preference away from metabolic utilization of fat as an energy source, and by increasing the mRNA expression of fat storage–promoting enzymes in white adipocytes (11, 23–27). Ghrelin’s orexigenic actions are rapid and trigger eating even at times of minimal spontaneous food intake (8). After an overnight fast, administration of a GHSR antagonist blocks rebound overeating (28). More chronic treatment with exogenous ghrelin also enhances feeding and body weight gain, suggesting that ghrelin participates in long-term body weight regulation (21). Although some studies have demonstrated little to no effect of genetic interference of ghrelin signaling on body weight and food intake (25, 26), other studies using genetically modified animal models suggest that intact ghrelin signaling is required for normal eating behaviors and body weight responses, especially responses to hedonically rewarding high-fat diets (27–29).
Of note, with the exception of obese individuals with Prader-Willi syndrome, in which ghrelin levels are high and may contribute to the extreme and debilitating food-seeking behaviors and hyperphagia characteristic of that disorder, in most obese subjects, ghrelin levels are lower than in lean individuals (30–32). That said, ghrelin levels do rise after weight loss from dieting, possibly contributing to rebound weight gain (24). In contrast, most studies have demonstrated a decline or lack of rise in ghrelin levels in subjects following weight loss resulting from Roux-en-Y gastric bypass (33). Such an atypical ghrelin response to the Roux-en-Y procedure, which also affects levels of other gastrointestinal hormones such as glucagon-like peptide 1 (GLP-1), GLP-2, and peptide YY (PYY), may be just one mechanism by which that bariatric surgical procedure produces such a profound and prolonged body weight effect (33).
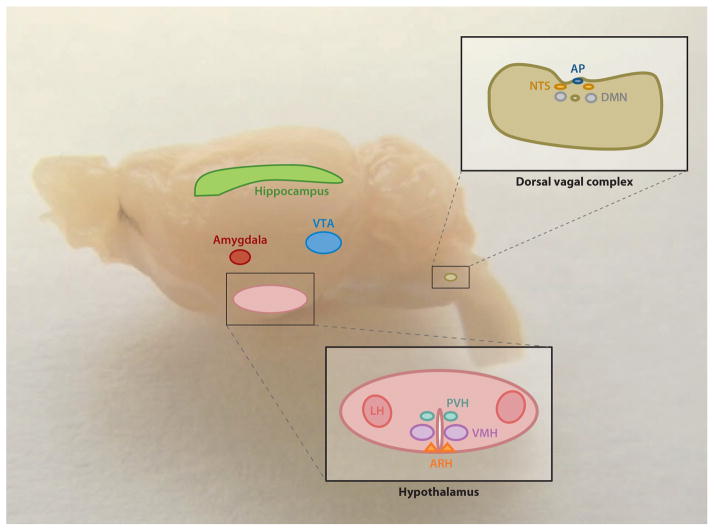
Ghrelin’s control of many varied behaviors related to eating suggests that one of its major roles is in shaping appetitive behavior and inducing motivation to seek and consume food (8). Also, the occurrence of ghrelin within a wide range of species suggests a strong biological necessity for ghrelin action (34). Furthermore, the known central nervous system (CNS) sites of GHSR expression, which include several regions previously linked to eating and reward, support this notion and are visualized in Figure 1 (8).
Figure 1.
The central nervous system sites of known direct ghrelin action are indicated here, superimposed onto a photomicrograph of a whole mouse brain. They include several hypothalamic nuclei (ARH, PVH, VMH, and LH), the hippocampus, the amygdala, the VTA, and the dorsal vagal complex [including AP, NTS, and DMN]. Abbreviations: AP, area postrema; ARH, arcuate nucleus of the hypothalamus; DMN, dorsal motor nucleus of the vagus; LH, lateral hypothalamic area; NTS, nucleus of the solitary tract; PVH, paraventricular nucleus of the hypothalamus; VMH, ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area.
In the current review, we discuss the CNS sites where ghrelin interacts to mediate its actions on food intake. For clarity, we focus on those CNS regions of direct ghrelin interaction as opposed to those downstream regions to which the directly engaged neuronal populations project. With the exception of the vagus nerve, we do not discuss known or potential peripheral sites of ghrelin action, because, to our knowledge, none has been linked to ghrelin’s orexigenic actions. In addition, although orexigenic control is a narrow category of ghrelin action, the ingestion of food is a rather complex behavioral process, including both homeostatic and hedonic feeding behaviors, and the current review discusses both of these categories.
INVOLVEMENT OF GHRELIN IN HOMEOSTATIC AND HEDONIC FEEDING BEHAVIORS
Homeostatic feeding is thought to be a primitive behavior required for survival, involving eating easily available food to replenish depleted energy stores (35). Supporting a role for ghrelin in this homeostatic realm of eating, acute administration of ghrelin potently and rapidly stimulates feeding. Also, plasma levels of endogenous ghrelin increase upon caloric restriction of various types and before set meals and decrease following ingestion of food (8). Both the magnitude and degree of the reduction in plasma ghrelin following food intake are directly related to the caloric content of the meal, which also affects the timing and magnitude of the subsequent rise in ghrelin that helps initiate the next meal (36–38). In addition, circulating ghrelin levels tend to be lower and the orexigenic effects of ghrelin tend to be blunted in cases of diet-induced obesity (39). GHSR expression occurs in several CNS nuclei long known to be involved in homeostatic feeding, including several located within the hypothalamus and the caudal brain stem, among others (40).
Hedonic feeding is seemingly a much more complex process and refers to a set of behaviors related to the pleasurable aspects of eating. A now vast literature has demonstrated the ability of both pharmacologically administered ghrelin and naturally elevated ghrelin—such as that which occurs in response to caloric restriction or to psychosocial stress—to enhance the rewarding value of fatty and sugary foods such that individuals exert more effort and learn methods to more efficiently obtain foods with hedonic appeal (41, 42). Established CNS nuclei mediating food-reward and other reward behaviors include the ventral tegmental area (VTA), the hippocampus, and the amygdala, among others, all of which contain GHSRs. A discussion of the specific CNS sites mediating ghrelin action on homeostatic and hedonic feeding behavior forms the basis of the current review.
Of note, it is still unclear how ghrelin crosses the blood-brain barrier to reach those CNS sites that are discussed in this review. Nonetheless, ghrelin from the periphery does reach the brain. For instance, radiolabeled ghrelin injected peripherally is later localized to the hippocampus, and direct microinjection of a GHSR antagonist into the VTA blocks the orexigenic actions of peripherally administered ghrelin (43, 44). It is assumed that entry to the brain occurs via circumventricular organs such as the median eminence, where capillaries are fenestrated and are thus more permissive to entry into the CNS (45, 46). However, a specific transporter that can move ghrelin across the blood-brain barrier has yet to be found (47). Also of interest, several studies have suggested that ghrelin is synthesized in one or more brain regions, although this finding is not generally reproducible, and thus the existence of centrally derived ghrelin and its physiological significance are uncertain (1, 48–50).
ACTION OF GHRELIN IN HYPOTHALAMIC FEEDING CENTERS
The hypothalamus is considered to be the master regulator of homeostasis. It consists of several nuclei, including the arcuate nucleus (ARH), the ventromedial nucleus (VMH), the paraventricular nucleus (PVH), and the lateral hypothalamic area (LH). Along with other neighboring hypothalamic regions, these hypothalamic nuclei receive signals from a plethora of sensory inputs and circulating nutritional hormones, such as ghrelin and leptin, and are believed to play pivotal roles in regulating appetite, feeding, and other metabolic processes (19, 51–54). Histological investigation of GHSR mRNA in the hypothalamus shows its rich distribution in the ARH, VMH, and PVH in rodents and primates, whereas some other studies also demonstrate GHSR expression in the LH (40, 48, 55–58). Any site with GHSR expression holds the potential to be directly engaged by ghrelin. Indeed, biotin-labeled ghrelin-binding assays and fluorescence-tagged ghrelin in vivo imaging have indicated that ghrelin can bind specifically to the ARH, PVH, and LH (45, 48). Intracerebroventricular injection of ghrelin and/or GHSR agonists into the third ventricle stimulates food intake and robustly induces c-fos—a marker of neuronal activation—in the ARH, PVH, dorsomedial hypothalamic nucleus (DMH), and LH, with a smaller c-fos induction in the VMH (19, 29, 59, 60). This ghrelin-induced activation seems to be independent of food intake because similar c-fos expression levels are also observed in these nuclei when animals are food restricted following ghrelin administration (59). The importance of GHSR in mediating the orexigenic actions of ghrelin is highlighted in GHSR-knockout models, in which there is absence of both ghrelin-triggered hyperphagia and hypothalamic c-fos induction (29, 61). Collectively, these studies suggest that ghrelin performs its functions in promoting feeding via direct actions on one or more hypothalamic nuclei. More detailed evidence is provided below.
Arcuate Nucleus
Out of all the potential CNS sites of direct ghrelin action, the ARH has been investigated the most. The ARH contains two major feeding-related neuronal subtypes. These include orexigenic agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons and anorexigenic pro-opiomelanocortin (POMC) neurons, in which the precursor polypeptide, POMC, is processed to several neuropeptides, including α-melanocyte-stimulating hormone (α-MSH), an agonist of melanocortin receptors 3 and 4 (MC3R and MC4R). The general importance of the ARH to feeding is well demonstrated by the severe reduction in food intake and body weight resulting from ablation of AgRP neurons in adult mice and by the rapid induction in food intake resulting from either optogenetic or pharmacogenetic activation of AgRP neurons (62–64). Meanwhile, optogenetic stimulation of POMC neurons inhibits food intake in mice (63). Importantly, comparison of the orexigenic effects of direct ghrelin microinjections into the ARH versus several other hypothalamic nuclei demonstrates that the lowest doses of ghrelin are most powerful when administered to the ARH (22). Also of note, the feeding-stimulating effect of both peripheral and central ghrelin applications is abrogated in animals with ARH lesions (65, 66).
The majority of GHSRs within the ARH are located on AgRP neurons; there are very few GHSRs on POMC neurons (67). Even before ghrelin was discovered, peripheral delivery of a GHSR agonist was shown to induce c-fos activity within the ARH, predominantly in AgRP neurons (68). Both central administration and peripheral administration of ghrelin have the same effect (19, 29, 59, 60, 69, 70). Additionally, central ghrelin application augments gene transcription of the orexigenic neuropeptides NPY and AgRP, and ghrelin increases electrical activity of AgRP neurons (48, 71–75). Ghrelin also increases PKA-dependent Ca2+ influx from NPY-containing, isolated ARH neurons (76). AgRP, NPY, and the inhibitory neurotransmitter GABA have been implicated in ghrelin action at the ARH. In particular, in NPY-deficient mice, the effect of peripheral ghrelin administration in stimulating appetite is attenuated, and this effect is completely abolished in NPY/AgRP double-knockout animals (77). In addition, antibodies and antagonists of both NPY and AgRP cancel ghrelin-induced feeding (20, 78, 79). Ghrelin-stimulated hyperphagia disappears upon disruption of GABA release from these AgRP neurons (80). Also, in mice that have experienced AgRP neuronal ablation during the neonatal stage, the usual feeding response to peripheral administration of ghrelin is lacking (81, 82). Altogether, these data support the hypothesis that ghrelin excites AgRP neurons, which in turn stimulates acute feeding behavior following the release of NPY, AgRP, and GABA.
Several studies have investigated possible postsynaptic targets of ghrelin-engaged AgRP neurons, with many data pointing to ARH POMC neurons. As such, application of ghrelin to hypothalamic brain slices increases the frequency of spontaneous GABA release from AgRP neurons onto POMC neurons and leads to hyperpolarization of these POMC neurons (48, 80). Also, ghrelin-induced feeding is blunted in POMC-knockout mice (83).
Although the prevailing perspective is that ghrelin initiates feeding due to its direct effects on increasing the electrical activity of AgRP neurons, alternative models have also been proposed. For instance, several data suggest that the effect of ghrelin on ARH AgRP neurons may instead involve indirect actions via presynaptic glutamatergic inputs. Electrophysiological recordings of AgRP neurons have demonstrated that ghrelin upregulates miniature excitatory synaptic current (mEPSC) frequency emanating from presynaptic glutamatergic axons (84). Future experiments are needed to further determine the importance of these indirect effects of ghrelin on AgRP neuronal activity in relation to the direct effects of ghrelin.
Paraventricular Nucleus
The PVH integrates information from many other CNS regions, including the ARH and caudal brain stem, to regulate feeding (54, 85–88). Not only does GHSR expression occur in the PVH, but also intra-PVH ghrelin injection stimulates acute feeding and induces c-fos in the PVH (22, 89, 90). Direct administration of either a serotonin or an MC3/4R agonist into the PVH blocks intra-PVH ghrelin-induced hyperphagia (91–93). Also, lesions of the PVH exaggerate food intake induced by peripheral ghrelin delivery (94).
Ventromedial Nucleus
In the rat, GHSRs are highly expressed in all regions of the VMH, whereas in the mouse, GHSR expression is far less prominent, and in lemurs, such expression is absent (40, 56). In the rat, direct VMH microinjection of ghrelin triggers food intake, and central ghrelin administration induces c-fos expression in the VMH, thus fitting with electrophysiological data demonstrating the ability of ghrelin to activate the majority of the VMH neurons (22, 95). Intracerebroventricular injection of ghrelin also activates the AMPK pathway in the rat, thus decreasing fatty acid synthase expression specifically in the VMH; when this AMPK signaling cascade is blocked in the VMH, the effect of central ghrelin is reduced (96). Also suggestive of a role of VMH in ghrelin action, peripheral injection of ghrelin increases arousal and activity in anticipation of a meal (food-anticipatory activity) in wild-type mice, but not in GHSR-knockout mice (97–99).
Lateral Hypothalamic Area
GHSR expression in the LH has been demonstrated by some but not all studies (40, 55, 56). Microinjection of ghrelin directly into the LH stimulates feeding, although much higher doses of ghrelin are required to achieve that effect compared with the amounts needed in intra-ARH, -PVH, and -VMH injections (22, 100). Ghrelin treatment also leads to depolarization of dispersed LH orexin neurons (101). Furthermore, suggestive of at least an indirect effect of LH orexin neurons on ghrelin action, ghrelin-induced intake of freely available food, ghrelin-induced food-reward behavior [including operant responding for fatty food (a measure of motivation to obtain foods that are liked)], and ghrelin-induced conditioned place preference (CPP) for fatty food (in which animals spend more time in an environment in which they have been trained to find liked foods) are blocked in orexin-deficient mice and/or wild-type mice administered orexin receptor antagonists (28, 102). An indirect effect of orexin neurons in mediating ghrelin action is supported by the observation that ghrelin-responsive neurons extensively overlap with orexin-responsive neurons in ARH AgRP/NPY neurons (76, 103).
LINKING THE LIMBIC SYSTEM WITH FEEDING CONTROL VIA GHRELIN
Hippocampus
As part of the limbic system, the hippocampus is believed to play an important role in feeding regulation, especially that which is associated with learning and emotion. GHSR mRNA is abundantly expressed in the hippocampus, and c-fos is significantly activated in the dentate gyrus and in other hippocampal regions after lateral ventricular injection of ghrelin (19, 104–106). Peripherally administered ghrelin gains access to the CNS and reaches hippocampal formations, where it binds, promotes dendritic spine formation, and generates long-term potentiation (43). Delivery of ghrelin into the hippocampus increases food intake, with specific effects on increasing meal frequency, combined with improved memory retention (107, 108). Ghrelin delivered to the ventral hippocampus in ad lib–fed rats increases spontaneous meals initiated by a discrete cue that was previously associated with meal access when the rats were food deprived (cue-potentiated feeding) (108). These effects on complex eating behavior are consistent with the known functions of the hippocampus in regulating cognition.
Amygdala
GHSRs are also highly expressed in the amygdala, another important part of the limbic system (109–111). Ghrelin treatment decreases the frequency of mEPSCs in pyramidal-like amygdalar neurons (110). Also, intra-amygdala administration of ghrelin stimulates regular chow intake and decreases anxiety-like behavior in food-restricted rats, although this ghrelin-induced feeding response has not been reproduced by all investigators (107, 110). A recent study also implies that ghrelin’s effects on cue-potentiated feeding behavior occur, at the least, via indirect action on the amygdala (111).
HINDBRAIN REGIONS MEDIATING THE OREXIGENIC EFFECT OF GHRELIN
In addition to hypothalamic and limbic system sites of ghrelin-induced food intake, expression of GHSRs in all three regions of the dorsal vagal complex (DVC)—the area postrema (AP), nucleus of the solitary tract (NTS), and dorsal motor nucleus of the vagus—suggests a role of the caudal brain stem in ghrelin action (112, 113). Infusion of ghrelin into the third ventricle (to probe both hypothalamic and more-caudal targets) or the fourth ventricle (to probe brain stem targets) potently induces hyperphagia, as does direct injection of ghrelin into the DVC (114). Importantly, the hyperphagia achieved by microinjection of ghrelin into the DVC can be elicited by using a dose much lower than the lowest effective dose previously shown to induce hyperphagia upon microinjection into the ARH (22, 114). Fourth-ventricular ghrelin delivery also increases number of meals and the size of meals during the first few hours after treatment and decreases the time until first-meal onset (114).
Upon its infusion into the fourth ventricle, ghrelin induces c-fos expression in the NTS, but not in the ARH or PVH, and it does not activate tyrosine hydroxylase (TH)-containing neurons in the brain stem (115). Ghrelin also induces c-fos expression in the NTS and in either the AP or the dorsal motor nucleus when it is infused into the lateral ventricle (59, 116). Fourth-ventricle infusion of ghrelin also increases NPY mRNA levels in the ARH (117).
Selective expression of GHSRs in mouse hindbrain cells accomplished by using Cre-lox technology does not result in an acute orexigenic response to peripherally administered ghrelin, indicating that hindbrain expression of ghrelin receptors is insufficient to mediate this effect of ghrelin (118). These mice do, however, demonstrate a muted lowering of fasting blood glucose that is similar to the glycemic response to fasting observed in wild-type mice and that is unlike the more marked lowering of blood glucose following overnight fasting of mice with global GHSR deletion (118).
Several studies have looked more directly at a possible role for the vagus nerve in mediating ghrelin’s actions on feeding, with mixed results. Presumably, these effects could include interaction with GHSRs on sensory vagal afferent neurons, which transmit information from the periphery to the CNS, in addition to interaction with GHSRs located on vagal efferent neurons, as discussed above. GHSR mRNA has been localized to the nodose ganglion, which houses the cell bodies of sensory vagal afferent neurons, and thus GHSR protein could presumably be expressed anywhere along those neurons, including where they innervate the gastrointestinal tract (119). In some studies, blockade of gastric vagal afferents via perivagal administration of the neurotoxin capsaicin prevents ghrelin-induced feeding (120), whereas administration of peripheral ghrelin to vagotomized mice fails to induce food intake (20). These latter studies suggest a necessity for vagal communication in transferring the peripheral ghrelin signal to central sites of appetite regulation. This communication presumably travels directly through the NTS to the ARH through a mainly noradrenergic pathway that is mediated via α1- and β2-adrenoreceptors, as suggested by peripheral ghrelin administration–induced increases in NTS dopamine β hydroxylase mRNA, by a subsequent increase in noradrenaline in the ARH, and by an ensuing activation of ARH NPY neurons (116). Similarly, in humans who have undergone truncal vagotomy, peripherally administered ghrelin fails to induce food intake (121). Countering the assertion that gut vagal afferents are required for the orexigenic effect of peripherally administered ghrelin, another study has demonstrated that in rats that have undergone a subdiaphragmatic vagal deafferentiation (a more selective procedure in which abdominal vagal afferents are disconnected), administration of peripheral ghrelin still induces feeding (120–122).
INTERACTION OF GHRELIN WITH MIDBRAIN DOPAMINERGIC REWARD CIRCUITRY
Much evidence exists for a role of midbrain dopaminergic reward circuits in ghrelin’s actions on both homeostatic and hedonic feeding behaviors. Receptors for ghrelin are highly expressed in the VTA and the substantia nigra (40, 44, 55) within both dopaminergic (TH-immunoreactive) neurons (40, 44) and GABAergic neurons (44). Centrally administered ghrelin and peripherally administered ghrelin induce dopamine release in the nucleus accumbens, to which VTA neurons project, and ghrelin increases action potential frequency in VTA dopamine neurons (123–126). Direct microinjection of ghrelin into the VTA increases intake of freely available food, whereas direct VTA microinjection of a GHSR antagonist decreases food intake in response to intraperitoneal ghrelin (44, 127). Selective knockdown of GHSR in rats, as achieved by transgenic expression of antisense GHSR driven by a TH promoter, results in reduced free-feeding food intake compared with such intake in wild-type controls and in failure of a GHSR agonist to induce food intake (128). Conversely, selective expression of GHSRs in dopaminergic neurons in the VTA and in a subset of other catecholaminergic cells, as achieved by using Cre-lox technology, partially restores food intake after peripheral administration of ghrelin (104). Also of note is the ability of administered ghrelin to induce CPP for high-fat diet, a behavioral task frequently used with drugs of abuse, in which an animal gravitates toward a chamber (place), with a particular set of visual and tactile cues previously associated with the pleasurable high-fat-diet reward (28, 129). The administration of ghrelin can induce CPP in wild-type mice, but not in GHSR-null animals, and this effect is fully restored in mice with selective expression of GHSRs in catecholaminergic neurons (28).
CONCLUSIONS
Ghrelin signaling in the CNS is critical for modulating both homeostatic feeding and hedonic feeding. Much evidence supports the hypothesis that the ARH plays a direct role in ghrelin-regulated homeostatic feeding and that the VTA directly mediates ghrelin-induced hedonic eating. Increasing evidence also suggests the involvement of other hypothalamic regions, the brain stem, the hippocampus, and the amygdala in ghrelin’s first-order appetite-stimulating actions. Indeed, ghrelin’s actions on homeostatic and hedonic eating likely involve a distributed network of multiple brain sites, some of which may be more directly engaged by ghrelin than are others. A next big challenge in the field of CNS control of ghrelin action will be to further dissect this complex network to better determine those CNS sites that are most critical for ghrelin’s effects, for instance, which sites are sufficient for and which sites are required for ghrelin’s varied orexigenic effects.
SUMMARY POINTS.
Ghrelin potently stimulates homeostatic feeding and induces food-reward behaviors via GHSRs in the CNS.
Ghrelin activates AgRP neurons in the ARH, where it can stimulate acute feeding behavior following the release of NPY, AgRP, and GABA.
Other evidence points to roles of other hypothalamic sites, including the VMH, PVH, and LH, in directly mediating ghrelin-induced food intake.
GHSRs in the hippocampus and amygdala mediate more complex behaviors related to food intake, including cue-potentiated feeding.
DVC GHSRs have the capacity to contribute to the orexigenic effect of ghrelin, although they do not appear to be sufficient for this action.
GHSRs in VTA dopaminergic neurons mediate ghrelin-induced homeostatic feeding and ghrelin-engaged food-reward behavior.
Acknowledgments
This work was supported by funding from the NIH (R01DA024680 and R01MH085298) and an International Research Alliance with the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen.
Glossary
- GHSR
growth hormone secretagogue receptor; also known as the ghrelin receptor
- CNS
central nervous system
- VTA
ventral tegmental area
- ARH
arcuate nucleus of the hypothalamus
- VMH
ventromedial nucleus of the hypothalamus
- PVH
paraventricular nucleus of the hypothalamus
- LH
lateral hypothalamic area
- AgRP
agouti-related peptide
- POMC
pro-opiomelanocortin
- DVC
dorsal vagal complex
- NTS
nucleus of the solitary tract
- TH
tyrosine hydroxylase
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
B.L. Mason, Email: Brittany.Mason@utsouthwestern.edu.
Q. Wang, Email: Qian.Wang5@utsouthwestern.edu.
J.M. Zigman, Email: jeffrey.zigman@utsouthwestern.edu.
LITERATURE CITED
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–25. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 5.Cruz CR, Smith RG. The growth hormone secretagogue receptor. Vitam Horm. 2008;77:47–88. doi: 10.1016/S0083-6729(06)77004-2. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 7.Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism—ghrelin versus leptin. Ann NY Acad Sci. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003;124:1532–35. doi: 10.1016/s0016-5085(03)00350-0. [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–19. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–38. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 12.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–53. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deleted in proof
- 14.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–73. [PubMed] [Google Scholar]
- 15.Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88:109–16. doi: 10.1210/jc.2002-020645. [DOI] [PubMed] [Google Scholar]
- 16.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–60. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strassburg S, Anker SD, Castaneda TR, Burget L, Perez-Tilve D, et al. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. Am J Physiol Endocrinol Metab. 2008;295:E78–84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–98. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 20.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 21.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 22.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–47. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 23.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 24.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–50. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albarran-Zeckler RG, Sun Y, Smith RG. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides. 2011;32:2229–35. doi: 10.1016/j.peptides.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, et al. Absence of ghrelin protects against early-onset obesity. J Clin Investig. 2005;115:3573–78. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–86. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Investig. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–44. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 31.Tauber M, Conte Auriol F, Moulin P, Molinas C, Delagnes V, Salles JP. Hyperghrelinemia is a common feature of Prader-Willi syndrome and pituitary stalk interruption: a pathophysiological hypothesis. Horm Res. 2004;62:49–54. doi: 10.1159/000078862. [DOI] [PubMed] [Google Scholar]
- 32.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–78. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 33.Thaler JP, Cummings DE. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 34.Palyha OC, Feighner SD, Tan CP, McKee KK, Hreniuk DL, et al. Ligand activation domain of human orphan growth hormone (GH) secretagogue receptor (GHS-R) conserved from pufferfish to humans. Mol Endocrinol. 2000;14:160–69. doi: 10.1210/mend.14.1.0412. [DOI] [PubMed] [Google Scholar]
- 35.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 36.Blom WAM, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HFJ. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 37.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89:1319–24. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 38.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–79. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs DI, Lemus MB, Kua E, Andrews ZB. Diet-induced obesity attenuates fasting-induced hyperphagia. J Neuroendocrinol. 2011;23:620–26. doi: 10.1111/j.1365-2826.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 40.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perello M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychiatry. 2012;72:347–53. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schellekens H, Dinan TG, Cryan JF. Ghrelin at the interface of obesity and reward. In: Gerald L, editor. Vitamins & Hormones. Philadelphia: Academic; 2013. pp. 285–323. [DOI] [PubMed] [Google Scholar]
- 43.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–88. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 44.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Investig. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci USA. 2013;110:1512–17. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norsted E, Gomuc B, Meister B. Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat. 2008;36:107–21. doi: 10.1016/j.jchemneu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–27. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 48.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 49.Furness JB, Hunne B, Matsuda N, Yin L, Russo D, et al. Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience. 2011;193:1–9. doi: 10.1016/j.neuroscience.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 50.Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–90. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 52.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 53.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–15. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 54.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 55.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, et al. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, Lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–89. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Bennett PA, Thomas GB, Howard AD, Feighner SD, van der Ploeg LH, et al. Hypothalamic growth hormone secretagogue-receptor (GHS-R) expression is regulated by growth hormone in the rat. Endocrinology. 1997;138:4552–57. doi: 10.1210/endo.138.11.5476. [DOI] [PubMed] [Google Scholar]
- 58.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–77. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–62. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 60.Scott V, McDade DM, Luckman SM. Rapid changes in the sensitivity of arcuate nucleus neurons to central ghrelin in relation to feeding status. Physiol Behav. 2007;90:180–85. doi: 10.1016/j.physbeh.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 61.Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2006;290:E317–25. doi: 10.1152/ajpendo.00181.2005. [DOI] [PubMed] [Google Scholar]
- 62.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–85. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 63.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–55. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Investig. 2011;121:1424–28. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–75. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- 66.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–91. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 67.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 68.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–77. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y–synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 70.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–49. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 71.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 72.Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144:544–51. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- 73.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–94. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 74.van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–39. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–51. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel–dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–56. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 77.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 78.Liu T, Kong D, Shah BP, Ye C, Koda S, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–22. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 80.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–25. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 82.Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012;31:4276–88. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tolle V, Low MJ. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57:86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 84.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 86.Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res. 1983;60:19–29. doi: 10.1016/S0079-6123(08)64371-X. [DOI] [PubMed] [Google Scholar]
- 87.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–53. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 88.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24:919–23. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 90.Melis MR, Mascia MS, Succu S, Torsello A, Muller EE, et al. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett. 2002;329:339–43. doi: 10.1016/s0304-3940(02)00673-0. [DOI] [PubMed] [Google Scholar]
- 91.Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007;27:6956–64. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Currie PJ, John CS, Nicholson ML, Chapman CD, Loera KE. Hypothalamic paraventricular 5-hydroxytryptamine inhibits the effects of ghrelin on eating and energy substrate utilization. Pharmacol Biochem Behav. 2010;97:152–55. doi: 10.1016/j.pbb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shrestha YB, Wickwire K, Giraudo SQ. Action of MT-II on ghrelin-induced feeding in the paraventricular nucleus of the hypothalamus. NeuroReport. 2004;15:1365–67. doi: 10.1097/01.wnr.0000127141.62476.d5. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Yuan Z, Dong J, Zhang D, Usami T, et al. Neuropeptide Y loses its orexigenic effect in rats with lesions of the hypothalamic paraventricular nucleus. Endocr Res. 2013;38:8–14. doi: 10.3109/07435800.2012.683226. [DOI] [PubMed] [Google Scholar]
- 95.Yanagida H, Morita T, Kim J, Yoshida K, Nakajima K, et al. Effects of ghrelin on neuronal activity in the ventromedial nucleus of the hypothalamus in infantile rats: an in vitro study. Peptides. 2008;29:912–18. doi: 10.1016/j.peptides.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 96.Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–99. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 97.Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci USA. 2007;104:20078–83. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA. 2009;106:13582–87. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Challet E, Pevet P, Lakhdar-Ghazal N, Malan A. Ventromedial nuclei of the hypothalamus are involved in the phase advance of temperature and activity rhythms in food-restricted rats fed during daytime. Brain Res Bull. 1997;43:209–18. doi: 10.1016/s0361-9230(96)00439-x. [DOI] [PubMed] [Google Scholar]
- 100.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 101.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 102.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–12. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 103.Kohno D, Suyama S, Yada T. Leptin transiently antagonizes ghrelin and long-lastingly orexin in regulation of Ca2+ signaling in neuropeptide Y neurons of the arcuate nucleus. World J Gastroenterol. 2008;14:6347–54. doi: 10.3748/wjg.14.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Investig. 2011;121:2684–92. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, et al. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–94. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol. 1997;133:1–7. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 107.Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–41. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 108.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73:915–23. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72:457–65. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 110.Alvarez-Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, et al. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE. 2012;7:e46321. doi: 10.1371/journal.pone.0046321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43:977–82. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- 113.Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–37. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–65. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 115.Faulconbridge LF, Grill HJ, Kaplan JM, Daniels D. Caudal brainstem delivery of ghrelin induces fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nuclei of the hypothalamus. Brain Res. 2008;1218:151–57. doi: 10.1016/j.brainres.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–31. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Lateral ventricular ghrelin and fourth ventricular ghrelin induce similar increases in food intake and patterns of hypothalamic gene expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1565–69. doi: 10.1152/ajpregu.00785.2005. [DOI] [PubMed] [Google Scholar]
- 118.Scott MM, Perello M, Chuang JC, Sakata I, Gautron L, et al. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS ONE. 2012;7:e44089. doi: 10.1371/journal.pone.0044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakata I, Yamazaki M, Inoue K, Hayashi Y, Kangawa K, Sakai T. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci Lett. 2003;342:183–86. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 120.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–28. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 121.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–24. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 122.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 124.Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology. 2012;153:1194–205. doi: 10.1210/en.2011-1606. [DOI] [PubMed] [Google Scholar]
- 125.King SJ, Isaacs AM, O’Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Horm Behav. 2011;60:572–80. doi: 10.1016/j.yhbeh.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 126.Kawahara Y, Kaneko F, Yamada M, Kishikawa Y, Kawahara H, Nishi A. Food reward–sensitive interaction of ghrelin and opioid receptor pathways in mesolimbic dopamine system. Neuropharmacology. 2013;67:395–402. doi: 10.1016/j.neuropharm.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 127.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–79. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Investig. 2002;109:1429–36. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–63. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]