Abstract
Paralleling the overall trend in allergic diseases, Eosinophilic Esophagitis is rapidly increasing in incidence. It is associated with food antigen-triggered, eosinophil-predominant inflammation and the pathogenic mechanisms have many similarities to other chronic atopic diseases, such as eczema and allergic asthma. Studies in animal models and from patients over the last 15 years have suggested that allergic sensitization leads to food-specific IgE and T-helper lymphocyte type 2 cells, both of which appear to contribute to the pathogenesis along with basophils, mast cells, and antigen-presenting cells. This review will outline our current understandings of the allergic mechanisms that drive eosinophilic esophagitis, drawing from clinical and translational studies in humans as well as experimental animal models.
Keywords: Eosinophilic Esophagitis, Allergic Mechanism, Atopic, Antigen Sensitization, Th2 Immunity, Pathogenesis
Introduction
Chronic tissue infiltration of eosinophils is the hallmark of allergic inflammatory diseases, which include asthma, atopic dermatitis, and eosinophilic gastrointestinal diseases (EGIDs). Eosinophilic esophagitis (EoE), a type of EGID, is characterized by eosinophil-predominant inflammation isolated to the esophagus, the only organ in the gastrointestinal tract that is homeostatically devoid of eosinophils. There is significant evidence of a role for allergic mechanisms as the driving force in EoE. The foundation for this understanding began almost 20 years ago, when Kelly et al. demonstrated that children with EoE resolved inflammation and clinical symptoms on amino acid-based diets.1 EoE is strongly linked to atopic disease and most often occurs co-morbidly with asthma, eczema, allergic rhinitis, and anaphylactic food allergy, and a strong family history of such disorders is equally as common.2, 3 The tissue inflammatory response in EoE is similar to other allergic inflammatory disorders and is characterized by a dysregulated immune response with both IgE-mediated and non-IgE-mediated T-helper cell type 2 (Th2) responses. Cross-sensitization to aeroallergens appears to be common and recent literature has begun to define antigen presentation, as well as the role of mast cells and basophils. This review will examine our understanding of the allergic mechanisms involved in the pathogenesis of EoE based on clinical and translational studies in humans as well as experimental models in genetically modified animals.
Body
Clinical Associations Of Eosinophilic Esophagitis With Allergic Diseases
Despite being frequently managed by gastroenterology specialists, there are several unique features of EoE that reflect the allergic nature of this disorder. During the last few decades, the prevalence of allergic diseases has been increasing at an incredible rate: in Westernized countries over a quarter of the population now has allergic eczema, allergic rhinitis, or food allergy.4 Paralleling this trend, epidemiologic studies show an increase in the number of children and adults with EoE.5, 6 In 2006, the prevalence of pediatric EoE in Australia was estimated to have increased 18-fold over the previous 10 years, while a US study reported a 35-fold increase over a similar time period.7, 8 This increasing prevalence is supported by a rising incidence; population-based data from Olmsted County, MN, USA shows the incidence of EoE increased from 0.35 to 9.45 per 100,000 persons from 1991–1995 to 2001–2005.9 Similarly, a population-based study of pediatric and adult EoE patients in Switzerland reported increased incidence from two to six per 100,000 persons over the period from 1989 to 2004,10 while findings from the Netherlands demonstrated an increased incidence from 0.01 per 100,000 in 1996 to 1.31 per 100,000 in 2010.5 This worldwide increase in EoE mirrors the trends in atopic diseases, although whether this is due to a true rise in disease incidence, increased disease recognition, or improved access to endoscopy remains unclear.11
Beyond these parallels in disease incidence, several clinical studies have demonstrated significant co-morbidity of EoE with other atopic diseases. While the prevalence of co-morbid atopic disease varies between these studies, perhaps due to regional population differences, they demonstrate that the majority (50–80%) of patients with EoE also have atopy and other allergic diseases, including rhinitis, asthma or eczema.12 Interestingly, while these allergic diseases can commonly be seen to follow the “atopic march”, whereby early in life appearance of eczema and food allergy are observed and predispose for rhinitis and asthma several years later, EoE displays a profoundly broad age range of onset and so seems to not adhere with this established concept. However, the strong clinical links between EoE and other well-described allergic diseases seems to imply shared pathogenic mechanisms.
Hypersensitivity in Eosinophilic Esophagitis: Immediate or Delayed?
According to the NIAID-convened “Guidelines for the diagnosis and management of food allergy in the United States”,13 food allergy was broadly defined as an abnormal immunologic hypersensitivity response to specific food proteins that leads to adverse clinical reactions. Within this definition, EoE is clearly an example of food antigen-driven hypersensitivity. However, hypersensitivity reactions can be further classified based on the mechanisms of antigen recognition: IgE (immediate-type) or the T-cell receptor (delayed-type).
In immediate-type IgE-mediated hypersensitivity, typically associated with anaphylaxis or urticaria, cross-linking of IgE and its receptor by antigen leads to the rapid release of pre-formed mediators from mast cells and basophils. Histamine is a key molecule in this process and histamine receptors are important in regulating the physiological responses.14 This early antigen-specific rapid response is followed by the subsequent de novo synthesis and release of lipid mediators, cytokines and chemokines that can drive a late phase response in some individuals, with infiltration of inflammatory cells, including eosinophils.
The majority of EoE patients have compelling evidence of IgE-mediated hypersensitivity to foods, as determined by elevated food-specific IgE or abnormal skin prick test (SPT), despite food-induced anaphylaxis only occurring in around 15% of these patients.2, 15, 16 Mechanistically, it has been shown that IgE-bearing mast cells are increased in the esophageal mucosa of EoE patients, particularly those that are atopic.17, 18 Thus, it may be that the immediate hypersensitivity response in EoE occurs in a localized fashion exclusively in the esophagus, similar to what is seen in oral allergy syndrome. While the involvement of IgE-mediated activation of mast cells in responses in the esophagus of EoE patients remains to be defined, the early phase reaction could enhance blood flow and muscle contractility via release of histamine,19, 20 while the late phase reaction could contribute to the recruitment of eosinophils, similar to processes that have been noted in allergen-induced eosinophil recruitment in atopic dermatitis.21
While the role of IgE-mediated hypersensitivity remains somewhat unclear, non-IgE-mediated reactions are increasingly understood to participate in EoE. These delayed-type reactions, often referred to as T-cell mediated hypersensitivity, are characterized by the activation of antigen-specific T-cells and subsequent recruitment of inflammatory cells. Delayed-type hypersensitivity (DTH) associated with allergic inflammatory disease is classically characterized by a Th2-predominant immune response, with elevated IL-4, IL-5, and IL-13, along with eosinophilic inflammation.22 In clinical diagnosis, patch testing, whereby antigen is applied to the skin so as to elicit a DTH-associated response, has been shown to significantly improve predictive values over SPT alone, highlighting the likely contribution of this arm of the immune response in EoE patient responses.23 Interestingly, the IgE and T cell-mediated arms may intersect, since IgE has been shown to enhance DTH responses in mice.24
Allergic Sensitization
Dependent on IgE or T-cells?
The loss of tolerance and subsequent sensitization to antigen are critical events in the initiation of allergic conditions, involving coordinated involvement of antigen-presenting cells, T-cells, and B-cells, to prime the adaptive immune system for subsequent responses to antigen exposures. In particular, allergic sensitization associates with the generation of allergen-specific T helper type (Th)2 cells which proliferate and differentiate into antigen-specific effector and memory T-cells. In addition, these Th2 cells play a critical role in B-cell production of allergen-specific IgE, through their ability to generate IL-4.
In EoE, allergic sensitization is clearly evident: regardless of atopic status, patients with EoE have increased density of B-cells and expression of IgE in the esophagus along with evidence of local class-switching.25 Specific IgEs for foods that trigger active disease are commonly detected in EoE patients in the absence of anaphylaxis, although they may be present at low levels, perhaps reflecting local production.26 Importantly, peripheral blood mononuclear cells from EoE patients exhibit allergen-specific cytokine responses that correlate with this elevation in specific IgE (although some patients have allergen-specific cytokine responses without elevated specific IgE, consistent with non-IgE mediated allergic sensitization).27 In addition, mouse models of EoE-like disease, whereby sensitization is elicited via cutaneous or respiratory allergen exposure, show increased antigen-specific IgE, and a clear dependency on T-cells, but are still able to traffic eosinophils to the esophagus in the absence of either B cells or IgE.28, 29 Thus, allergic sensitization in EoE drives the formation of allergen-specific IgE and T-cells, however they potentially exhibit, independent roles in the underlying disease pathogenesis.
Tolerance
In animal models, allergic sensitization commonly occurs to an antigen for which the animal is naïve; however, in EoE where the mean age at diagnosis is 33.5 years, it is interesting to note that sensitization often occurs to antigens already in the diet or environment that have been tolerated.30 This suggests that loss of tolerance may be critical to facilitate the subsequent immune sensitization. A number of early life risk factors have been found for EoE, including antibiotic use in infancy, cesarean delivery, preterm birth, and lack of breast-feeding,31 all of which have been suggested by other studies to alter the development of tolerance. One critical cell type that appears to play a role in the maintenance of tolerance is the regulatory T-cell (Treg), characterized by expression of Foxp3. In EoE, an imbalance between effector and regulatory T-cells was shown by Stuck et al., who found the proportion of Foxp3+CD3+ T-cells was 50% reduced in EoE compared to healthy controls.32 Using an intranasal aeroallergen-induced murine model of EoE, Zhu et al. found a similar alteration in the frequency of CD4+ T cell subsets in the esophagus of allergen-challenged mice compared with saline-challenged mice.33 Not surprisingly, it was FOXP3+ cells that were critically reduced among these cell populations. While these studies suggest there may be a relative lack of Treg-type T-cells at the site of inflammation, it remains to be determined whether this alteration in regulatory T-cells has simply a sustaining effect in EoE or whether Treg imbalances are critical to promoting allergic sensitization.
Route of Sensitization
An interesting question that remains in EoE is the site at which allergic sensitization occurs. To date, clinical studies of early life risk factors have not supported a specific route of sensitization in EoE. However, a variety of animal models of allergic disease have demonstrated that allergic sensitization can occur via the skin, airway, or gut.28, 34, 35 Experimental animal models of EoE suggest that sensitization may not require esophageal allergen exposure. Akei et al. found that epicutaneous antigen sensitization in the form of repeated allergen administration to the shaved back of mice followed by an intranasal allergen challenge, facilitated an EoE-like disease that was IL-5-dependent and, to a lesser extent, IL-4 and IL-13 dependent.36 More recently, Noti et al. found that sensitization to egg or peanut protein could occur during skin inflammation or injury (tape stripping) in a TSLP-dependent, basophil-dependent, and IgE-independent manner.28 Sensitization also appears to be effective via the lungs as repeated exposure to aeroallergens via intranasal administration led to increase antigen-specific IgG1 and was sufficient to induce eosinophilic inflammation in the esophagus.35 Interestingly, neither intragastric nor oral administration of aeroallergens was sufficient to induce EoE-like disease. It is likely therefore that antigen sensitization can occur at several sites including lung and skin and likely depends on a variety of host and environmental factors, but will require further studies to determine what is clinically relevant in EoE.
Aeroallergens
A significant number of EoE patients show evidence of polysensitization, not just to multiple foods but also to environmental aeroallergens.37 While the functional role of allergic sensitization to aeroallergens is not entirely clear, there is evidence both in animal models and clinically that it may participate in driving elements of the EoE disease. Several studies have described seasonal variation in a subset of patients and in whom disease worsening occurred during pollen season regardless of therapy.38, 39 It remains unclear whether swallowed aeroallergen directly promotes active disease or inhaled aeroallergen exacerbates concomitant airway disease, as could be interpreted from the murine studies with intranasal aeroallergen. Primary allergic sensitization to aeroallergens may also contribute to food sensitization as a result of cross-reactivity or cross-sensitization. Detection of this phenomenon has become possible with the use of protein microarrays, which allow for simultaneous assessment of specific IgE antibodies against multiple recombinant or purified natural allergen components, so-called component-resolved diagnostics. Using this technique, Simon et al. found IgE against food-specific allergen components were rare in Swiss EoE patients while cross-reactive responses were common.40 They noted the dominant pattern of cross-reactivity was to profilins, pathogenesis-related (PR)-10 and lipid transfer proteins (LTP). These findings were validated by a group in the Netherlands who also found that the majority of food sensitizations in EoE patients are a result of cross-sensitization to PR-10 proteins present in birch pollen.41 Interestingly these proteins can pass through the esophagus intact, but are degraded in the stomach, which could limit inflammation to the esophagus. Thus, sensitization to aeroallergens, which can occur via the skin or airway, may be a significant factor in the development of EoE and explain some of the co-morbidity with atopic disease.
Antigen Presentation in the Esophagus
Antigen presentation plays a crucial role in initiating a highly specific immune response to a foreign protein. Antigen presenting cells (APCs) engulf, process, and display peptides coupled to major histocompatibility complex (MHC) class II peptides on their cell membrane. Cell surface co-stimulatory molecules also help determine whether presented antigen provokes an immunogenic or tolerogenic T-lymphocyte response. In EoE, the mechanistic understanding of this process is quite limited, but it appears that both professional APCs such as dendritic cells (DCs) and non-professional APCs such as epithelial cells play a role.
The primary professional APC in the esophagus seems to be the Langerhans cell, a type of dendritic cell found in all squamous epithelia,42 particularly the epidermis. Langerhans cells of the esophagus are structurally similar to those in the skin and are located along the papillae of the lamina propria and in the suprabasal region.43 These myeloid DCs, identified by the surface marker CD1a, are increased in density in children with EoE as compared to controls and are reduced post-treatment.44 However, there is contradictory data from a study in adults with EoE, which found no differences in CD1a density pre/post-treatment or with controls.45 This may be due histopathologic variability or may represent a key etiologic difference between children and adults with EoE. Additionally, Langerhans cells of the upper GI tract express the high-affinity IgE receptor, FcεRI,46 for which expression increases in active EoE.47 IgE signaling on APCs has been proposed to enhance antigen uptake and enhance the development and activation of allergen-specific T-cells.48 While the role of Langerhans cells in EoE is unclear, they are critical to the pathogenesis of atopic diseases such as eczema, and their close proximity to T-cells in the esophagus suggests a possible interaction and pathologic function.
Under pathological conditions, epithelial cells at mucosal surfaces act as nonprofessional APCs, and can regulate immune responses at the site of exposure. Antigen presentation by small bowel epithelium is well established and likely plays a role in food hypersensitivity.49, 50 Mulder et al. found that basal epithelial cells in EoE biopsies express the MHC class II protein HLA-DR.51 Using the human esophageal epithelial HET-1A cell line, which maintains characteristics of basal esophageal epithelium, they demonstrated the ability of these cells to engulf, process, and present antigen in an IFNγ-dependent manner, as well as stimulate T-helper cell activation. IFNγ, which is increased in biopsy tissue of EoE patients, enhanced expression of MHC class II, while IL-4 enhanced co-stimulatory molecule expression. Thus, while the esophageal epithelium is unlikely to play a role in the early initiation of an immune response, given the dependence on cytokine priming, it may potentially to play a role in perpetuating EoE-associated inflammation.
Interestingly, and rather controversially, recent literature has suggested that eosinophils may also function as APCs at the site of inflammation.52 In asthmatics, eosinophils appear to express the MHC Class II protein, HLA-DR, dependent on stimulation by GM-CSF, as well as co-stimulatory molecules CD40, CD80, and CD86, and can traffic to regional lymph nodes after exposure to antigen bringing them in proximity to T-cells for presentation.53, 54 Likewise in EoE, tissue eosinophils have been shown to express increased HLA-DR,55 as well as CD40 and CD80,56 supporting the potential capacity for antigen presentation. However, the evidence to support the ability of eosinophils to engulf and process protein antigens seems lacking and would limit their ability to function in similar ways to professional antigen presenting cells, such as DCs. One postulated mechanism, whereby MHC II loading occurs from exogenous peptides generated from the protease-rich milieu in EoE, remains to be fully established. Additionally, the nature of the interaction between T-cells and antigen-presenting eosinophils is not well understood; thus it is unclear the extent to which eosinophils act as APCs to initiate or even sustain the inflammatory process.
T-lymphocyte immune responses
Murine studies on mice lacking various components of the adaptive immune system have established a critical role for T-cells in EoE.29 Similar to other atopic diseases, such as allergic asthma and eczema, tissue inflammation in EoE is characterized by a Th2-type inflammatory response. This was initially described by Straumann et al. in 2001, who observed increased T-cells and IgE+ mast cells in esophageal biopsies of EoE patients associated with IL-5 expression in the infiltrating inflammatory cells.57 Since that initial study, several reports have confirmed these findings and described elevated levels of IL-4 and IL-13 in biopsy samples.25, 58, 59 Peripheral blood mononuclear cells from EoE patients also produce IL-5 and IL-13 in response to specific allergen stimulation,27 and murine models of EoE have provided additional support that a Th2-mediated response is required for pathogenicity.29, 36, 60 Blanchard et al. examined a large cohort of EoE patients and found enhanced expression of both IL-4 and IL-5 in atopic individuals with EoE compared to non-atopic.61 In addition the study noted concerted expression of IL-5 and IL-13, suggesting a common cell type is responsible for their production.
While much of the focus has been on the Th2 cell in EoE, Th1-associated cytokines are also an important part of the inflammatory response, and likely play a critical role in pathogenesis. This includes TNF, which is expressed by esophageal epithelial cells,57 and is involved in remodeling,62 and IFNγ, which is expressed by T-cells following stimulation with IL-15,63 and is involved in priming the epithelium for antigen presentation.51
IL-4
Critical to the initiation of a Th2 response, IL-4 promotes differentiation of naïve T-helper cells into Th2 cells as well as B-cell class-switching to produce IgE. While the initial source of IL-4 in atopic disease is not entirely clear, recent work has proposed that TSLP-elicited basophils may be important.28, 64, 65 In the esophagus, IL-4 not only sustains the Th2 response, but also contributes directly to recruitment of eosinophils by stimulating eotaxin production in esophageal epithelial cells.66 Th2 cells are considered to be an important source of IL-4 in EoE, which is enhanced by IL-15 stimulation.63 Countering this concept, IL-4 expressions levels correlate poorly with the other Th2-associated cytokines, IL-5 or IL-13,59 and suggest other cells may be relevant sources.
IL-5
Of the Th2 cytokines, IL-5 is the most well studied in EoE and appears to be central to the disease. In the GI tract, IL-5+ allergen-specific T-cell responses differentiate EGIDs from IgE-mediated immediate hypersensitivity, characterized by IL-5− Th2 responses.67 IL-5 is produced primarily by Th2 cells, although additional sources include mast cells and eosinophils. It acts on the bone marrow to stimulate eosinophil proliferation and differentiation and regulates survival and activation of eosinophils.68 Mice lacking IL-5 fail to recruit eosinophils to the esophagus in intranasal aeroallergen-induced EoE, whereas mice transgenic for IL-5 under the control of the T-cell specific CD2 promoter (CD2-IL5) develop chronic esophageal eosinophila and mastocytosis.69, 70 Trafficking of eosinophils by IL-5 likely occurs by priming of eosinophil responses to chemokines such as eotaxins (CCL11, CCL24 and CCL26), or by up-regulating homing receptors.60 IL-5 also has a role in tissue remodeling, since mice with CD2-IL-5 mediated esophageal eosinophilia have increased collagen accumulation in the lamina propria and extended stromal papillae, whereas IL-5 deficient mice do not in similar model studies.60, 70 Although clinical and murine studies has shown a central role for IL-5 in the allergic mechanisms of EoE, and there is variable down-regulation of IL-5 expression following treatment with fluticasone,71 anti-IL-5 biologic therapy has shown limited clinical efficacy,72 suggesting that, while IL-5 plays an important role in EoE disease, blocking IL-5 may not be sufficient to prevent the immunopathology of EoE in humans.
IL-13
IL-13 is a pleotropic cytokine that exerts pathologic effects when excessively produced by activating local tissue inflammatory responses; its cellular sources include Th2 cells and activated eosinophils.27, 57, 73 In EoE, active inflammation is self-perpetuating as infiltrating eosinophils secrete IL-13 which acts to enhance further recruitment of eosinophils to the esophagus by inducing STAT6-dependent eotaxin expression in the epithelium.74–76 The critical interaction between IL-13 and the esophageal epithelium in driving EoE was highlighted by Blanchard et al. who found a significant overlap between the transcriptome of primary esophageal epithelial cells treated with IL-13 and total RNA from EoE patient biopsies, which was reversible with steroid therapy.58 IL-13 also recruits eosinophils by promoting fibroblasts to produce periostin, which increases eosinophil adhesion to fibronectin.77 Intra-tracheal IL-13 was sufficient to induce experimental EoE in mice in an eotaxin/IL-5/STAT-6 dependent manner,74 but was not required, as intranasal aeroallergen-induced EoE had only mildly reduced esophageal eosinophilia in IL-13 deficient mice,36, 78 which may be due to exaggerated Th17 responses.79 IL-13 also plays key roles in barrier function, by downregulating genes involved in the epithelial cell differentiation, such as desmoglein-1, filaggrin, and involucrin,76, 80 and eosinophil-independent tissue remodeling by promoting collagen deposition, angiogenesis, and epithelial hyperplasia.81 IL-13 is a prominent T-cell mediator in EoE pathogenesis with broad function, and may be predicted as a likely factor that prevented efficacy from targeted IL-5 therapy. Ongoing clinical trials with anti-IL-13 will serve to better understand its role in pathogenesis and relevance as a therapeutic target.
B-lymphocytes and IgE
Formation of antigen-specific IgE is a principal effector function of Th2 cells, which promote class switching in B-cells by stimulation with IL-4. IgE can bind to its high affinity receptor, FcεRI, on the surface of mast cells and basophils, where subsequent engagement with polyvalent antigen triggers hypersensitivity responses as described above. This process is central to the pathogenesis of many atopic disorders including anaphylaxis, allergic bronchospasm, and urticaria. Additionally, there are a number of antigen-independent immune functions of IgE, which include enhancement of mast cell survival, maintenance of mast cell location, and dendritic cell migration.82 Clinical studies have documented the presence of allergen-specific IgE in patients with EoE, and skin prick testing (SPT) is commonly abnormal, providing evidence of immediate hypersensitivity to a variety of foods, and specific IgE has been suggested to contribute to symptoms.40 Interestingly, a clinical study of omalizumab, a monoclonal antibody directed against IgE, in the treatment of EoE, found clinical but not histologic or endoscopic improvement.83 This may have pathogenic implications, as it suggests that some acute symptoms in EoE may be associated with IgE-mediated activation of mast cells or basophils.18 However, many patients have no evidence of food-specific IgE, nor abnormal SPT, and as described previously, experimental studies in mice have demonstrated that eosinophil recruitment is IgE-independent, indicating a critical need for more translational and murine studies to fully understand the contribution of IgE to disease pathogenesis.
Eosinophils
Both acute and chronic allergic reactions are associated with tissue eosinophilia. Antigen-driven recruitment of eosinophils occurs through critical mediators like IL-5 and the eotaxin family of chemokines, as well as lipid mediators like prostaglandin D2 (PGD2). At the site of inflammation, eosinophils become activated where they modulate immune responses and can damage the surrounding tissue.84 Activation promotes secretion of various mediators that have been linked to EoE, including cytokines such as IL-4, IL-5, IL-13, and transforming growth factor (TGF)-β, chemokines such as CCL5/RANTES and CCL11/eotaxin-1, and lipid mediators such as leukotriene C4.59, 85 These molecules have profound effects on the inflammatory response that include upregulation of adhesion molecules, enhanced cellular trafficking and activation, and regulation of vascular permeability and muscle contraction. In addition, eosinophils secrete toxic granule protein that damage gut epithelium, including eosinophil peroxidase, eosinophil cationic protein, eosinophil-derived neurotoxin, and major basic protein, all of which have been shown to be increased in EoE tissues.86
The regulation over eosinophil recruitment to the esophagus seems similar to other atopic inflammatory diseases and largely associated with the chemotactic effects of the eotaxin proteins, which are critical for maintenance of tissue eosinophilia. In EoE, eotaxin-3 (CCL26) was shown to be the most highly expressed gene in the esophagus, and expression levels strongly correlated with disease severity.87 Eotaxin-deficient mice have been described as having markedly impaired esophageal eosinophilia,88 even when overexpression of IL-5 is genetically introduced.60 Interestingly, the relative increase in esophageal eosinophils in these IL-5 transgenic/eotaxin-deficient mice, when compared to control mice, suggest that additional chemotactic factors participate. This may include histamine, generated from mast cells or basophils, as thioperamide, a non-selective blocker of the histamine 3- and 4-receptor, inhibited eosinophil infiltration to the esophagus in an allergen-inhalation model of EoE in guinea pigs.89 In addition PGD2, a prostanoid largely produced by mast cells, is sufficient to drive eosinophils to the esophagus since injection of a PGD2 agonist into the esophagus led to rapid recruitment of eosinophils in guinea pigs, and pretreatment with a selective antagonist limited this experimental EoE-like disease.90 Also, in adults with corticosteroid-resistant EoE, treatment for 8 weeks with OC000459, a selective antagonist of CRTH2, the receptor for PGD2, led to reduced tissue eosinophilia and clinical symptoms.91
The eosinophil is critical to many aspects of the histopathology of EoE. Mice deficient in the eosinophil lineage have a significant reduction in basal layer and lamina propria collagen thickness and fail to develop esophageal strictures as detected by barium esophagram.69, 70 Notably, these mice still maintain evidence of esophageal motility dysfunction, suggesting that this aspect of the disease is eosinophil-independent.69 In oral ovalbumin-induced EoE in mice, antibody targeting of Siglec-F, the mouse homolog of Siglec-8, which is highly expressed on eosinophils, and which mediates their apoptosis and clearance, reduced esophageal eosinophilia associated with reduced angiogenesis, basal zone hyperplasia, and fibronectin deposition.92 These studies implicate the eosinophil as a critical damage-inducing mediator of EoE.
Mast cells
Despite being derived from the same CD34+ progenitor cell type as eosinophils, mast cells are normally resident in the mucosa and submucosa of the esophagus. Their best-characterized function involves crosslinking of IgE, which binds to the high-affinity receptor, FcεRI, and becomes activated after engagement with antigen. Similar to eosinophils, mast cells store and produce an abundant number of inflammatory mediators that might participate in the pathogenesis of EoE. This includes TGF-β1, Th2 cytokines IL-4, IL-5, and IL-13, as well as eotaxins, histamine, leukotrienes, lipid mediators, and proteases, which can collectively contribute to fibrosis, tissue inflammation and/or recruitment and activation of eosinophils.93
Mast cells are considered to be a major player in allergic disease, although the exact nature of their role EoE pathogenesis remains unclear. Numerous studies have observed an increase in mast cell density in biopsies from EoE patients,18, 57, 87 and this correlates with both eosinophil density and basal zone hyperplasia and is responsive to swallowed steroids.45 Mast cells activation occurs locally in EoE, as ultrastructural changes in cytoplasmic granules are detectable by electron microscopy,18 and genes such as carboxypeptidase A3, tryptase and histamine decarboxylase are elevated in esophageal tissue, and normalize with therapy.94 In the smooth muscle of patients with EoE, mast cell numbers are increased and express TGF-β1, which is capable of enhancing muscle contraction, suggesting a potential role in symptoms.85 Atopic EoE patients can be distinguished from non-atopic patients by the presence of IgE on esophageal mast cells, suggesting mast cell activation may occur by alternate mechanisms in non-atopic patients.17
Mechanistically, the role of mast cells in EoE has been studied in mice and, similar to humans, there is an increase in density paralleling the increase in eosinophils.69, 78, 88, 95, 96 In the intranasal aeroallergen-induced model of EoE, the increase in esophageal mast cells was time- and challenge-dependent. While mast cell-deficient mice had reduced muscle cell hyperplasia and hypertrophy in this model, consistent with a role in remodeling, interestingly there was no effect on eosinophil recruitment.96 Likewise, eosinophils are not required to recruit mast cells to the esophagus, but IL-5 seems to participate in recruiting mast cells, as CD2-IL5 transgenic/eosinophil-deficient mice had similar elevations esophageal mast cell density as controls.69 This was supported clinically by sub-analysis of a trial of mepolizumab in children, which also found reduced esophageal mast cells in treated patients.97 Thus it appears that mast cells have a multifunctional role in EoE, contributing to symptoms, remodeling, and tissue inflammation.
Basophils
Despite an abundance of work defining the role of basophils in allergic inflammation, there have been limited studies investigating their pathogenic function in EoE. Recently, their potential involvement was demonstrated using a novel murine model of EoE, whereby mice are sensitized to food antigen via the skin and EoE-like disease is initiated by repeated oral challenge with antigen.28 In this model, antigen-specific IgE is detectable, but the mice develop EoE-like inflammation that is IgE-independent. Instead, the authors show that EoE responses were associated with a significant expansion of basophils, driven by exposure to epithelial-derived TSLP, along with a Th2 response that was dominated by IL-4. They establish that depletion of basophils or deficiency of TLSP signaling resulted in loss of responses and included translational studies also demonstrating increased TSLP and basophils in EoE patient biopsies. This new model provides unique insight into the mechanism by which IgE-independent allergic sensitization to antigen can facilitate eosinophil recruitment to the esophagus driven by basophils.
Summary
Eosinophilic esophagitis is a chronic inflammatory disease isolated to the esophagus that impacts both children and adults. Animal models as well as studies in patients have helped to uncover various aspects of its pathogenesis, although many questions remain. EoE appears to share a significant clinical link with other atopic diseases and there is strong evidence that it is caused by immune dysregulation secondary to allergic sensitization to dietary and/or aeroallergens. While the role of IgE remains unclear, EoE is dominated by T-lymphocyte mediated pathology with significant participation from mast cells, basophils, epithelial cells, and dendritic cells, summarized in Figure 1. What remains poorly understood is how the disease develops and while there is some literature to suggest a role for loss of tolerance, further translational research is necessary to better characterize this aspect of the disease. As the complex immune mechanisms that govern EoE are uncovered, novel diagnostic and treatment options will move from bench to bedside.
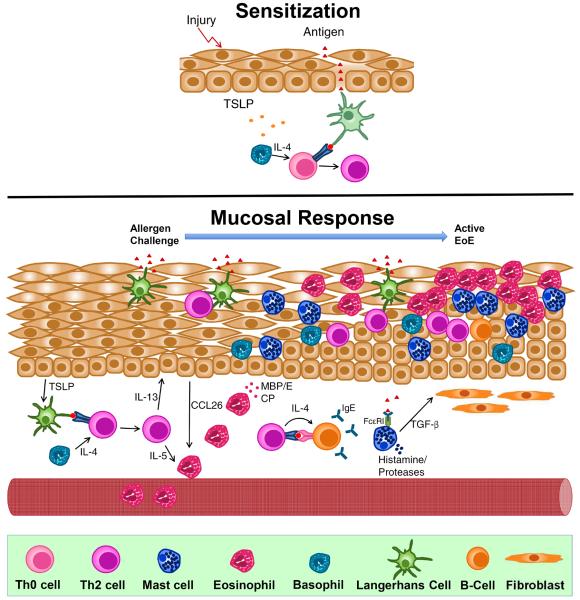
Fig 1.
Proposed allergic mechanisms involved in the pathogenesis of Eosinophilic Esophagitis (EoE). TOP: Proposed mechanism of allergic sensitization in EoE. In the presence of epithelial injury, TSLP production is elicited from epithelium. This primes basophils to produce IL-4 which promotes allergic sensitization after antigen presentation to a naïve T-cell which generates antigen-specific Th2 cells. BOTTOM: Proposed mucosal response in EoE. Subsequet antigen challenge leads to recruitment and expansion of Th2 cells which secrete IL-5 and IL-13, both critical in the recruitment of eosinophils and remodeling of the esophagus. Th2 cells locally promote class-switching of B-cells to produce antigen-specific IgE, which binds to the surface of mast cells. Activation of mast cells leads to the releast of pro-inflammatory mediators such as TGF-β, which promotes remodeling and enhances muscle cell contractility.
Key Points
Eosinophilic esophagitis shares a clinical link with other atopic diseases and is caused by immune dysregulation secondary to allergic sensitization to dietary and/or aeroallergens.
Allergic sensitization, which may occur via multiple routes, drives the formation of allergen-specific IgE and T-cells that appear to participate in the esophageal hypersensitivity response.
Loss of tolerance is likely critical to pathogenesis, and may be secondary to regulatory T-cell imbalance.
Eosinophilic esophagitis is dominated by T-helper lymphocyte type 2-mediated eosinophil-predominant inflammation with key contributions from mast cells, basophils, epithelial cells, and dendritic cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–1512. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 2.Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. The Journal of allergy and clinical immunology. 2007;119(3):731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Simon D, Marti H, Heer P, et al. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. The Journal of allergy and clinical immunology. 2005;115(5):1090–1092. doi: 10.1016/j.jaci.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Kiyohara C, Tanaka K, Miyake Y. Genetic susceptibility to atopic dermatitis. Allergology international : official journal of the Japanese Society of Allergology. 2008;57(1):39–56. doi: 10.2332/allergolint.R-07-150. [DOI] [PubMed] [Google Scholar]
- 5.van Rhijn BD, Verheij J, Smout AJ, et al. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(1):47–52. e45. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 6.Soon IS, Butzner JD, Kaplan GG, et al. Incidence and prevalence of eosinophilic esophagitis in children. Journal of pediatric gastroenterology and nutrition. 2013;57(1):72–80. doi: 10.1097/MPG.0b013e318291fee2. [DOI] [PubMed] [Google Scholar]
- 7.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Archives of disease in childhood. 2006;91(12):1000–1004. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3(12):1198–1206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 9.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(10):1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? The Journal of allergy and clinical immunology. 2005;115(2):418–419. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Syed AA, Andrews CN, Shaffer E, et al. The rising incidence of eosinophilic oesophagitis is associated with increasing biopsy rates: a population-based study. Alimentary pharmacology & therapeutics. 2012;36(10):950–958. doi: 10.1111/apt.12053. [DOI] [PubMed] [Google Scholar]
- 12.Jyonouchi S, Brown-Whitehorn TA, Spergel JM. Association of eosinophilic gastrointestinal disorders with other atopic disorders. Immunology and allergy clinics of North America. 2009;29(1):85–97. x. doi: 10.1016/j.iac.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. The Journal of allergy and clinical immunology. 2010;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler JB, Schroeder HA, Byrne AJ, et al. Anaphylactic responses to histamine in mice utilize both histamine receptors 1 and 2. Allergy. 2013;68(10):1338–1340. doi: 10.1111/all.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spergel JM, Andrews T, Brown-Whitehorn TF, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2005;95(4):336–343. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Current opinion in allergy and clinical immunology. 2007;7(3):274–278. doi: 10.1097/ACI.0b013e32813aee4a. [DOI] [PubMed] [Google Scholar]
- 17.Mulder DJ, Mak N, Hurlbut DJ, et al. Atopic and non-atopic eosinophilic oesophagitis are distinguished by immunoglobulin E-bearing intraepithelial mast cells. Histopathology. 2012;61(5):810–822. doi: 10.1111/j.1365-2559.2012.4303.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch R, Bokhary R, Marcon MA, et al. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. Journal of pediatric gastroenterology and nutrition. 2007;44(1):20–26. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- 19.Feldman MJ, Morris GP, Dinda PK, et al. Mast cells mediate acid-induced augmentation of opossum esophageal blood flow via histamine and nitric oxide. Gastroenterology. 1996;110(1):121–128. doi: 10.1053/gast.1996.v110.pm8536848. [DOI] [PubMed] [Google Scholar]
- 20.Percy WH, Warren JM, Brunz JT. Characteristics of the muscularis mucosae in the acid-secreting region of the rabbit stomach. The American journal of physiology. 1999;276(5 Pt 1):G1213–1220. doi: 10.1152/ajpgi.1999.276.5.G1213. [DOI] [PubMed] [Google Scholar]
- 21.Barata LT, Ying S, Meng Q, et al. IL-4- and IL-5-positive T lymphocytes, eosinophils, and mast cells in allergen-induced late-phase cutaneous reactions in atopic subjects. The Journal of allergy and clinical immunology. 1998;101(2 Pt 1):222–230. doi: 10.1016/s0091-6749(98)70387-2. [DOI] [PubMed] [Google Scholar]
- 22.Uzzaman A, Cho SH. Chapter 28: Classification of hypersensitivity reactions. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2012;33(Suppl 1):S96–99. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 23.Spergel JM, Brown-Whitehorn T, Beausoleil JL, et al. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007;119(2):509–511. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Bryce PJ, Miller ML, Miyajima I, et al. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20(4):381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 25.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59(1):12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erwin PC, Greene SB, Mays GP, et al. The association of changes in local health department resources with changes in state-level health outcomes. American journal of public health. 2011;101(4):609–615. doi: 10.2105/AJPH.2009.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki K, Murray JA, Arora AS, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Digestive diseases and sciences. 2006;51(11):1934–1941. doi: 10.1007/s10620-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 28.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nature medicine. 2013;19(8):1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra A, Schlotman J, Wang M, et al. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. Journal of leukocyte biology. 2007;81(4):916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 30.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of Eosinophilic Esophagitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013 doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen ET, Kappelman MD, Kim H, et al. Early life exposures as risk factors for pediatric eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2013;57(1):67–71. doi: 10.1097/MPG.0b013e318290d15a. [DOI] [PubMed] [Google Scholar]
- 32.Stuck MC, Straumann A, Simon HU. Relative lack of T regulatory cells in adult eosinophilic esophagitis - no normalization after corticosteroid therapy. Allergy. 2011;66(5):705–707. doi: 10.1111/j.1398-9995.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Wang M, Crump CH, et al. An imbalance of esophageal effector and regulatory T cell subsets in experimental eosinophilic esophagitis in mice. American journal of physiology Gastrointestinal and liver physiology. 2009;297(3):G550–558. doi: 10.1152/ajpgi.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganeshan K, Neilsen CV, Hadsaitong A, et al. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. The Journal of allergy and clinical immunology. 2009;123(1):231–238. e234. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rayapudi M, Mavi P, Zhu X, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. Journal of leukocyte biology. 2010;88(2):337–346. doi: 10.1189/jlb.0110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akei HS, Mishra A, Blanchard C, et al. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129(3):985–994. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Slack MA, Erwin EA, Cho CB, et al. Food and aeroallergen sensitization in adult eosinophilic esophagitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;111(4):304–305. doi: 10.1016/j.anai.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. Journal of pediatric gastroenterology and nutrition. 2009;48(1):30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 39.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? Journal of clinical gastroenterology. 2007;41(5):451–453. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 40.Simon D, Straumann A, Dahinden C, et al. Frequent sensitization to Candida albicans and profilins in adult eosinophilic esophagitis. Allergy. 2013;68(7):945–948. doi: 10.1111/all.12157. [DOI] [PubMed] [Google Scholar]
- 41.van Rhijn BD, van Ree R, Versteeg SA, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy. 2013;68(11):1475–1481. doi: 10.1111/all.12257. [DOI] [PubMed] [Google Scholar]
- 42.de Fraissinette A, Schmitt D, Thivolet J. Langerhans cells of human mucosa. The Journal of dermatology. 1989;16(4):255–262. doi: 10.1111/j.1346-8138.1989.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 43.Terris B, Potet F. Structure and role of Langerhans' cells in the human oesophageal epithelium. Digestion. 1995;56(Suppl 1):9–14. doi: 10.1159/000201295. [DOI] [PubMed] [Google Scholar]
- 44.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122(5):1216–1225. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 45.Lucendo AJ, Navarro M, Comas C, et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. The American journal of surgical pathology. 2007;31(4):598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 46.Bannert C, Bidmon-Fliegenschnee B, Stary G, et al. Fc-epsilon-RI, the high affinity IgE-receptor, is robustly expressed in the upper gastrointestinal tract and modulated by mucosal inflammation. PloS one. 2012;7(7):e42066. doi: 10.1371/journal.pone.0042066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen EH, Hornick JL, Dehlink E, et al. Comparative analysis of FcepsilonRI expression patterns in patients with eosinophilic and reflux esophagitis. Journal of pediatric gastroenterology and nutrition. 2010;51(5):584–592. doi: 10.1097/MPG.0b013e3181de7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI) Immunobiology. 2007;212(6):499–503. doi: 10.1016/j.imbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Buning J, von Smolinski D, Tafazzoli K, et al. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class II-restricted antigen processing and origin of exosomes. Immunology. 2008;125(4):510–521. doi: 10.1111/j.1365-2567.2008.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heyman M. Symposium on `dietary influences on mucosal immunity'. How dietary antigens access the mucosal immune system. The Proceedings of the Nutrition Society. 2001;60(4):419–426. [PubMed] [Google Scholar]
- 51.Mulder DJ, Pooni A, Mak N, et al. Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis. The American journal of pathology. 2011;178(2):744–753. doi: 10.1016/j.ajpath.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akuthota P, Wang H, Weller PF. Eosinophils as antigen-presenting cells in allergic upper airway disease. Current opinion in allergy and clinical immunology. 2010;10(1):14–19. doi: 10.1097/ACI.0b013e328334f693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi HZ, Humbles A, Gerard C, et al. Lymph node trafficking and antigen presentation by endobronchial eosinophils. The Journal of clinical investigation. 2000;105(7):945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HB, Ghiran I, Matthaei K, et al. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. Journal of immunology. 2007;179(11):7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel AJ, Fuentebella J, Gernez Y, et al. Increased HLA-DR expression on tissue eosinophils in eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2010;51(3):290–294. doi: 10.1097/MPG.0b013e3181e083e7. [DOI] [PubMed] [Google Scholar]
- 56.Le-Carlson M, Seki S, Abarbanel D, et al. Markers of antigen presentation and activation on eosinophils and T cells in the esophageal tissue of patients with eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2013;56(3):257–262. doi: 10.1097/MPG.0b013e3182758d49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. The Journal of allergy and clinical immunology. 2001;108(6):954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 58.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. The Journal of allergy and clinical immunology. 2007;120(6):1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2011;127(1):208–217. 217, e201–207. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra A, Hogan SP, Brandt EB, et al. IL-5 promotes eosinophil trafficking to the esophagus. Journal of immunology. 2002;168(5):2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 61.Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. The Journal of allergy and clinical immunology. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 62.Persad R, Huynh HQ, Hao L, et al. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. Journal of pediatric gastroenterology and nutrition. 2012;55(3):251–260. doi: 10.1097/MPG.0b013e31824b6391. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Wang M, Mavi P, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139(1):182–193. e187. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giacomin PR, Siracusa MC, Walsh KP, et al. Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. Journal of immunology. 2012;189(9):4371–4378. doi: 10.4049/jimmunol.1200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62(6):824–832. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. The Journal of allergy and clinical immunology. 2009;124(6):1326–1332. e1326. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. The Journal of allergy and clinical immunology. 2001;108(4):503–508. doi: 10.1067/mai.2001.119149. [DOI] [PubMed] [Google Scholar]
- 69.Mavi P, Rajavelu P, Rayapudi M, et al. Esophageal functional impairments in experimental eosinophilic esophagitis. American journal of physiology Gastrointestinal and liver physiology. 2012;302(11):G1347–1355. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134(1):204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucendo AJ, De Rezende L, Comas C, et al. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. The American journal of gastroenterology. 2008;103(9):2184–2193. doi: 10.1111/j.1572-0241.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 72.Reddy V, Ghaffari G. Eosinophilic esophagitis: review of nonsurgical treatment modalities. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2013;34(5):421–426. doi: 10.2500/aap.2013.34.3690. [DOI] [PubMed] [Google Scholar]
- 73.Straumann A, Kristl J, Conus S, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflammatory bowel diseases. 2005;11(8):720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 74.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125(5):1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Blanchard C, Durual S, Estienne M, et al. Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. The international journal of biochemistry & cell biology. 2005;37(12):2559–2573. doi: 10.1016/j.biocel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. Journal of immunology. 2010;184(7):4033–4041. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blanchard C, Mingler MK, McBride M, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal immunology. 2008;1(4):289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niranjan R, Rayapudi M, Mishra A, et al. Pathogenesis of allergen-induced eosinophilic esophagitis is independent of interleukin (IL)-13. Immunology and cell biology. 2013;91(6):408–415. doi: 10.1038/icb.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He R, Kim HY, Yoon J, et al. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. The Journal of allergy and clinical immunology. 2009;124(4):761–770. e761. doi: 10.1016/j.jaci.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal immunology. 2013 doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuo L, Fulkerson PC, Finkelman FD, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. Journal of immunology. 2010;185(1):660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bryce PJ, Oettgen HC. Antigen-independent effects of immunoglobulin E. Current allergy and asthma reports. 2005;5(3):186–190. doi: 10.1007/s11882-005-0036-6. [DOI] [PubMed] [Google Scholar]
- 83.Rocha R, Vitor AB, Trindade E, et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. European journal of pediatrics. 2011;170(11):1471–1474. doi: 10.1007/s00431-011-1540-4. [DOI] [PubMed] [Google Scholar]
- 84.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 85.Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. The Journal of allergy and clinical immunology. 2010;126(6):1198–1204. e1194. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 86.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62(10):1395–1405. doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. The Journal of clinical investigation. 2006;116(2):536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajavelu P, Rayapudi M, Moffitt M, et al. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. American journal of physiology Gastrointestinal and liver physiology. 2012;302(7):G645–654. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu S, Stahl E, Li Q, et al. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life sciences. 2008;82(5–6):324–330. doi: 10.1016/j.lfs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S, Wu X, Yu S. Prostaglandin D2 receptor d-type prostanoid receptor 2 mediates eosinophil trafficking into the esophagus. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2013 doi: 10.1111/dote.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Straumann A, Hoesli S, Bussmann C, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–385. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 92.Rubinstein E, Cho JY, Rosenthal P, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2011;53(4):409–416. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shakoory B, Fitzgerald SM, Lee SA, et al. The role of human mast cell-derived cytokines in eosinophil biology. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2004;24(5):271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]
- 94.Hsu Blatman KS, Gonsalves N, Hirano I, et al. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. The Journal of allergy and clinical immunology. 2011;127(5):1307–1308. e1303. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho JY, Rosenthal P, Miller M, et al. Targeting AMCase reduces esophageal eosinophilic inflammation and remodeling in a mouse model of egg induced eosinophilic esophagitis. International immunopharmacology. 2013;18(1):35–42. doi: 10.1016/j.intimp.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niranjan R, Mavi P, Rayapudi M, et al. Pathogenic role of mast cells in experimental eosinophilic esophagitis. American journal of physiology Gastrointestinal and liver physiology. 2013;304(12):G1087–1094. doi: 10.1152/ajpgi.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Otani IM, Anilkumar AA, Newbury RO, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2013;131(6):1576–1582. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]