Abstract
In the adult nervous system, chemical neurotransmission between neurons is essential for information processing. However, neurotransmission is also important for patterning circuits during development, but its precise roles have yet to be identified, and some remain highly debated. Here, we highlight viewpoints that have come to be widely accepted or still challenged. We discuss how distinct techniques and model systems employed to probe the developmental role of neurotransmission may reconcile disparate ideas. We underscore how the effects of perturbing neurotransmission during development vary with model systems, the stage of development when transmission is altered, the nature of the perturbation, and how connectivity is assessed. Based on findings in circuits with connectivity arranged in layers, we raise the possibility that there exist constraints in neuronal network design that limit the role of neurotransmission. We propose that activity-dependent mechanisms are effective in refining connectivity patterns only when inputs from different cells are close enough spatially to influence each other’s outcome.
Keywords: neuronal connectivity, synaptic transmission, circuit refinement, synapse formation, synapse elimination
Introduction
The functioning of the adult nervous system requires the creation of precise patterns of connectivity during development. A complete understanding of how circuits develop requires knowledge of the mechanisms that coordinate multiple developmental events that act together to shape connectivity patterns unique to each circuit. As such, a large body of work over several decades has focused on elucidating these developmental mechanisms. Cellular and molecular mechanisms that regulate the morphology of axons and dendrites, the output and input structures of neurons, respectively, have been explored in detail. Ascertaining the mechanisms that control the number, efficacy, location and molecular composition of synapses, the sites of neurotransmission, continues to be of prime importance.
Of much interest since the 1960s has been the role of neuronal activity in circuit development, underscored by classic studies in the visual system [1, 2] and in the neuromuscular junction [3]. Subsequent studies in diverse model systems have, however, provided disparate viewpoints about the importance of neuronal activity in patterning circuits. While neuronal activity often describes a broad range of cellular activities, we focus here on the role of chemical neurotransmission between pre- and postsynaptic cells. We outline briefly at what stages of circuit development, neurotransmission has been found to play a role. Specifically, we discuss whether transmission regulates synapse formation, differentiation, elimination and maintenance. Next, we consider the techniques that are commonly used to ascertain the role of neurotransmission and compare observations drawn from distinct methods. We then highlight a few recent studies that exemplify what can be learnt from applying contrasting experimental strategies to solve the problem. We speculate on what factors might limit a developmental role for neurotransmission in circuit development. Finally, we summarize some current perspectives and future directions in the field.
What stages of circuit assembly are regulated by neurotransmission
Synapse formation and differentiation
Synaptogenesis commences upon contact between pre- and postsynaptic neurons (Fig. 1). Thereafter, pre- and postsynaptic proteins accumulate at the site of contact, leading to differentiation of the synapse. However, even prior to physical contact, axonal growth cones can release neurotransmitters [4, 5] and dendrites are lined with transmitter receptors. Thus, it is conceivable that prior to axonal-dendritic contact, transmitter release influences synaptogenesis. One way it may do so is to regulate the probability of contact between cells. For example, the dendrites of hippocampal neurons in culture are covered with highly motile filopodia that can facilitate contact between axons and dendrites [6]. Furthermore, focal application of glutamate, an excitatory neurotransmitter, can locally induce filopodial formation in these neurons [7]. In retinal ganglion cells, dendritic filopodia motility is reduced upon blocking neurotransmitter receptors [8]. The decrease in filopodia motility theoretically reduces the potential contact volume presented by the postsynaptic cell. Thus, transmission may promote synapse formation by regulating filipodial formation and motility. In contrast, the loss of cholinergic neurotransmission at the rodent skeletal neuromuscular junction (NMJ) from embryonic stages increases the prevalence of myopodia, thin protrusions from muscle fibers [9]. The increase in myopodia is matched by an increase in the number of receptor-bearing junctions on the myotube. Transmission at early stages of NMJ development is therefore needed to suppress hyperinnervation of muscle fibers.
Figure 1. Stages of circuit development.
Simplified view of the major developmental events underlying the assembly of neuronal circuits. I. Both axons and dendrites show marked motility during early stages of neuronal growth. Some sites of contact differentiate into synapses (e.g. red arrow) upon the recruitment of pre- (yellow) and postsynaptic (red) proteins. II. Many circuits undergo a period of refinement in their initial pattern of connectivity by the removal of erroneous contacts (compare with III). Individual presynaptic cells may contact more postsynaptic cells than at maturity (greater divergence), and single postsynaptic cells may receive inputs from inappropriate presynaptic cells (greater convergence). Note that synapse elimination (blue arrow) and synapse formation (red arrow) can take place concurrently. III. Mature patterns of circuits are established not only by synapse elimination but also by the subsequent growth and maintenance of appropriate connections.
Although transmitter release could play a role in facilitating the rate and regulating the extent of initial contact between cells, neurotransmission is not required for synaptogenesis per se [10]. Synapses still form in mouse mutants where transmitter release is perturbed from the very beginning [11, 12]. It is clear, however, that once formed, transmission is involved in the maturation of nascent synapses [13, 14].
Refining connectivity: Inappropriate connections are removed with maturation
Although from the very beginning, some circuits are generated with near precision, others undergo varying degrees of reorganization before attaining their mature connectivity patterns. Circuit development thus involves not only the formation and maintenance of synaptic connections but in many instances, necessitates the elimination of erroneous contacts (Figs. 1, 2). There is much evidence to support a role for neurotransmission in circuit refinement across diverse model systems. The skeletal NMJ is, however, arguably the most well-defined system where transmission-mediated circuit refinement occurs (Fig. 2A). Somewhat unique to the NMJ is that innervation of each muscle fiber is confined to a single pretzel-shaped postsynaptic area bearing acetylcholine receptors. At birth, muscle fibers are initially multi-innervated, and subsequent refinement requires the withdrawal of all but one motor axon [3, 15]. The remaining axon terminal expands and takes over the synaptic area previously occupied by the other axons [16]. This ‘synaptic takeover’ behavior at the NMJ implicates motor neurons engaging in a competition for contact with the muscle fiber. The competition, producing winners and losers, is fueled by neurotransmission, without which, refinement does not occur.
Figure 2. Circuits that refine their connectivity based on neurotransmission.
In many systems, postsynaptic cells receive erroneous connections that are eliminated by maturity. A: At the mammalian neuromuscular junction (NMJ), multiple motor neurons (MN) contact a muscle fiber (MF) at a single junction in early development but only one axon remains at maturity. B: In some vertebrate visual systems, connections from retinal ganglion cells (RGC) representing the left and right eyes are segregated into eye-specific layers in the dorsal lateral geniculate nucleus (dLGN) and ocular dominance columns (ODCs) in the visual cortex (VC). Eye-specific layers form prior to eye-opening and before ODCs appear. C: In the rodent cerebellum, multiple cells from the inferior olivary nucleus make climbing fiber (CF) connections onto the cell body of each Purkinje cell (PC). All but one input is subsequently removed, and the remaining climbing fiber expands its territory to innervate the proximal dendrites of the Purkinje cell.
Refinement of the initial patterns of connectivity in the mammalian visual system (Fig. 2B) is also thought to involve a process of activity-dependent competition [17–19]. At maturity, retinal ganglion cells from the left and right eyes form synaptic connections with different postsynaptic cells in their subcortical target, the dorsal lateral geniculate nucleus (dLGN). Likewise, in the cortex, dLGN axons representing the left and right eyes remain segregated and are distributed into ocular dominance columns. Eye-specific layers and ocular dominance columns emerge during development from initially diffuse patterns of innervation, and perturbing transmission along the visual pathway disrupts these refinements in connectivity. Depriving one eye of vision (monocular deprivation) leads to a loss of axonal territory in the cortex representing the deprived eye and an expansion of territory subserving the seeing eye [1, 2, 17]. Thus, as in the NMJ, some dLGN axons gain whereas others lose territory during development via a process that is mediated by neurotransmission.
Implicit in the observation that postnatal refinement always results in singly-innervated NMJs is that neurotransmission shapes the convergence of inputs within a circuit. Transmission-mediated refinement resulting in stereotypic convergence can be found in the visual system. Mouse dLGN neurons can receive inputs from as many as 20 retinal ganglion cells at birth but only maintain connections with 1–3 cells in the adult [20]. This refinement takes place even after eye specific layers are formed, and although it does not depend on visual experience, it does require spontaneous retinal activity [21]. Similarly, adult Purkinje cells in the cerebellum are contacted by a single climbing fiber, but earlier in development are innervated by as many as 5 climbing fibers [22] (Fig. 2C). Disrupting postsynaptic neurotransmission at Purkinje cells using a variety of methods [23–25] results in persistent innervation from multiple climbing fibers. Thus, across several systems, neurotransmission regulates the stage of circuit refinement that determines the number of afferents converging onto an individual postsynaptic cell.
It is important to note that circuits must also regulate the number of divergent connections each presynaptic cell makes. In the mature cerebellum, each inferior olivary cell contacts ~7 Purkinje cells [26], yet initially each climbing fiber axon contacts upwards of 100 Purkinje cells [27]. Much less is known about the developmental patterns of divergence in the visual system and at the NMJ. But, because transmission regulates ‘afferent convergence’ in these model systems, it is possible that transmission also plays a pivotal role in customizing the number of cells each individual axon contacts within its respective network.
The notion that transmission evokes mechanisms that can bring about circuit refinement begs the question, ‘what factors determine who wins?’ Multiple observations across the systems we discuss here suggest that the more active input wins. At the NMJ, axons unable to synthesize neurotransmitter lose to those that transmit [28]. Likewise, the non-deprived eye clearly gains more territory in the visual cortex. Also, the efficacy of neurotransmission of the climbing fiber that will eventually solely innervate the Purkinje cell is greater than that of other co-innervating fibers [29]. However, it is also apparent that the ability to transmit better than one’s neighbors is not the sole factor underlying the outcome of competition. In these systems, it is crucial that the postsynaptic cell is able to respond to its inputs in order to promote the appropriate refinement process. For example, inhibiting cortical neurons during monocular deprivation favors the deprived, rather than the non-deprived, eye. Thus, imbalances of transmission underlie transmission-dependent refinement in connectivity, but such imbalances need to be suitably detected by the postsynaptic cell [30].
Whereas multiple manipulations can nicely unmask a role for neurotransmission in circuit refinement, it is not yet clear how imbalances in transmission are normally created between competing axons. One thought is that within each cell there are limited resources of the molecular machinery underlying transmitter release. Intriguingly, at a specific NMJ, the larger axon that contacts more muscle fibers ends up losing, possibly because larger axons can provide relatively little resource to each of its terminals, compared to smaller axons [31]. In circuits in which more than one axon remains connected to a common target cell, it may be that there isn’t much difference in the efficacy of transmission between individual axons but rather, winning axons fire axon potentials synchronously, together leading to a more effective depolarization of the postsynaptic cell [32]. Despite the fact that the molecular, physiological and cellular factors causing imbalances in transmission from competing axons are still elusive, it is evident that physiological changes often appear prior to structural alterations. For example, Layer IV neurons lose responses to the deprived eye prior to structural changes in dLGN axons [33], and functional differences between climbing fiber inputs onto Purkinje cells appear prior to the elimination of connections [34, 35].
Maintaining connectivity patterns and strengthening connections require transmission
The stability of the initial contact between an axon and a dendritic filopodium does not require excitatory neurotransmission [36]. But, transmission is eventually needed to initiate pathways (such as CAMKII, small GTPases) that stabilize the cytoskeletal network necessary for the overall maintenance of axons and dendrites [37, 38]. Maintenance of axonal and dendritic structure involves the release of trophic factors such as brain-derived neurotophic factor [39]. In mouse mutants lacking transmitter release, synapses are precipitously lost shortly after formation [12]. This decline may result from destabilization of connections in the absence of neurotransmission, or a consequence of cell death. Indeed, distinguishing between transmission-dependent trophic support and selective synapse maintenance under conditions of activity blockade is not trivial.
However, there is evidence to suggest that transmission influences the maintenance of connectivity patterns that are newly established. In the mouse retinogeniculate pathway, visual deprivation commencing past the period of initial circuit refinement results in a weakening of synaptic strength of inputs onto a single dLGN neuron [40]. Thus, even remaining ‘winning’ connections within this circuit need to be maintained by visually-evoked transmission, at least within a critical period when they still appear malleable. Strengthening can occur via a variety of mechanisms, including increasing the number of synapses between the winning axon and the postsynaptic cell [20] and increasing efficacy of transmission pre- and/or postsynaptically.
Formulating defined roles for neurotransmission in circuit patterning thus requires assessing potential effects at all stages of circuit development. It is important to emphasize that synapse formation and elimination occur concurrently within a network and that activity-dependent mechanisms may function in tandem with activity-independent mechanisms [17, 19]. We have thus far emphasized support for a role for transmission at various stages of circuit development, but as discussed next, there remains much debate when ascribing precise roles for transmission even at stages where the effects of transmission are most well-studied. Disparate findings may have come about simply because circuits draw upon transmission differentially during development, but they may also be due in part to how transmission is perturbed.
Perturbing neurotransmission: Many ways to probe multiple roles
The availability of diverse approaches to alter neurotransmission has been invaluable but can complicate comparisons across manipulations. As mentioned earlier, a classic approach that uncovered the importance of neurotransmission in circuit development is blockade of sensory stimuli. However, because there is spontaneous activity in immature sensory circuits before sensory stimulation occurs [41], pharmacological approaches have been utilized to block transmission completely. One advantage, of course, with pharmacological agents is having temporal control of the manipulation. A pharmacological manipulation that has led to seminal discoveries is the application of the neurotoxin, tetrodotoxin (TTX). TTX blocks voltage gated sodium channels and thus the generation of action potentials that lead to synchronized release of transmitters at the axon terminal. In the presence of TTX, however, transmission onto a cell may still occur at sub-threshold levels of activity, or if the presynaptic cells have TTX-insensitive sodium channels. Additionally, it can be difficult to isolate TTX treatment to specific cell types or brain regions because this agent is readily diffusible. Neurotransmitter receptor antagonists have therefore been used widely and effectively to selectively inhibit transmission along specific pathways (e.g. glutamatergic or cholinergic). However, caveats include the possibilities that pre- and postsynaptic cells may possess the same receptors, and that disruption does not block all transmission.
Genetic techniques have greatly facilitated both cell specific and location specific suppression of neurotransmission, in addition to temporal control of the manipulation. Using transfection or transgenic techniques, it is possible to express exogenous proteins in specific cell populations, which produces relatively well-characterized effects on neurotransmission. For example, over-expression of the inward rectifying potassium channel (Kir2.1) can hyperpolarize cells and reduce their output [42]. Alternatively exogenous expression of tetanus toxin light chain (TeTx) that cleaves an integral component of the molecular machinery needed for vesicular release of transmitters (exocytosis), can also suppress transmission [43]. There are, however, physiological differences between their actions. Kir2.1 over-expression lowers the cell’s resting membrane potential, and thus the cell’s response to inputs is also diminished. Although TeTx allows a cell to maintain its respones to presynaptic inputs, it may also prevent the release of trophic agents necessary for neuronal survival [44].
Recent studies have applied the various combinations of perturbations summarized in Figure 3, and have revealed novel insights into how transmission regulates circuit development. Two important conclusions arose from these studies. The first is that different manipulations even within the same system may yield opposing results. The second is that global disruption of neurotransmission can lead to different outcomes compared to when transmission is altered in a few cells. Indeed, in vivo and in vitro observations underscore an important point raised earlier – that the development of each cell’s connectivity is highly dependent upon the relative activity of its neighbors. Examples of the findings that underlie these two major conclusions are provided in Box 1.
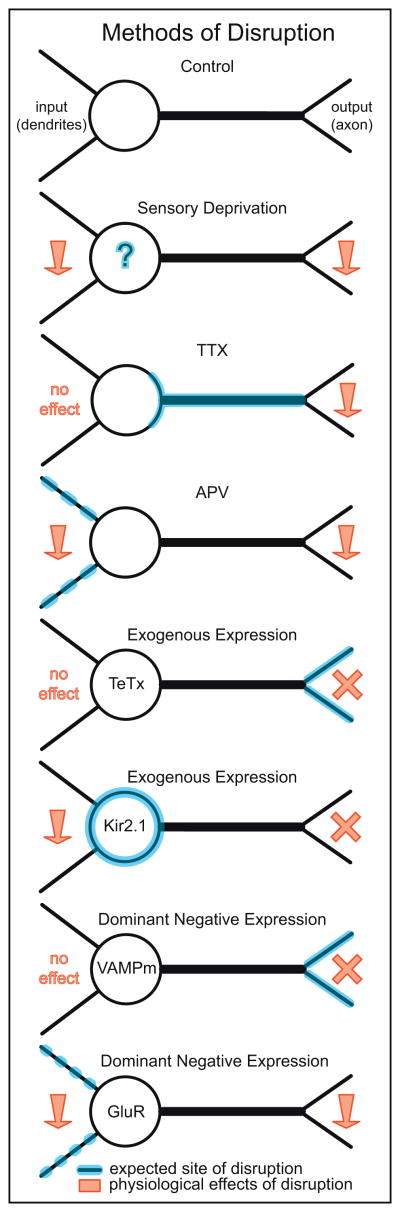
Figure 3. Methodologies for disrupting neurotransmission.
Summary of common methods used to disrupt neurotransmission. Some approaches suppress transmission onto a cell (input), perturb transmission from the cell (output) or affect both input and output. Methods that suppress release of transmitters include the use of Tetanus toxin, TeTx; Dominant negative expression of mutated vesicle associated membrane protein, VAMPm [91], and Tetrodotoxin (TTX). Sensory Deprivation, N-methyl-D-aspartate (NMDA) receptor blocker (APV) and over-expression of the inward rectifying potassium channel Kir2.1 or nonfunctioning forms of postsynaptic receptors (e.g. the C terminal of glutamate receptors (GluR) [48,92]) alter transmission by reducing both input and output. Downward red arrows, decrease neurotransmission; red crosses, blockade of transmission.
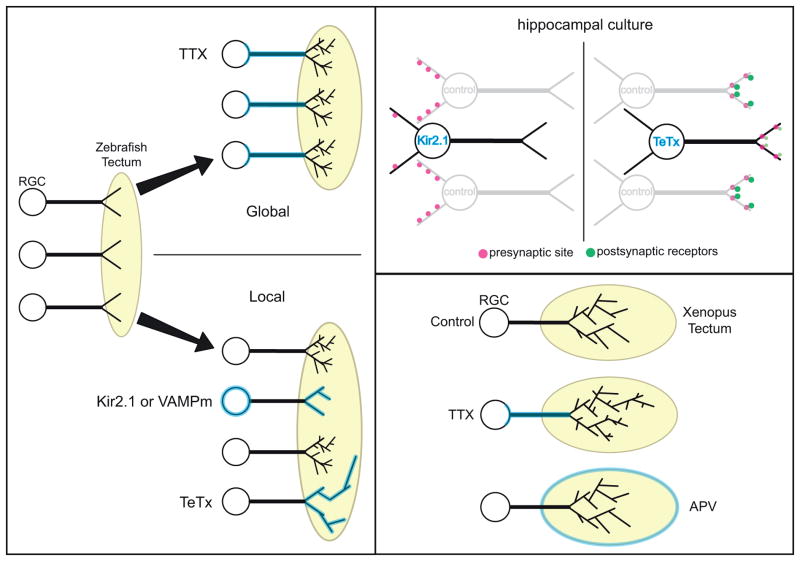
Box 1. Circuits are differentially perturbed by distinct methods of disrupting transmission.
The left panel schematizes the effects of global versus local perturbation of neurotransmission on axonal arborization of developing zebrafish retinal ganglion cells (RGC) within the optic tectum. The exposure of RGCs to tetrodotoxin (TTX) does not alter their axonal development [93,94], although this finding is debated [95]. However, there is decreased net growth and axonal branching when the excitability of isolated RGCs is reduced (Kir2.1), or if the cells fail to release transmitter (VAMPm) [93]. Conversely, although exocytosis is also impaired by expression of tetanus toxin (TeTx), toxin expression in isolated RGCs causes an increase in axonal growth [96]. Intriguingly these deficits that are a consequence of perturbed transmission in isolated cells, are rescued if transmission from their neighbors is additionally suppressed [93,96].
The upper right panel illustrates the different effects of disrupting neurotransmission with TeTx expression or Kir2.1 over-expression in hippocampal cultures. Isolated Kir2.1 over-expressing cells receive fewer synapses compared to control cells [97]. The axon of isolated TeTx expressing cells makes the same number of synapses but these ‘silenced’ synapses are apposed to postsynaptic sites with fewer glutamate receptors [67]. The number of synapses onto a postsynaptic cell does not change when TTX is applied to the entire culture [67], though this may depend upon the duration of exposure [98, 99].
In the lower right panel, chronic blockade of Xenopus retinal activity with intraocular injection of TTX [39] alters the development of retinal axonal morphology, whereas blocking glutamate receptors on tectal neurons by APV infusion to the tectum does not [46]. Note also that TTX increases RGC axonal arborization in Xenopus but not in zebrafish.
Box 1.
It is also evident that differential effects of global and selective manipulation can take place even at a single synapse. At the NMJ, selective blockade of the receptors of a small region of the postsynaptic area on a single muscle fiber results in the loss of connectivity only at the area of receptor blockade, while blockade of the entire postsynaptic region results in no loss [45]. But, differences in global and local blockade do not extend to all circuits examined. For example, chronic infusion of APV, an antagonist of NMDA receptors, into the Xenopus optic tectum results in less elaborate tectal dendritic arbors [46] and prevents sensory stimulated dendritic growth [47]. In comparison, genetically altering NMDA receptor function in isolated tectal neurons also produced similar effects on dendritic morphology [48]. Corresponding findings have also been observed in the mammalian somatosensory system, where the disruption of NMDA-dependent neurotransmission either in all postsynaptic cortical cells [49, 50], or in a single cell [51] results in disruptions in dendritic arborization.
Studies in which either suppressing presynaptic transmitter release or disrupting postsynaptic transmitter receptors have also, at times, presented disparate findings, despite both approaches effectively reducing neurotransmission (Box 1). While it is possible that some pharmacological manipulations are not specific to pre- or postsynaptic sites, recent studies utilizing genetic manipulation of thalamocortical and cortical circuits demonstrated that this reason may not explain contrasting observations. When perturbing neurotransmission by Kir2.1 over-expression in either presynaptic cortical projecting neurons or in their postsynaptic cortical target cells, there is decreased growth and branching of the axonal arbors of the projection neurons [52, 53]. Thus, transmission from presynaptic cells and depolarization of postsynaptic cells are intricately linked in patterning axonal and dendritic components of their circuit. Indeed, many studies have shown that signaling molecules can also be transmitted from post to presynaptic cells, and that such ‘retrograde transmission’ can alter synaptic and cellular function [54]. Intriguingly, retrograde transmission can even influence the dendritic development of the presynaptic cell [55] and the efficacy of transmission onto those dendrites [56]. Therefore, in order to understand how neurotransmission affects circuit development, it is necessary to assess both presynaptic and postsynaptic effects of any given manipulation.
Uncovering unexpected roles of neurotransmission by different approaches
One assay, multiple manipulations
One approach to dissect the contributions of transmission is to assay a single feature of the circuit and then probe systematically the effects of neurotransmission on this feature. Here we highlight two model systems that have been studied with such an approach, the first assessing effects on axonal projections, and the second on dendritic arrangements.
In the mature mammalian olfactory system, olfactory sensory neurons (OSNs) in the nasal olfactory sensory epithelium (OSE) project their axons to the olfactory bulb (OB). Each mature OSN expresses a single odorant receptor (OR), and the axons of all OSNs expressing the same OR converge onto the same postsynaptic specialization in the olfactory bulb called a glomerulus (Fig. 4A, [57]). Genetic mutation in mechanisms that allow OSNs to detect odorants effectively silences OSNs, but the axons of OSNs still converge onto the correct glomeruli [58, 59]. However, subsequent studies revealed that sensory stimuli are necessary for [60] and can modulate the timing of refinement of connections between OSNs and glomeruli [61]. Furthermore, Yu and colleagues [62] demonstrated that expression of TeTx in a subset of OSNs, but not all OSNs, disrupted the targeting of the axons to specific glomeruli (Figs. 4B, C). Intriguingly, if Kir2.1 was expressed by the subset of OSNs instead, their axons were not even able to target the olfactory bulb. These observations exemplify two of the features we previously highlighted - that even for the same presynaptic cell type, different manipulations of neurotransmission may have distinct outcomes, and that local manipulations may have effects distinct from those of global disruptions.
Figure 4. One type of in vivo perturbation with distinct effects across two model systems.
A: In the mammalian olfactory system, subpopulations of olfactory sensory neurons (OSNs) (blue and yellow cells) express a single type of olfactory receptor. Each population projects their axons from the olfactory sensory epithelium (OSE) to the olfactory bulb (OB) where they converge at separate postsynaptic specializations or glomeruli (green and purple ovals). TeTx expression in a subpopulation (C) but not all (B) OSNs leads to mistargeting of axons [62]. D: In the vertebrate retina, ON and OFF bipolar cells (yellow) receive their inputs from photoreceptors (Ph) and contact retinal ganglion cells (RGCs). ON bipolar cells stratify their axons in the inner half of the inner plexiform layer (IPL). RGC dendrites stratify in either the ON or OFF or both sublayers (ON/OFF) of the IPL. Expression of TeTX in all ON bipolar cells does not disrupt their axonal morphology and lamination nor alter the dendritic structure of RGCs [64]. However, compared to wildtype animals (E) ON RGCs make fewer connections (cyan dots) in the TeNT retina (F).
A second system in which an unprecedented number of manipulations was utilized to assess connectivity within a single circuit is the development of the aCC motor neurons in the Drosophilia ventral cord. Using a range of genetic manipulations, Landgraf and colleagues [63] elegantly separated transmission-dependent versus transmission-independent effects on dendritic growth of the aCC neuron in Drosophila. Dendritic growth of the aCC neuron was constrained by contact with presynaptic cholinergic interneurons throughout development, but neurotransmission was only necessary after an initial phase of synaptogenesis. Furthermore, genetic manipulation of the density or location of synapses on the aCC dendritic arbor revealed that transmission-independent contact locally restricts dendrite extension. Neurites bearing contacts become stabilized while their neighboring non-contacted neurites continue to grow. However, when transmission is blocked later in development, non-contacted dendrites show increased growth because transmission dependent inhibition of dendritic growth is lost. Their study also went on to show that increasing the density of contacts but not overall transmitter levels causes a correlated decrease in the size of the dendritic arbor. Taken together, these observations in the fly: (i) emphasized when during development transmission is important, (ii) distinguished global from local effects, and (iii) separated the effects of cell-cell contact from neurotransmission.
One manipulation, multiple assays
A different approach to isolating the role of neurotransmission in circuit development is to assay all features of the circuit while disrupting transmission with one type of manipulation. This can be achieved by studying circuits in which connectivity is organized into stereotypic patterns for which axons, dendrites and their synapses can be reconstructed in detail. Indeed, connectivity within the vertebrate retina is highly arranged, compact, and its cellular and subcellular components are easily visualized within a single field of view. Additionally, altered cellular morphology often reflects perturbed visual function. Within the retina are two parallel excitatory pathways separately encoding increments (ON) or decrements (OFF) of light levels (Fig. 4D). Photoreceptors synapse onto ON or OFF bipolar cells that converge onto the output cells of the retina, the retinal ganglion cells (RGCs). While some RGCs only receive direct input from either ON or OFF cone bipolar cells, there also exists a subset of RGCs that receive input from both bipolar cell subclasses. Within the inner plexiform layer (IPL), the axons, dendrites and synapses of ON and OFF cells occupy distinct sublaminae. The compact nature of the bipolar-RGC circuit makes it additionally plausible to address how transmission affects connectivity at multiple levels including: (i) Axonal structure of the presynaptic cell, (ii) dendritic arbor of postsynaptic cell, (iii) the number of synapses an individual axon makes with a particular postsynaptic cell, (iv) the total number of inputs on the postsynaptic cell and (v) direct comparison of distinct inputs onto the same postsynaptic cell to assay for ‘competition’.
Using transgenic expression of TeTx to suppress glutamatergic neurotransmission from ON but not OFF bipolar cells in the mouse retina (Grm6:TeTx transgenic line) in vivo, a study by Kerschensteiner et al. [64] produced several unexpected findings. Strikingly, neurotransmission does not regulate the axonal development of bipolar cells nor the dendritic arbors of RGCs (Figs. 4 E, F). Bipolar cell axons and RGC dendrites remained stratified and were unchanged in their basic branching patterns and size. The unaffected dendritic stratification of RGCs in Grm6:TeTx retinas contrasts with previous observations when glutamatergic transmission is perturbed chronically by intraocular injections of APB, a metabotropic glutamate receptor 6 (mGluR6) agonist that blocks transmission onto ON bipolar cells [65]. However, RGC dendritic stratification appears normal in a mutant in which mGluR6 receptors are absent [66]. The difference in observations across studies may be due to species differences, cell type differences or the nature of the perturbation.
Not unexpectedly, differentiated synapses are present in the Grm6:TeTX retina. However, in contrast to hippocampal cultures [10, 67], the number of synapses made between the transmission-defective bipolar cells and the RGCs is reduced despite normal axonal and dendritic morphologies (Figs. 4 E, F). Individual ON bipolar cell axons had fewer output sites and ON RGC dendrites show a corresponding decrease in the number of postsynaptic sites. Few other studies have assayed for the density of postsynaptic sites after specific presynaptic blockade [68, 69]. Perhaps most unexpected was that the reduced synapse density in ON RGCs in Grm6:TeTx retinas is explained by a decrease in the rate of formation and not elimination of synapses. This finding is fundamentally different from the generally accepted view that transmission primarily shapes connectivity by regulating synapse elimination. Also, it provides evidence that the rate of synaptogenesis, as reported by the appearance of glutamatergic postsynaptic sites, can be influenced by transmission. Moreover, like the aCC circuit in the fly ventral cord, the rate of synapse formation in the bipolar cell-ganglion cell circuit is only regulated by transmission after an initial activity-independent phase.
The reduction in synapse density in the Grm6:TeTx retina is localized to ON connections only, even for RGCs that are contacted by both ON and OFF bipolar cells. Thus, axons that converge onto the same postsynaptic cell may not necessarily compete for synaptic territory, as apparent at the NMJ and in the mammalian visual pathway. The absence of activity-based competition between ON and OFF bipolar cell axons in the mouse retina prompts the question of why some converging inputs compete and others do not.
Constraints on activity-dependent competition by network design
One reason why mouse ON and OFF bipolar cells do not compete for synaptic territory on a common postsynaptic RGC is that their inputs are restricted to separate laminae. This raises the possibility that molecular cues dictating the formation of synaptic laminae [70] may preclude axons from competing by keeping their inputs far apart. Perhaps the direct targeting of ON and OFF bipolar cell inputs onto separate dendritic arbors of an individual bistratified ganglion cell early in development [71, 72] does not encourage competition between the ON and OFF bipolar cell axons because punishment signals, as hypothesized for the NMJ [73], cannot spread far enough to destabilize the synapses of the lesser competitor (Fig. 5). This possibility is supported by the observation that transmission is not necessary for localizing inputs representing the left and right ears onto separate dorsal and ventral arbors of Nucleus Laminaris (NL) neurons (Fig. 5C). Indeed, differential expression of molecular guidance cues is found in the dorsal and ventral layers in the NL [74, 75]. In contrast, transmission-dependent emergence of eye-specific layers in the dLGN may proceed because left and right inputs are initially intermingled on the dendrites of an individual dLGN neuron prior to segregation. Indeed, potentiation upon stimulation of an input during development of Xenopus retinotectal circuits can spread to inputs that are close-by on the dendritic arbor [76].
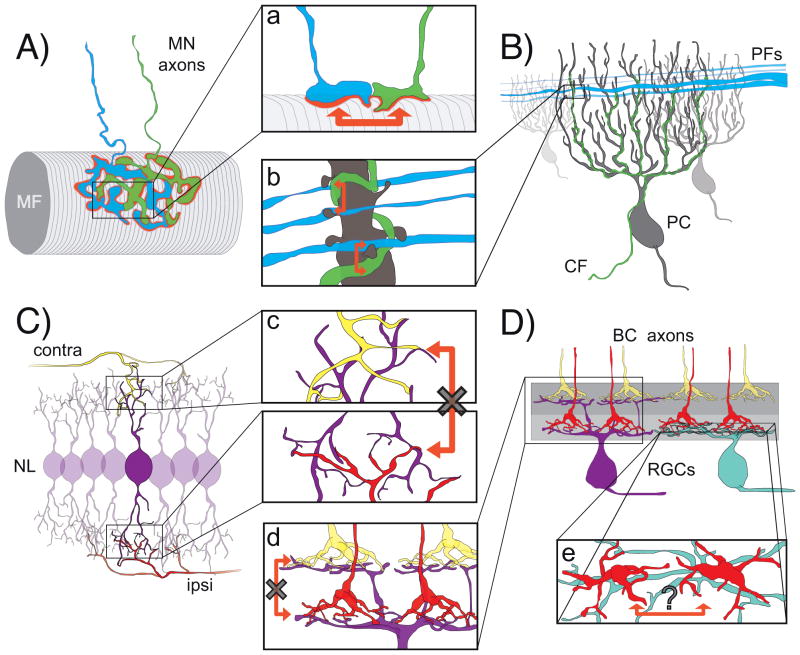
Figure 5. Is neurotransmission-mediated circuit refinement dependent on input proximity?
A: Neurotransmission dependent elimination of motor neuron (MN) axonal inputs that initially overlap at the neuromuscular junction is thought to employ local ‘punishment’ signals (red arrows, a) [73]. MF, muscle fiber. B: Parallel fibers (PFs) make connections primarily onto the distal dendrites and a single climbing fiber (CF) innervates the proximal dendrites of cerebellar Purkinje cells. While climbing fibers are the first to innervate Purkinje cells, their territory becomes intermingled with the inputs of parallel fibers later in development. Disruptions to neurotransmission from either PFs or CFs result in a local take over of territory by the more active input (red arrows, b; [100]). C & D: Not all circuits with converging afferents display neurotransmission-mediated competition. C: Inputs representing the ipsilateral (ipsi) and contralateral (contra) ears contact separate dendritic arbors of Nucleus Laminaris (NL) neurons of the auditory brainstem. These inputs do not intermingle even during development. If transmission is disrupted in one set of inputs, synaptic takeover does not occur [101]. D: Similarly, in the mammalian retina, the axons of OFF bipolar cells (BCs) do not innervate the retinal ganglion cell (RGC) dendrites contacted by ON BC axons with suppressed transmitter release [64]. The apparent absence of activity-dependent mechanisms (crossed arrows in c,d) in shaping the selectivity of inputs in the NL and retina may be because distinct types of afferents in their circuits show little to no spatial overlap. The axonal arbors of neighboring BCs of the same subtype tile at maturity but whether or not these converging inputs intermingle during development, and utilize neurotransmission to determine their relative territories has yet to be determined (e, en face view of the retina).
In the vertebrate retina, there is an additional constraint on the patterning of inputs even from neighboring afferents of the same presynaptic cell types. For example, the axonal arbors of each morphologically defined subtype of retinal bipolar cell tile, i.e. their arbors do not overlap (Fig. 5E). This tiling is thought to arise from homotypic interactions between cells of the same subtype [77, 78]. We hypothesize that axonal tiling could restrict the developmental effects of transmission by limiting the potential overlap of converging inputs. If the axonal territories of bipolar cells are ‘released’ from tiling, it is conceivable that the terminal branches of neighboring cells extend, and their synapses inter-digitate on the postsynaptic dendrite. The freedom to expand may allow every bipolar cell to increase their connectivity, implicating a role for homotypic interactions in constraining the number of connections each bipolar cell can make. Alternatively, individual bipolar cells may continue to acquire their stereotypic number of connections in the absence of axonal tiling. This would suggest that tiling alone does not regulate the number of synapses each bipolar makes. For either scenario, creating an imbalance in activity of neighboring bipolar cells of the same subtype could further uncover a latent role for transmission, if the active cell makes more synapses than its silenced neighbors.
Experiments separating interactions between synaptic partners, and homotypic interactions between presynaptic cells, are thus important [78]. Certainly, in the past, inherent competitiveness between afferents has been revealed when the situation promotes this behavior. Notably, in the frog retinotectal system, eye-specific stripes appear when retinal axons from an implanted third eye was forced to co-innervate a single tectum [79]. Whether the extent of synaptic overlap is a factor that circumscribes a role for activity-mediated competition remains a hypothesis to be explored experimentally but we think, worth testing. Further investigations will greatly benefit from knowledge of whether synapses from different presynaptic cells are initially intermingled on the dendrite prior to synapse elimination. As such, visualizing the locations of all the synapses formed by each presynaptic cell onto an individual dendritic arbor, and where synapses of individual axons are added and eliminated over time, will be immensely helpful. Efforts to ascertain the type of wires and location of synapses within major brain circuits are underway [80].
Conclusions
Despite the immense complexity in defining the roles of neurotransmission in circuit development, several fundamental findings and thought-provoking ideas have emerged. First, there exists transmission-dependent and transmission-independent phases of circuit development that do not overlap, but not in every system studied to date [81]. Second, the developmental effects of transmission may be local or global; regulation of connectivity between a pair of pre and postsynaptic cells may depend not only on transmission between the cell-pairs but also transmission arising from other neurons in the circuit. Third, it is also evident that that the outcome of suppressing transmission in an experimental model could vary depending on the nature of the perturbation. However, recent advances in genetic strategies have enabled better targeting of the site of activity-blockade. Finally, recent findings in the visual and auditory systems raise the possibility that the effects of transmission may be limited by spatial constraints built into the circuit design. We raise the notion that axons may not compete for synaptic space on the postsynaptic cell if their synapses are not near enough to affect each other.
The future of this field is immense and many questions remain unresolved. There is a major effort to ascertain how the spatiotemporal firing patterns of presynaptic cells and the depolarization of the postsynaptic cell influence the maintenance or elimination of synapses [32,82]. The intercellular pathways that ‘translate’ neurotransmission into structural and physiological changes in wiring need to be fully explored. Of much current interest is defining the roles of activity-mediated local protein synthesis [83, 84] and transcriptional events in patterning axons, dendrites and synapses [85, 86]. Also, because neurons generally receive inhibitory inputs, determining how the balance of excitation and inhibition comes about during development is essential [87]. Furthermore, it is now evident that complex physiological properties of circuits can be shaped developmentally by activity patterns [88–90] of their inputs, but how this occurs is unknown.
In summary, while it is clear that neurotransmission influences circuit development, it is unlikely that there is a unified role (or roles) for neurotransmission that applies to all circuits, within and across species. Nevertheless, perhaps the goal in determining how transmission shapes neuronal connectivity is not to pursue common effects but rather to appreciate that diverse strategies are utilized by organisms to build their nervous system.
Acknowledgments
Supported by NIH grants (EY 10699,17101,14358) to R.O.L.W. and A.B. is supported a Developmental Biology Predoctoral Training Grant T32HD007183 from the National Institute of Child Health and Human Development. We thank Felice Dunn for the many helpful comments on the manuscript.
Abbreviations
- APB
2-amino-4-phosphonobutyrate
- APV
DL-2-Amino-5-phosphonopentanoic acid
- CAMKII
calcium/calmodulin dependant protein kinase II
- dLGN
dorsal lateral geniculate nucleus
- GTPases
guanosine triphosphate hydrolyzing enzymes
- Kir2.1
inward rectifying potassium channel 2.1
- NMDA
N-methyl-D-aspartic acid
- NMJ
neuromuscular junction
- RGC
retinal ganglion cell
- TeTx
tetanus toxin light chain
- TTX
tetrodotoxin
- VAMPm
dominant-negative vesicle-associated membrane protein
References
- 1.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 2.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 3.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 4.Hume RI, Role LW, Fischbach GD. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–4. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 5.Taylor J, Docherty M, Gordon-Weeks PR. GABAergic growth cones: release of endogenous gamma-aminobutyric acid precedes the expression of synaptic vesicle antigens. J Neurochem. 1990;54:1689–99. doi: 10.1111/j.1471-4159.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 6.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 7.Smith SJ, Jahr CE. Rapid induction of filopodial sprouting by application of glutamate to hippocampal neurons. In: Letourneau PC, Kater SB, Macagno ER, editors. The Nerve Growth Cone. Raven Press; New York: 1992. pp. 19–26. [Google Scholar]
- 8.Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–2. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- 9.Misgeld T, Burgess RW, Lewis RM, Cunningham JM, et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–48. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 10.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–12. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 11.Varoqueaux F, Sigler A, Rhee JS, Brose N, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99:9037–42. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhage M, Maia AS, Plomp JJ, Brussaard AB, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 13.Okabe S, Kim HD, Miwa A, Kuriu T, et al. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci. 1999;2:804–11. doi: 10.1038/12175. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–6. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 15.Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- 17.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 19.Leamey CA, Van Wart A, Sur M. Intrinsic patterning and experience-dependent mechanisms that generate eye-specific projections and binocular circuits in the visual pathway. Curr Opin Neurobiol. 2009;19:181–7. doi: 10.1016/j.conb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–66. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 21.Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–91. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto K, Ichikawa R, Takechi H, Inoue Y, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–12. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzetto E, Caselli L, Feng G, Yuan W, et al. Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum. Proc Natl Acad Sci USA. 2009;106:16475–80. doi: 10.1073/pnas.0907298106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki T, Hashimoto K, Shin HS, Kano M, et al. P/Q-type Ca2+ channel alpha1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24:1734–43. doi: 10.1523/JNEUROSCI.4208-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugihara I, Wu HS, Shinoda Y. The entire trajectories of single olivocerebellar axons in the cerebellar cortex and their contribution to Cerebellar compartmentalization. J Neurosci. 2001;21:7715–23. doi: 10.1523/JNEUROSCI.21-19-07715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugihara I. Organization and remodeling of the olivocerebellar climbing fiber projection. Cerebellum. 2006;5:15–22. doi: 10.1080/14734220500527385. [DOI] [PubMed] [Google Scholar]
- 28.Buffelli M, Burgess RW, Feng G, Lobe CG, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–4. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 29.Bosman LW, Takechi H, Hartmann J, Eilers J, et al. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hata Y, Stryker MP. Control of Thalamocortical Afferent Rearrangement by Postsynaptic Activity in Developing Visual Cortex. Science. 1994;265:1732–5. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 31.Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–30. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- 32.Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci. 2001;24:139–66. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 33.Silver MA, Stryker MP. Synaptic density in geniculocortical afferents remains constant after monocular deprivation in the cat. J Neurosci. 1999;19:10829–42. doi: 10.1523/JNEUROSCI.19-24-10829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–96. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, et al. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63:106–18. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–60. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–34. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 38.Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J Neurosci. 1999;19:8909–18. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen-Cory S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J Neurosci. 1999;19:9996–10003. doi: 10.1523/JNEUROSCI.19-22-09996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–17. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johns DC, Marx R, Mains RE, O’Rourke B, et al. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–7. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiavo G, Benfenati F, Poulain B, Rossetto O, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–5. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 44.Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–7. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- 45.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–24. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 46.Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38:357–68. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–80. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 48.Ewald RC, Van Keuren-Jensen KR, Aizenman CD, Cline HT. Roles of NR2A and NR2B in the development of dendritic arbor morphology in vivo. J Neurosci. 2008;28:850–61. doi: 10.1523/JNEUROSCI.5078-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002;21:477–92. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005;25:2304–11. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–17. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizuno H, Hirano T, Tagawa Y. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. Eur J Neurosci. 2010;31:410–24. doi: 10.1111/j.1460-9568.2009.07070.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamada A, Uesaka N, Hayano Y, Tabata T, et al. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc Natl Acad Sci USA. 2010;107:7562–7. doi: 10.1073/pnas.0900613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao HW, Poo M. Retrograde signaling at central synapses. Proc Natl Acad Sci USA. 2001;98:11009–15. doi: 10.1073/pnas.191351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lom B, Cogen J, Sanchez AL, Vu T, et al. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–49. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du JL, Wei HP, Wang ZR, Wong ST, et al. Long-range retrograde spread of LTP and LTD from optic tectum to retina. Proc Natl Acad Sci USA. 2009;106:18890–6. doi: 10.1073/pnas.0910659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou DJ, Chesler A, Firestein S. How the olfactory bulb got its glomeruli: a just so story? Nat Rev Neurosci. 2009;10:611–8. doi: 10.1038/nrn2666. [DOI] [PubMed] [Google Scholar]
- 58.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 59.Lin DM, Wang F, Lowe G, Gold GH, et al. Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron. 2000;26:69–80. doi: 10.1016/s0896-6273(00)81139-3. [DOI] [PubMed] [Google Scholar]
- 60.Zou DJ, Feinstein P, Rivers AL, Mathews GA, et al. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–9. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]
- 61.Kerr MA, Belluscio L. Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat Neurosci. 2006;9:484–6. doi: 10.1038/nn1673. [DOI] [PubMed] [Google Scholar]
- 62.Yu CR, Power J, Barnea G, O’Donnell S, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–66. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 63.Tripodi M, Evers JF, Mauss A, Bate M, et al. Structural homeostasis: compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol. 2008;6:e260. doi: 10.1371/journal.pbio.0060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, et al. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–20. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–6. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- 66.Tagawa Y, Sawai H, Ueda Y, Tauchi M, et al. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci. 1999;19:2568–79. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- 68.Riccio RV, Matthews MA. Effects of intraocular tetrodotoxin on dendritic spines in the developing rat visual cortex: a Golgi analysis. Brain Res. 1985;351:173–82. doi: 10.1016/0165-3806(85)90189-0. [DOI] [PubMed] [Google Scholar]
- 69.Ultanir SK, Kim JE, Hall BJ, Deerinck T, et al. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA. 2007;104:19553–8. doi: 10.1073/pnas.0704031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–62. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mumm JS, Williams PR, Godinho L, Koerber A, et al. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–21. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jennings C. Developmental neurobiology. Death of a synapse. Nature. 1994;372:498. doi: 10.1038/372498a0. [DOI] [PubMed] [Google Scholar]
- 74.Cramer KS, Karam SD, Bothwell M, Cerretti DP, et al. Expression of EphB receptors and EphrinB ligands in the developing chick auditory brainstem. J Comp Neurol. 2002;452:51–64. doi: 10.1002/cne.10399. [DOI] [PubMed] [Google Scholar]
- 75.Cramer KS, Bermingham-McDonogh O, Krull CE, Rubel EW. EphA4 signaling promotes axon segregation in the developing auditory system. Dev Biol. 2004;269:26–35. doi: 10.1016/j.ydbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Tao HW, Zhang LI, Engert F, Poo M. Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron. 2001;31:569–80. doi: 10.1016/s0896-6273(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 77.Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–17. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reese BE. Development of the Retina and Optic Pathway. Vision Res. 2010 doi: 10.1016/j.visres.2010.07.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reh TA, Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985;5:1132–43. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–22. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–8. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Ghosh A. Regulation of dendritic development by neuronal activity. J Neurobiol. 2005;64:4–10. doi: 10.1002/neu.20150. [DOI] [PubMed] [Google Scholar]
- 86.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–28. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–36. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 88.Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–5. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Van Hooser SD, Mazurek M, White LE, et al. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–6. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White LE, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56:327–38. doi: 10.1016/j.neuron.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Sørensen JB, Matti U, Wei SH, Nehring RB, et al. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc Natl Acad Sci USA. 2002;99:1627–32. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA. 2006;103:12127–31. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–6. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 94.Stuermer CA, Rohrer B, Münz H. Development of the retinotectal projection in zebrafish embryos under TTX-induced neural-impulse blockade. J Neurosci. 1990;10:3615–26. doi: 10.1523/JNEUROSCI.10-11-03615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gnuegge L, Schmid S, Neuhauss SC. Analysis of the activity-deprived zebrafish mutant macho reveals an essential requirement of neuronal activity for the development of a fine-grained visuotopic map. J Neurosci. 2001;21:3542–8. doi: 10.1523/JNEUROSCI.21-10-03542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ben Fredj N, Hammond S, Otsuna H, Chien CB, et al. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J Neurosci. 2010;30:10939–51. doi: 10.1523/JNEUROSCI.1556-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–8. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 98.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–82. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 99.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, et al. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 100.Hashimoto K, Yoshida T, Sakimura K, Mishina M, et al. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009;162:601–11. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 101.Deitch JS, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: time course and specificity of dendritic atrophy following deafferentation. J Comp Neurol. 1984;229:66–79. doi: 10.1002/cne.902290106. [DOI] [PubMed] [Google Scholar]