Abstract
Microglia have been implicated in various neurological and psychiatric disorders in rodent and human postmortem studies. However, the dynamic actions of microglia in the living human brain have not been clarified due to a lack of studies dealing with in situ microglia. Herein, we present a novel technique for developing induced microglia-like (iMG) cells from human peripheral blood cells. An optimized cocktail of cytokines, GM-CSF and IL-34, converted human monocytes into iMG cells within 14 days. The iMG cells have microglial characterizations; expressing markers, forming a ramified morphology, and phagocytic activity with various cytokine releases. To confirm clinical utilities, we developed iMG cells from a patient of Nasu-Hakola disease (NHD), which is suggested to be directly caused by microglial dysfunction, and observed that these cells from NHD express delayed but stronger inflammatory responses compared with those from the healthy control. Altogether, the iMG-technique promises to elucidate unresolved aspects of human microglia in various brain disorders.
Microglia, immune cells in the brain, play major immunological/inflammatory roles as brain macrophage in the central nervous system (CNS). The origin of resident microglia have been proven to be from primitive myeloid progenitors (primitive macrophage) that arise in the yolk sac before embryonic day 81. Resident microglia form as a ramified type (called ramified microglia), whose branches constantly move and survey the microenvironment under physiological conditions in the CNS2, and once activated, shift to an ameboid type, phagocytose, and release various mediators such as pro-inflammatory cytokines3,4,5. Microglia are suggested to contribute to the pathophysiology of various neurological and psychiatric disorders6,7,8. Nasu–Hakola disease (NHD) which is a very rare autosomal recessive disorder, initially reported in Finland and Japan9,10, is believed to be caused by microglial dysfunction. Until now, only about 200 cases have been reported worldwide and the majority of cases are in the Finnish and Japanese populations11. NHD is characterized by formation of multifocal bone cysts and progressive early-onset dementia with various psychiatric symptoms including personality changes11,12, caused by mutations of DNAX-activation protein 12 (DAP12)13 or triggering receptor expressed on myeloid cells 2 (TREM2)14, both of which are expressed in human microglia. A rodent brain study showed that DAP12 is expressed only in microglia and deletion of DAP12 induces synaptic impairments possibly due to microglial dysfunction15. A human postmortem study has revealed the absence of DAP12 expression on ramified microglia in the brains of NHD patients16.
The above-mentioned reports have strongly supported the theory that human microglia maladaptively contribute to a variety of neurological and psychiatric disorders including NHD, while dynamic analysis of microglial dysfunction in the human brain has yet to been clarified. The most significant limitation in human brain research is the difficulty in obtaining living brain cells including microglial cells from living human brains due to ethical and technical considerations. To solve this limitation, alternative methods have long been warranted. Presently, human neuronal cells can be established from somatic cells (not from the brain) such as skin fibroblasts by utilizing the gene-modification technique of induced pluripotent stem (iPS) cells17,18. In addition, recently, neuronal cells are more easily established from direct conversion of human skin fibroblasts, called induced neuronal (iN) cells19,20,21. Novel methods of establishing ramified microglia from human somatic cells are strongly warranted, based on iPS or direct conversion techniques, while none have yet been reported. Herein, we show a novel technique for developing induced microglia-like (iMG) cells easily and quickly from adult human peripheral blood cells. In addition, by utilizing this iMG-technique, we present the first translational analysis of the dynamic actions of microglia from a patient of NHD.
Results
Inducing ramified microglia-like cells
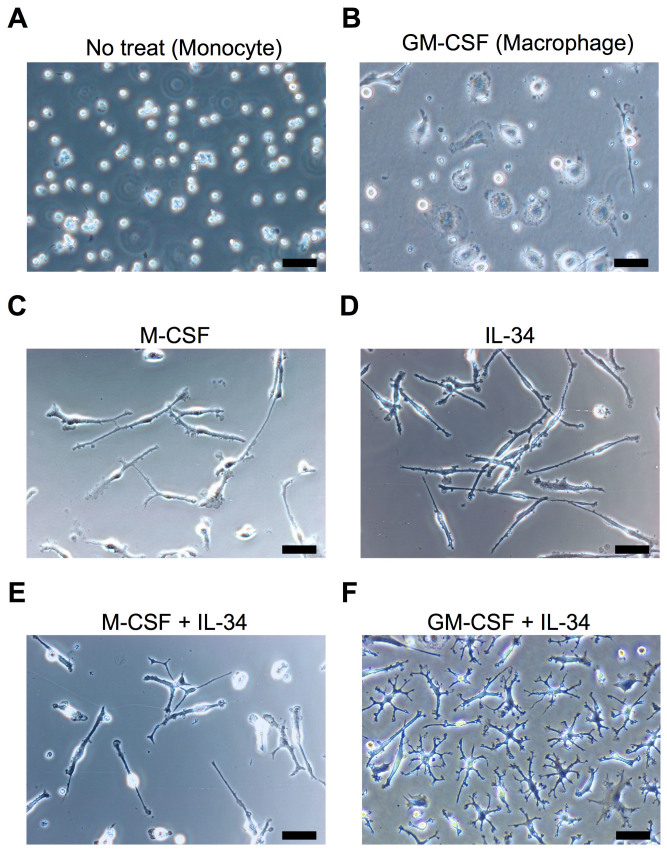
To determine what cytokines induce ramified microglia from human peripheral monocytes, we selected and tested the effects of the following candidate cytokines; granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF) and interleukin (IL) -34, all of which are suggested to be essential for developing and maintaining ramified microglia22,23,24,25. Untreated monocytes showed round shapes (Fig. 1A). Macrophages, induced by GM-CSF (10 ng/ml), shifted to an ameboid morphology on DAY 14 (Fig. 1B). On the other hand, treatment of M-CSF (10 ng/ml) alone or IL-34 (100 ng/ml) alone showed a spindle morphology (Fig. 1C and D), and the cocktail of both cytokines induced more complicated morphologies than the single treatment (Fig. 1E). Surprisingly, the cocktail of both GM-CSF (10 ng/ml) and IL-34 (100 ng/ml) induced small soma bodies bearing numerous branched collaterals (Fig. 1F), which expressed the specific morphology of ramified microglia- small soma with extensive radial ramifications. The viability of these cells post 14 days was 16.7% ± 4.2 (n = 3, mean ± SEM) as compared to the initial cell number (DAY 0). Interestingly, the earliest branched cells were observed on DAY 3 after GM-CSF and IL-34 treatment (Supplementary Fig. S1A). In addition, we confirmed that these cells survive at least one month when medium change was performed once a week (Supplementary Fig. S1B).
Figure 1. Inducing ramified microglia from human peripheral monocytes.
The monocytes on the day of isolation (A) were incubated with the following candidate cytokines; GM-CSF (10 ng/ml; B), M-CSF (10 ng/ml; C), IL-34 (100 ng/ml; D), M-CSF + IL-34 (E) and GM-CSF + IL-34 (F) for 14 days. The optimal cytokine conditions were tested by morphological changes using phase-contrast microscopy. The cocktail of both GM-CSF and IL-34 induced small soma body bearing numerous branched collaterals, which expressed the specific morphology of ramified microglia (F). Scale bar, 50 μm.
Phenotyping of the induced microglia-like (iMG) cells
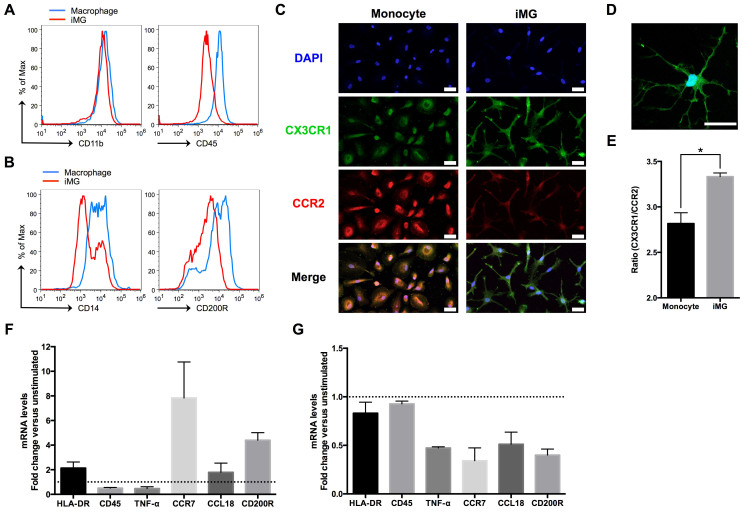
Next, we tested whether the ramified microglia-like cells, named induced microglia-like (iMG) cells, have microglial characterization. Generally, it is difficult to distinguish between macrophage and microglia, because useful and specific microglial markers are very limited. Traditionally, CD11b and CD45 are used as a distinction marker between macrophage and microglia26. Recently, the phenotype of human microglial cells, isolated from the fresh postmortem brain, has been revealed to have lower expression of CD14 and CD200R compared to macrophage27. Thus, we compared the expression level of surface markers between iMG cells and induced macrophage using flow cytometry. The expression level of CD11b on iMG cells did not differ from that on macrophage, while that of CD45 decreased on iMG cells (Fig. 2A). The expression levels of CD14 and CD200R were also decreased on iMG cells compared to those on macrophage (Fig. 2B), which support that iMG cells have the specific phenotype of microglia27. Furthermore, Mizutani et al.28 recently reported a clear-cut distinction between monocytes (CCR2high, CX3CR1low) and resident microglia (CCR2low, CX3CR1high) using CX3CR1+/GFPCCR2+/RFP knockin fluorescent protein reporter mice. Therefore, we compared the expression pattern of CCR2 and CX3CR1 between monocytes and iMG cells. Monocytes were stained with bright red fluorescence (CCR2) bearing round or elliptic morphology (Fig. 2C), and iMG cells were stained with bright green fluorescence (CX3CR1) bearing highly branched forms (Fig. 2, C and D). In addition, we confirmed that the expression ratio (CX3CR1/CCR2) of iMG cells is significantly higher than that of monocytes by flow cytometry (Fig. 2E). These results indicate that the iMG cells induced by GM-CSF and IL-34 show the essential characteristics of resident microglia28.
Figure 2. The iMG cells show the character of human resident microglia.
(A and B) The expression levels of surface markers on the iMG cells and induced macrophage were performed by flow cytometer. Peripheral monocytes were incubated with GM-CSF (macrophage) or cocktail of GM-CSF and IL-34 (iMG cells) for 14 days. The iMG cells showed the specific phenotypes of microglia compared to macrophage. (C to E) The expression pattern of CCR2 and CX3CR1 between monocytes and iMG cells were observed by immunocytochemistry. The monocytes and iMG cells were cultured for 14 days, and stained with specific antibodies. (C and D) The iMG cells were stained with bright green fluorescence (CX3CR1) bearing highly branched forms. Scale bar, 50 μm. (E) The expression ratio (CX3CR1/CCR2) of iMG cells was significantly higher than that of monocytes by flow cytometry (n = 3). The iMG cells were incubated with IL-4 (F) or dexamethasone (G) for 72 hours, and extracted RNA was analyzed by qRT-PCR (n = 6). Fold changes were depicted in mRNA levels after stimulation compared with unstimulated cells. *P < 0.05. Error bars, standard error of the mean (SEM).
Melief et al.27 have also revealed that IL-4 and dexamethasone alter specific gene expressions in fresh human microglial cells ([IL-4] HLA-DR, CCR7, CCL18, and CD200R are upregulated, and CD45 and TNF-α are downregulated; [dexamethasone] CCL18 is upregulated, and HLA-DR, CCR7, CD45, TNF-α, and CD200R are downregulated). Therefore, we assessed the above gene expression patterns in iMG cells incubated with IL-4 and dexamethasone using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Except for CCL18 (treated with dexamethasone), almost all gene expression patterns (HLA-DR, CCR7, CD200R, CD45 and TNF-α) in the iMG cells were in agreement with the above data using fresh human microglia27 (Fig. 2F, G).
Functional analysis of the iMG cells
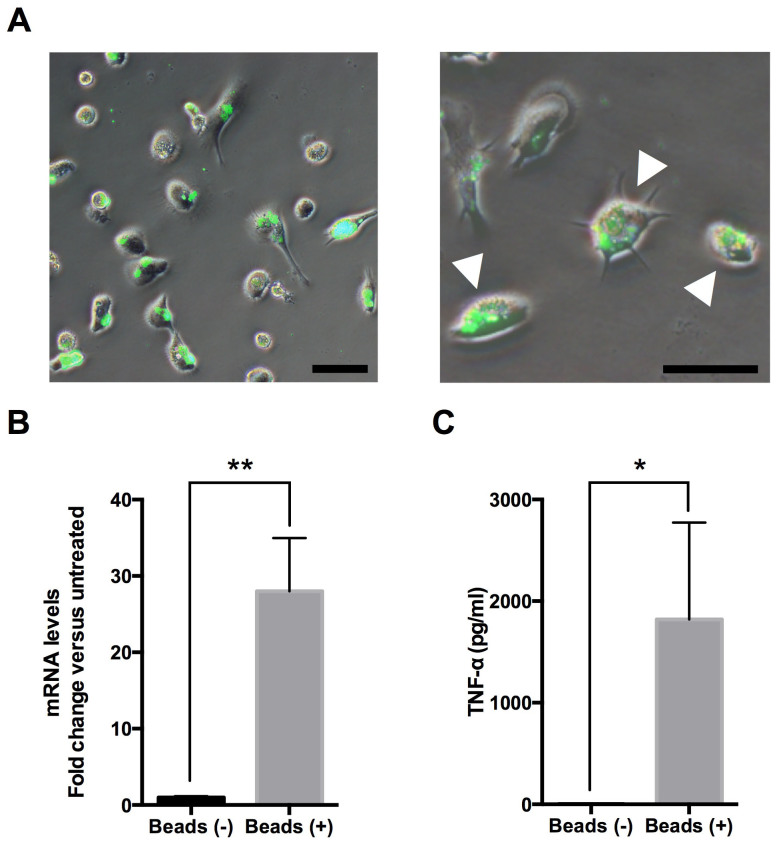
Microglia reside as a ramified form, and various molecules activate microglia into an ameboid form, phagocytizing and releasing various cytokines3, and overactivation of microglia induces neuronal damage and various brain pathologies via pro-inflammatory cytokines such as tumor necrosis factor (TNF) -α6,7. To examine whether iMG cells have these dynamic functions, we tested the phagocytosis ability and the following TNF-α secretion. Interestingly, the iMG cells showed the ability of phagocytosis with morphological changes into an ameboid form (Fig. 3A). Next, we tested the ability for TNF-α production during phagocytosis on the iMG cells, and revealed that the mRNA expression and protein level of TNF-α on the iMG cells during phagocytosis are significantly higher compared to those on non-treated cells (Fig. 3, B and C).
Figure 3. Dynamic functional analysis of the iMG cells.
(A) The iMG cells were incubated with FITC-conjugated latex beads for 24 hours, and phagocytic activity was observed by fluorescent microscopy. The iMG cells showed the ability of phagocytosis with morphological changes into an ameboid form (arrow head). Scale bar, 50 μm. (B and C) The ability of TNF-α production during phagocytosis was measured on the iMG cells. The iMG cells were incubated with latex beads for 72 hours. The extracted RNA and culture supernatant were analyzed by qRT-PCR and Cytometric Beads Array System (CBA), respectively. The mRNA expression (B) and protein level of TNF-α (C) on the iMG cells were significantly higher compared to controls (B, n = 4; C, n = 6). *P < 0.05, **P < 0.01. Error bars, SEM.
Analysis of the iMG cells from a patient of NHD
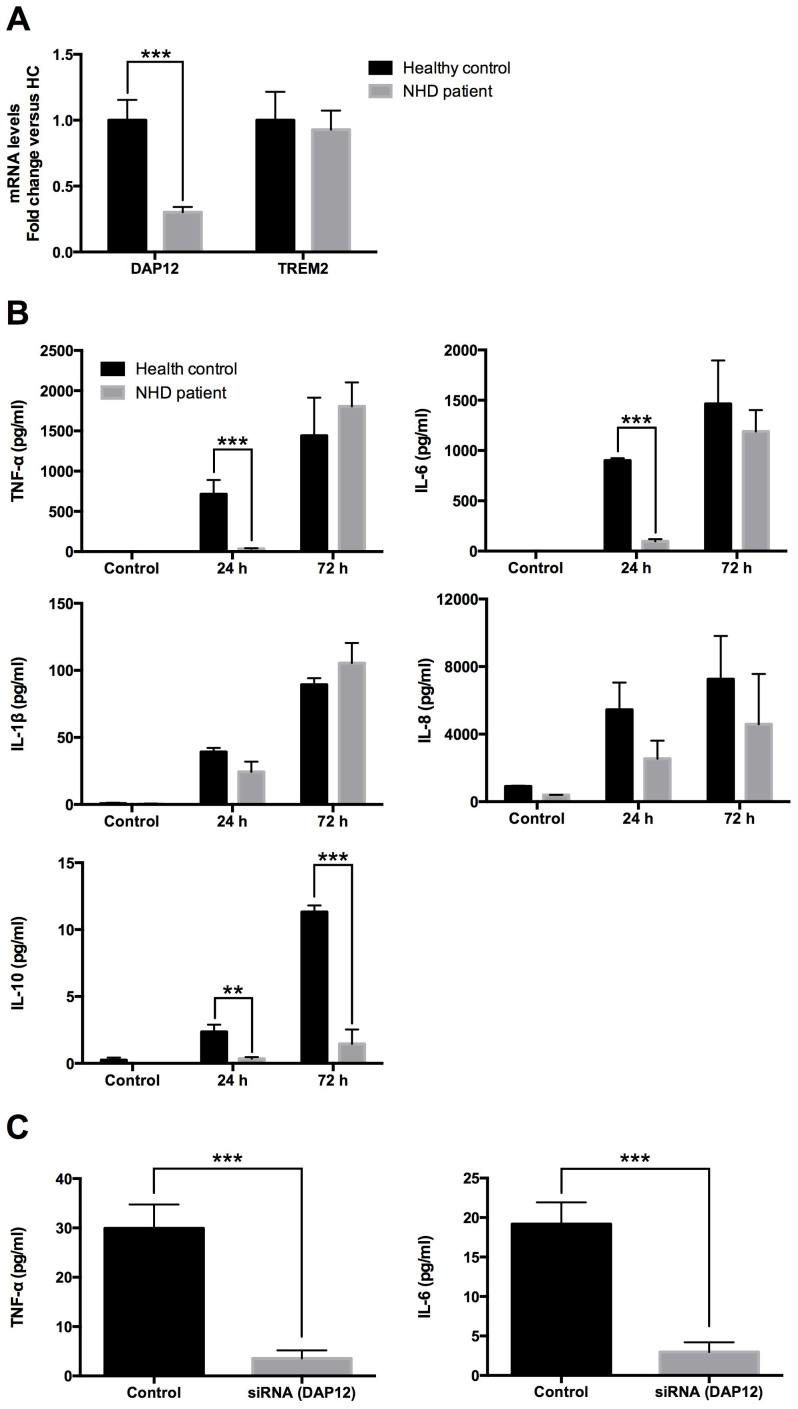
The above results demonstrated that the iMG cells have the dynamic functions of human microglia, and we suggest that iMG cells have the possibility to be utilized for analyzing the underlying microglial pathophysiology of brain disorders. As the initial step, we conducted the first translational analysis of the iMG cells derived from a patient of NHD. NHD is believed to be caused by microglial dysfunction, while no investigation exists using living human microglial cells from patients of NHD. We analyzed the dynamic functions of microglia using the iMG cells from a patient of NHD (141delG in DAP12 gene), after obtaining informed consent (under the permission of the Institutional Review Board of Kyushu University and Osaka University). In agreement with genetic diagnosis, the iMG cells from the NHD patient showed significantly lower expression of DAP12 than those from a healthy control, and there was no difference in TREM2 expression (Fig. 4A). Interestingly, the production of pro-inflammatory cytokines (TNF-α and IL-6) was delayed in the iMG cells from the NHD patient as compared to those from the healthy control after 24 hours. Furthermore, the iMG cells from the patient showed a significantly lower level of anti-inflammatory cytokine (IL-10) than those from the healthy control. The production levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-8) had no significant differences between the NHD and healthy control after 72 hours (Fig. 4B). Next, we examined whether it is possible for iMG cells from the healthy control to silence the target gene with siRNA (DAP12). DAP12 was successfully downregulated in the iMG cells from the healthy control (Supplementary Fig. S2). We assessed the cytokine production of iMG cells treated with siRNA. Predictably, the production of pro-inflammatory cytokines (TNF-α and IL-6) was delayed in the DAP12-silenced iMG cells as compared to the control (Fig. 4C).
Figure 4. Dynamic functional analysis of the iMG cells from a patient of NHD.
(A) The iMG cells from the NHD patient showed significantly lower gene expression of DAP12 compared to those from the healthy control (n = 6). (B) Cytokine production from the iMG cells were compared between the NHD patient and the healthy control. The iMG cells from the NHD patient and the healthy control were incubated with latex beads for 24 or 72 hours, and culture supernatants were analyzed by CBA. In the iMG cells from the NHD patient, the production of pro-inflammatory cytokines (TNF-α and IL-6) was delayed, and that of anti-inflammatory cytokine (IL-10) was decreased (n = 4). (C) The effects of DAP12 silencing on the proinflammatory cytokine production. The iMG cells treated with siRNA were incubated with latex beads for 24 hours, and culture supernatants were analyzed by CBA In the iMG cells treated with siRNA, the production of pro-inflammatory cytokines was delayed (n = 8) **P < 0.01, ***P < 0.001. Error bars, SEM.
Discussion
We have shown a novel technique for developing directly induced microglia-like cells from human peripheral blood cells. GM-CSF and IL-34 converted human monocytes into iMG cells within 14 days. The iMG cells have microglial characterizations; expressing markers, forming a ramified morphology, and phagocytic activity with various cytokine releases. Until now, some attempts to induce microglia-like cells from hematopoietic cells have been performed using an astrocyte co-culture system or astrocyte-conditioned media29,30, and GM-CSF and IL-34 are known to be derived from astrocytes30,31. Especially, IL-34 has recently come to be known as a key molecule for the proliferation of microglia32. The present data have suggested that both GM-CSF and IL-34 from astrocytes are the minimum essential inducing-factors for microglia-like cells from hematopoietic cells.
The present results indicate that the iMG cells from a patient of NHD show slower (24 h) but not weaker (72 h) pro-inflammatory cytokines' responses compared to those from the healthy control, possibly due to the deletion of DAP12. In addition, suppression of IL-10 production from the iMG cells from the NHD patient indicates that human brain microglia of NHD patients tend to be shifted to pro-inflammatory reactions compared to those of healthy controls. Furthermore, the situation observed in the iMG cells from a NHD patient was replicated with iMG cells from a healthy control using siRNA. These data have suggested that DAP12 expression is a key factor in from the perspective of microglial immunoresponse such as cytokine production in NHD patients. In the present study, we examined the iMG cells from a female patient of NHD. Recent studies have suggested microglial functional differences between sexes33. Further investigations should focus on sex differences related to microglial dysfunction of NHD. DAP12 and TREM2 are the responsible genes of NHD, which mediate various important roles such as phagocytosis and cytokine production in osteoclasts, macrophages, dendritic cells and microglia34. A rodent study showed that deletion of DAP12 induces synaptic impairments due to microglial dysfunction15. Hamerman et al.35 demonstrated that macrophage from DAP12-deficient mice increase inflammatory cytokines' responses, which suggest that DAP12-deleted microglia increase similar inflammatory response. These previous reports and our present findings based on the iMG cells from a NHD patient suggest that human NHD microglia has the potential to induce stronger and long-acting pro-inflammatory reactions compared to those of healthy human subjects.
In sum, we have shown a novel technique of developing directly induced microglia-like cells, named “iMG cells”, with a combination of GM-CSF and IL-34 from adult human monocytes, easily and quickly without any virus, feeder cells, and genetic engineering. The iMG cells proved to have many characterizations of microglial cells, such as expressing CD11bhigh/CD45low and CX3CR1high/CCR2low. Moreover, the iMG cells expressed dynamic functions such as phagocytosis and releasing pro- and anti- inflammatory cytokines. Further investigations such as microarray analysis should be conducted to validate the closeness of iMG cells to human primary microglial cells in the brain. Finally, we presented the translational utilities of the iMG cells for analyzing the underlying microglial pathophysiology of NHD. We believe that this novel technique will shed new light on solving unknown dynamic aspects of human microglial cells in various brain disorders.
Methods
Subjects
The present study was conducted in accordance with the World Medical Association's Declaration of Helsinki and was approved by the Ethics Committee of the Graduate School of Medical Sciences, Kyushu University and Osaka University. We recruited a middle-aged female patient, who was diagnosed with Nasu-Hakola disease (141delG in DAP12 gene) in her thirties. Based on informed consents both from the patient and a family member, we took a blood sample. Healthy adult volunteers including an age-matched female were also recruited.
Induction of induced microglia-like (iMG) cells from human peripheral blood
Peripheral blood was collected using a heparinized tube from healthy adult volunteers and a patient of NHD. Peripheral blood mononuclear cells (PBMC) were isolated by Histopaque-1077 (Sigma Chemical Co., St. Louis, MO) density gradient centrifugation. PBMC were resuspended with RPMI-1640 (Nacalai Tesque, Kyoto, Japan), 10% heat-inactivated fetal bovine serum (FBS; Endotoxin = 0.692 EU/ml; Japan Bio Serum, Hiroshima, Japan) and 1% antibiotics/antimycotic (Invitrogen, Carlsbad, CA). PBMC were plated onto culture chambers at a density of 4 × 105 cells/ml and cultured overnight in standard culture conditions (37°C, 5% CO2). After overnight incubation, culture supernatant and non-adherent cells were removed. The adherent cells (monocytes) were cultured with RPMI-1640 Glutamax (Invitrogen) supplemented with 1% antibiotics/antimycotic and a mixture of the following candidate cytokines; recombinant human GM-CSF (10 ng/ml; R&D Systems, Minneapolis, MN), recombinant human IL-34 (100 ng/ml; R&D Systems) and M-CSF (10 ng/ml; Peprotec, Rocky Hill, NJ) in order to develop iMG cells. We also developed induced macrophage from human monocytes; monocytes were cultured with RPMI-1640 Glutamax supplemented with 1% antibiotics/antimycotic and recombinant human GM-CSF (10 ng/ml). All cells were cultured in standard culture conditions for up to 14 days.
Cell morphology
Morphological changes of cytokines-treated cells were examined using phase-contrast microscopy (TS100-F; Nikon Instech, Tokyo, Japan). Images were taken with a DS-Vi1 digital camera (Nikon Instech) and a DS-L3 control unit (Nikon Instech).
Flow cytometry
Flow cytometry was performed using a FACS Aria (BD Biosciences, Bedford, MA) with FACS Diva software (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, San Carios, CA). For iMG phenotyping, fluorochrome conjugated monoclonal antibodies specific for human CD11b (APC-Vio770; Miltenyi Biotec, Gladbach, Germany), CD14 (FITC; Sigma), CD45 (PE; Miltenyi Biotec) and CD200R (Alexa647; Serotec, Oxford, UK) were used. Induced macrophage and iMG cells were cultured in 6-well plates (Corning, NY) at a density of 4 × 105 cells/ml. Cells were harvested by non-enzymatic cell dissociation solution (Sigma) and cell lifter (Corning). The cells were washed with MACS buffer (Miltenyi Biotec) and incubated for 5 minutes at 4°C in FcR-blocking reagent (Miltenyi Biotec). Antibodies were incubated with cell suspension for 30 minutes at 4°C, washed with calcium-magnesium-free phosphate-buffered saline (PBS(-)), resuspended and fixed with 1% paraformaldehyde (Wako, Osaka, Japan) in PBS(-). The fluorescence intensity of the cells was measured.
Indirect immunofluorescence for flow cytometry was performed using the following antibodies: rabbit anti-CX3CR1 antibody (Immuno-Biological Laboratories, Gunma, Japan) and mouse anti-CCR2 antibody (R&D Systems). The monocytes and iMG cells were treated with the same process until the primary antibody staining. After primary staining, washed with MACS buffer and were stained with Alexa488- or Alexa546-conjugated secondary antibodies (Invitrogen). The ratio of CX3CR1 to CCR2 was calculated by the fluorescent intensity of each fluorochrome.
Immunocytochemistry
In immunocytochemistry, iMG cells and monocytes were cultured in 8-well chambers (LabTec chamber slide system; Nalge Nunc International, Rochester, NY) at a density of 4 × 105 cells/ml. These cells were fixed with 4% paraformaldehyde (Wako) for 20 minutes and then rinsed thrice with PBS(-) for 5 minutes. Indirect immunofluorescence was performed using the following antibodies: rabbit anti-CX3CR1 antibody (1:500 dilution; Immuno-Biological Laboratories, Gunma, Japan) and mouse anti-CCR2 antibody (1:500 dilution; R&D Systems). Cells were incubated in primary antibodies diluted in 0.1% Triton-X 100 in PBS containing 5% normal goat serum at 4°C overnight. After rinsing thrice with PBS(-) for 5 min, Alexa488- or Alexa546-conjugated secondary antibodies (Invitrogen) were used for detection. Fluorescent images were taken with a confocal laser scanning microscope (LSM-780; Carl Zeiss, Jena, Germany).
Quantitative real time-polymerase chain reaction (qRT-PCR)
To assess the gene expression patterns in iMG cells after the treatment of IL-4, dexamethasone or during phagocytosis, we performed qRT-PCR using a LightCycler 480 system (Roche Diagnostics, Mannheim, Germany). IL-4 (40 ng/ml; Peprotec), dexamethasone (2 nM; Sigma) or latex beads-rabbit IgG-FITC solution (Cayman Chemical) was added to the iMG cells and incubated for 72 hours in standard culture conditions. After incubation, the iMG cells were washed and the total RNA was extracted using a High Pure RNA Isolation kit (Roche Diagnostics) according to the manufacturer's protocol, and subjected to cDNA synthesis using a Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics). qRT-PCR for HLA-DR, CD45, TNF-α, CCR7, CCL18 and CD200R was performed using each primer (Supplementary Table 1). Beta 2-microglobulin of Universal ProbeLibrary (Roche Diagnostics) was used as a house-keeping control gene. Fold changes were depicted in mRNA levels after stimulation compared with unstimulated cells.
Using the iMG cells from a female NHD patient and an age-sex matched healthy control, we examined the gene expression of DAP12 and TREM2 by qRT-PCR. The iMG cells were washed and the total RNA was extracted respectively, and qRT-PCR was performed using each primer (Supplementary Table 1). Beta 2-microglobulin was used as a house-keeping control gene. Fold changes were depicted in mRNA levels after stimulation compared with unstimulated cells.
Phagocytosis
Phagocytosis was examined by fluorescent microscopy using Phagocytosis Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. The iMG cells were cultured in 8-well chambers (Nalge Nunc International) at a density of 4 × 105 cells/ml. We added 50 μl of the latex beads-rabbit IgG-FITC solution to each well of the chamber, and incubated the cells in standard culture conditions for 24 hours. After discarding the supernatant by careful aspiration, we quenched surface-bound fluorescence, added 125 μl of trypan blue solution to each well of the chamber, and incubated for two minutes at room temperature. Each well was analyzed by using a fluorescence microscope (Olympus IX-71, Tokyo, Japan) and DP71 digital camera system (Olympus).
Cytokine measurement
Secretion of pro- and anti- inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8 and IL-10) during phagocytosis was measured from culture supernatants using Cytometric Beads Array System (CBA; BD Biosciences) according to the manufacturer's protocol. Latex beads-rabbit IgG-FITC solution (Cayman Chemical) was added to the iMG cells and incubated for 24 or 72 hours in standard culture conditions. After incubation, culture supernatants were centrifuged at 10000 × g for 10 minutes and kept frozen at −80°C until assayed. The culture supernatants were incubated with the cytokine capture beads and PE-conjugated detection antibodies for 3 hours at room temperature. Afterwards, the capture beads were washed and measurement data were acquired using a FACS Canto™ flow cytometer (BD Biosciences). The data analysis was performed using FCAP Array software (BD Biosciences).
Gene silencing of DAP12
Gene silencing assay was performed using siRNA (DAP12; Santa Cruz, USA) and RNAiMAX (Invitrogen) according to the manufacturer's protocol. The mix solution of siRNA and RNAiMAX was added to the iMG cells. After overnight incubation, the medium was changed to the culture medium and incubated for 48 hours. The siRNA-modified iMG cells were used for cytokine assay.
Statistical analysis
Analysis of comparisons between groups were conducted by two-tailed Student's t-test.
Author Contributions
All authors contributed substantially to the scientific process leading up to the writing of the present paper. T.A.K., the principal investigator of the present research, and M.O., the first author, created the conception and design of the project and wrote the protocol. The performance of experiments and the data analysis/interpretation were performed by M.O., T.A.K., D.S., N.S., K.H. and N.S. M.O. wrote the first draft of the manuscript. Clinical recruitments were conducted by R.H., K.S., T.Y., K.H. and N.S. Critical revisions of the manuscript were made by T.A.K., D.M., H.U. and S.K. All authors have approved to submit the final manuscript.
Supplementary Material
Supplementary Data
Acknowledgments
The authors would like to thank Ms. Mayumi Tanaka and Ms. Mayumi Inenaga for their technical assistances. We appreciate the technical support from Department of Dermatology (Prof. Masutaka Furue) and the Research Support Center, Graduate School of Medical Sciences, Kyushu University. This work was supported by Grant-in-Aid for Scientific Research on (1) the Japan Society for the Promotion of Science (to TAK,MO and SK), (2) Innovative Areas ‘‘Glia Assembly’’ of The Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 25117011 to SK), (3) the Health and Labour Sciences Research Grant (No. (H24-Seishin-Jitsuyouka (Seishin)-Ippan-001 to SK), (4) Young Principal Investigators' Research Grant of Innovation Center for Medical Redox Navigation, Kyushu University (to TAK), (5) Takeda Science Foundation – Medical Research (to TAK), and (6) SENSHIN Medical Research Foundation (to TAK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Ginhoux F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F. & Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Hanisch U. K., Noda M. & Verkhratsky A. Physiology of microglia. Physiol Rev 91, 461–553 (2011). [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. & Perry V. H. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27, 119–145 (2009). [DOI] [PubMed] [Google Scholar]
- Graeber M. B. Changing face of microglia. Science 330, 783–788 (2010). [DOI] [PubMed] [Google Scholar]
- Block M. L., Zecca L. & Hong J. S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8, 57–69 (2007). [DOI] [PubMed] [Google Scholar]
- Hanisch U. K. & Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10, 1387–1394 (2007). [DOI] [PubMed] [Google Scholar]
- Kato T. A. et al. Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. Curr Med Chem 20, 331–344 (2013). [DOI] [PubMed] [Google Scholar]
- Hakola H. P. Neuropsychiatric and genetic aspects of a new hereditary disease characterized by progressive dementia and lipomembranous polycystic osteodysplasia. Acta Psychiatr Scand Suppl 232, 1–173 (1972). [PubMed] [Google Scholar]
- Nasu T., Tsukahara Y. & Terayama K. A lipid metabolic disease-“membranous lipodystrophy”-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol Jpn 23, 539–558 (1973). [DOI] [PubMed] [Google Scholar]
- Kaneko M., Sano K., Nakayama J. & Amano N. Nasu-Hakola disease: The first case reported by Nasu and review. Neuropathology 30, 463–470 (2010). [DOI] [PubMed] [Google Scholar]
- Paloneva J. et al. CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology 56, 1552–1558 (2001). [DOI] [PubMed] [Google Scholar]
- Paloneva J. et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet 25, 357–361 (2000). [DOI] [PubMed] [Google Scholar]
- Paloneva J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71, 656–662 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A. et al. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci 24, 11421–11428 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J. et al. Immunohistochemical characterization of microglia in Nasu-Hakola disease brains. Neuropathology 31, 363–375 (2011). [DOI] [PubMed] [Google Scholar]
- Mattis V. B. & Svendsen C. N. Induced pluripotent stem cells: a new revolution for clinical neurology? Lancet Neurol 10, 383–394 (2011). [DOI] [PubMed] [Google Scholar]
- Dimos J. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008). [DOI] [PubMed] [Google Scholar]
- Pfisterer U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 108, 10343–10348 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L. et al. Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell 146, 359–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pang Z. P. et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13, 753–760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F., De Simone R., Columba-Cabezas S., Penna G. & Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol 164, 1705–1712 (2000). [DOI] [PubMed] [Google Scholar]
- Erblich B., Zhu L., Etgen A. M., Dobrenis K. & Pollard J. W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6, e26317 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S. et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol 367, 100–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D. et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88, 7438–7442 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief J. et al. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia 60, 1506–1517 (2012). [DOI] [PubMed] [Google Scholar]
- Mizutani M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol 188, 29–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze A. et al. Microglia differentiation using a culture system for the expansion of mice non-dadherent bone marrow stem cells. J Inflamm 9, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto D. et al. Development of a culture system to induce microglia-like cells from haematopoitic cells. Neuropathol Appl Neurobiol. 10.1111/nan.12086. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilemini G. et al. Granulocyte macrophage colony stimulating factor stimulates in vitro proliferation of astrocytes derived from simian mature brains. Glia 16, 71–80 (1996). [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D. et al. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci 33, 2481–2493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. M. & Bilbo S. D. Sex, glia, and development: interactions in health and disease. Horm Behgav 62, 243–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradowska-Gorycka A. & Jurkowska M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum Immunol 74, 730–737 (2013). [DOI] [PubMed] [Google Scholar]
- Hamerman J. A., Tchao N. K., Lowell C. A. & Lanier L. L. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol 6, 579–586 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data