Abstract
PURPOSE
To determine if clinical and reference strains of Pseudomonas aeruginosa, Serratia marcescens, and Staphylococcus aureus form biofilms on silicone hydrogel contact lenses, and ascertain antimicrobial activities of contact lens care solutions.
METHODS
Clinical and American Type Culture Collection (ATCC) reference strains of Pseudomonas aeruginosa, Serratia marcescens, and Staphylococcus aureus were incubated with lotrafilcon A lenses under conditions that facilitate biofilm formation. Biofilms were quantified by quantitative culturing (colony forming units, CFUs), and gross morphology and architecture were evaluated using scanning electron microscopy (SEM) and confocal microscopy. Susceptibilities of the planktonic and biofilm growth phases of the bacteria to five common multipurpose contact lens care solutions and one hydrogen peroxide care solution were assessed.
RESULTS
P. aeruginosa, S. marcescens, and S. aureus reference and clinical strains formed biofilms on lotrafilcon A silicone hydrogel contact lenses, as dense networks of cells arranged in multiple layers with visible extracellular matrix. The biofilms were resistant to commonly used biguanide preserved multipurpose care solutions. P. aeruginosa and S. aureus biofilms were susceptible to a hydrogen peroxide and a polyquaternium preserved care solution, whereas S. marcescens biofilm was resistant to a polyquaternium preserved care solution but susceptible to hydrogen peroxide disinfection. In contrast, the planktonic forms were always susceptible.
CONCLUSIONS
P. aeruginosa, S. marcescens, and S. aureus form biofilms on lotrafilcon A contact lenses, which in contrast to planktonic cells, are resistant to the antimicrobial activity of several soft contact lens care products.
Keywords: bacterial biofilm, silicone hydrogel contact lens, contact lens care solutions
INTRODUCTION
Silicone hydrogel lenses are the predominant lens type prescribed for extended wear in the global marketplace1 and they lead the United States contact lens market for all modes of wear.2 However, problems associated with infection and inflammation have not been resolved with the introduction of these polymers. In fact, the incidence of microbial keratitis with silicone hydrogel extended lens wear has not changed and the incidence of non-infectious corneal inflammatory events has doubled compared to extended wear with traditional hydrogel contact lenses.3–6
One explanation for contact lens induced infection despite good compliance is the ability of organisms to form biofilms on lens surfaces.7–13 Biofilms are microbial communities adhering to a surface, contain an extracellular matrix of polymeric substances, and are associated with differential expression of genes and proteins.14–19 Importantly, bacteria in biofilms have increased resistance to antimicrobials and host immune responses.8, 12, 13
The ability of bacteria to attach to contact lenses and subsequently form biofilms has been previously demonstrated.13 Studies using scanning electron microscopy have shown distinct biofilms on contact lens surfaces recovered from patients with microbial keratitis even in those patients with good compliance.10, 11 Bacterial biofilms have also been recovered from lens cases used by patients during an episode of microbial keratitis,10 and lens case associated biofilms are more resistant to contact lens care solutions.20, 21
It is well known that bacteria within biofilms are more resistant to biocides and antibiotics.22 However, there is no accepted contact lens model to assess the susceptibility of bacterial biofilms bound on hydrogel contact lenses to contact lens care solutions. In a recent study, we reported on a fungal biofilm-contact lens model using Fusarium species and found that lens bound fungal biofilm was resistant to two biguanide-based contact lens care products while the planktonic cells were susceptible.23 In the current study, we used a bacterial biofilm-contact lens model to determine whether 3 different bacterial strains, commonly associated with contact lens keratitis and inflammation, can form biofilm on silicone hydrogel contact lenses. Additionally, we assessed the antimicrobial activities of contact lens care solutions against bacterial cells grown under planktonic or biofilm conditions. Our results show that all the selected reference and clinical bacterial strains form biofilms on the lotrafilcon A silicone hydrogel material. Furthermore, these biofilms were resistant to common biguanide preserved multipurpose care solutions, while the planktonic forms were susceptible. Our studies suggest that adverse events associated with silicone hydrogel lenses may be related to the propensity of bacteria to adhere and form resistant biofilms on contact lens surfaces.
METHODS
Bacterial Strains
The following bacterial species were used in the current study: Pseudomonas aeruginosa, Serratia marcescens, and Staphylococcus aureus (Table 1). These species were selected because they are common causative agents of contact lens-associated infection and inflammation.24–31 The ATCC strains used were based on recommendations by the FDA guidance document FDA Premarket Notification 510(k) Guidance Document for Contact Lens Care Products.32
Table 1.
List of strains tested in this study
| Species | Isolate | Source |
|---|---|---|
| P. aeruginosa | ATCC 9027 | ATCC (from outer ear infection)* |
| MRL8620 | CMM* | |
| S. marcescens | ATCC 13880 | ATCC (from pond water) |
| MRL9195 | CMM | |
| 056SM | Contact lenses of a patient with contact lens acute red eye (CLARE) in the Longitudinal Analysis of Silicone Hydrogel (LASH) contact lens study§ | |
| 094SM | Contact lenses of a patient with infiltrative keratitis the Longitudinal Analysis of Silicone Hydrogel (LASH) contact lens study§ | |
| S. aureus | ATCC 6538 | ATCC (from a human lesion) |
| ATCC 43300 | ATCC (clinical isolate) | |
| 094SA | Contact lenses of a patient with infiltrative keratitis the Longitudinal Analysis of Silicone Hydrogel (LASH) contact lens study§ |
ATCC – American Type Culture Collection, Rockville, MD; CMM – Center for Medical Mycology (CMM), University Hospitals Case Medical Center. Source for ATCC isolates are based on the description provided at the ATCC website (http://www.atcc.org).
The LASH study (performed by LSF at University Hospitals Case Medical Center) is an ongoing prospective cohort study of 208 users of lotrafilcon A lenses worn continuously for up to 30 days of wear.
Bacterial Growth Conditions
Bacteria were grown overnight at 37°C in tryptic soy broth (TSB, MP Biomedicals, OH) washed 3 times with phosphate buffered saline (PBS) and the number of cells suspension was adjusted spectrophotometrically (wavelength 660 nm).
Contact Lenses
Lotrafilcon A (CIBA Vision, Duluth, GA) soft contact lenses were used in this study. All lenses were manufactured in an 8.6 mm base curve and +1.50 D. This lens is a U.S. Food and Drug Administration (FDA) Group 1 non-ionic silicone hydrogel polymer with 24% water and a permanent 25nm plasma surface coating. It was chosen to represent a leading extended wear silicone hydrogel material and is one of the two soft lens types approved by the FDA for up to 30 days of extended wear.
Lens Care Solutions
The five most common multipurpose solutions (MPSs) or multipurpose disinfecting solutions (MPDSs) available at the time of this study were used: ReNu Multiplus and ReNu with MoistureLoc (Bausch & Lomb, Rochester, NY), Complete MoisturePlus (Advanced Medical Optics, Santa Ana, CA), AQuify (Ciba Vision, Duluth, GA), and Opti-Free Replenish (Alcon Laboratories, Fort Worth, TX). Additionally, Clear Care (Ciba Vision, Duluth, GA), a hydrogen peroxide-based care system was tested. Since the completion of study, ReNu with MoistureLoc and Complete MoisturePlus have been withdrawn from the global market due to association with outbreaks of Fusarium and Acanthamoeba keratitis, respectively.33, 34 Table 2 lists the specific care systems used and their respective antimicrobial agents. Note that ReNu Multiplus and MoistureLoc, Complete MoisturePlus, and AQuify contain a polymeric biguanide, Opti-Free Replenish contains polyquaternium-1 and myristamidopropyl dimethylamine, and Clear Care contains 3% hydrogen peroxide (with a platinum coated disc for rapid and effective neutralization before placement of lenses on the eye) as active disinfection ingredient.
TABLE 2.
Contact lens care solutions tested
| Product | Disinfectants |
|---|---|
| ReNu MultiPlus | DYMED® (polyaminopropyl biguanide) 0.0001% |
| ReNu MoistureLoc | Alexidine 0.00045% |
| AQuify | Polyhexanide (polyhexamethylene biguanide) 0.0001% |
| COMPLETE MoisturePlus | Polyhexamethylene biguanide 0.0001% |
| OPTI Free Replenish | Polyquad® (polyquaternium-1) 0.001%; Aldox®(myristamidopropyl dimethylamine) 0.0005% |
| CLEAR CARE | Hydrogen peroxide 3% |
Biofilm Formation and quantification
Lotrafilcon A lenses were washed with PBS, placed in 12-well tissue culture plates with 4 ml standardized cell suspensions (absorbance was 0.1 at 660nm) and incubated for 120 min at 37°C to allow adhesion of cells to the lens surface (adherence phase). Next, these lenses (all of which contained adherent bacteria) were transferred to new 12-well plates containing fresh PBS (4 mL). Next, lenses were immersed in 1% TSB and incubated for different time periods (2, 6, 18 and 24 hours) at 37°C on a rocking platform. At indicated time points lenses with bacterial biofilm were washed with PBS and transferred to a 1.5 ml conical tube with 1.0 ml of PBS. To quantify biofilms, lenses on which biofilms were formed by different bacterial species and strains were sonicated for 5 min and vortexed for 3 min. The resulting cell suspensions were collected, serially diluted in PBS, and aliquots were spread on Mueller-Hinton (MH) agar plates. The plates were incubated at 37°C for 24 h, and the number of colony forming units (CFUs) was recorded.
Scanning electron microscopy (SEM) analysis of biofilms formed on contact lenses
Surface topography of bacteria grown as biofilms (on soft contact lenses) and planktonic cells were investigated using SEM as described previously.35, 36 Briefly, bacterial biofilms utilizing the FDA recommended reference strains (P. aeruginosa, ATCC 9027; S. marcescens, ATCC 13880; and S. aureus, ATCC 6538) were formed to mature phase (18 h) on contact lenses. Contact lenses with mature biofilms were fixed with 2% glutaraldehyde, followed by fixing with osmium tetraoxide, tannic acid, and uranyl acetate. These steps were followed by a series of ethanol dehydration steps as described previously,35, 36 and the prepared samples were sputter coated with Au-Pd (60/40 ratio) and viewed with a model XL3C ESEM Philips microscope.
Confocal scanning laser microscopic (CSLM) analyses of biofilm architecture and thickness
Architecture and thickness of biofilms formed utilizing the FDA recommended reference strains on contact lenses were evaluated by confocal scanning laser microscopy (CSLM). Briefly, biofilms grown on contact lenses were transferred to 12-well plates and stained with the LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Eugene, OR). After incubation with the dyes, the biofilms were placed on a 35-mm-diameter glass-bottom Petri dish (MatTek Corp., Ashland, Mass.). Stained biofilms were observed with a Zeiss LSM510 confocal scanning laser microscope equipped with argon and HeNe lasers and mounted on a Zeiss Axiovert100 M microscope (Carl Zeiss Inc.). The objective used was a water immersion C-apochromat lens (403; numerical aperture of 1.2). Depth measurements were taken at regular intervals across the width of the device. For orthogonal analyses, a series of horizontal (xy) optical sections were obtained throughout the full length of the biofilm, and analyzed using LSM 510 software (Carl Zeiss). Confocal images of green (ConA) and red (FUN-1) fluorescence were conceived simultaneously using a multitrack mode. Planktonically grown cells were used as comparators in these studies.
Evaluation of Antibacterial Activity of Contact Lens Care Solutions
Lotrafilcon A lenses with biofilm were washed by PBS (for at least 5 seconds) to simulate the rinsing step, put in 4 ml of one of the indicated contact lens care solutions in 12-well plates and incubated at room temperature for 4 h (for ReNu MultiPlus and MoistureLoc, AQuify, and COMPLETE MoisturePlus) or 6 h (for OPTI-Free and Clear Care) according to manufacturer recommendations. In the case of Clear Care, lenses with biofilms were put into the lens case supplied by the manufacturer using their recommended amount of solution because this solution is based on a peroxidase system and the specified solution volume must be inactivated with a platinum coated disk. After treatment, lenses were washed by PBS as above, transferred to 1.5 ml tube with 1.0 ml of PBS, sonicated for 5 min and vortexed for 3 min. Cell suspensions were treated with Dey-Engley Neutralizing Broth (DEB, Difco Laboratories) for 15 min and serial dilutions were spread on Tryptic Soy Agar (TSA) plates to evaluate viability. Each strain was tested three independent times.
The effect of contact lens care solutions against planktonically grown cells was evaluated according to International Organization for Standardization (ISO 14729) Stand Alone Procedure guidelines. Briefly, absorbances of cell suspensions were adjusted to obtain 4.0 × 107 cfu/ml. Subsequently, a 0.1 ml suspension of each strain was mixed with 10 ml of each lens care solution and incubated at room temperature for 4 h (for ReNu MultiPlus and MoistureLoc, AQuify, and COMPLETE MoisturePlus) or 6 h (for OPTI-Free and Clear Care), as suggested by the respective manufacturer. In the case of Clear Care, the cell suspensions were placed in the manufacturer’s platinum coated disk container with their specified volume of solution so that the neutralization step was effectively accomplished. After treatment, the resultant mixture was treated with DEB and serial dilutions were spread on TSA plates for CFU counting. Each strain was tested three independent times.
Statistical Analyses
For the comparison of the various strains to form biofilm, an ANOVA was performed with Bonferroni/Dunn post-hoc comparisons. Additionally an ANOVA with Bonferroni/Dunn post-hoc comparisons was performed on the reduction of Log CFU/ml for each lens solution for each growth form and organism. All statistical analyses were performed using SPSS software, ver. 16.0.
RESULTS
Time Course of Bacterial Biofilm Development on Lotrafilcon A Lenses
In order to evaluate the time course of bacterial biofilm development on lotrafilcon A lenses, we monitored biofilm formation at 0, 2, 6, 18 and 24 h using quantitative culturing. As shown in Figure 1, there were three distinct patterns of growth for each organism: biofilm formation by P. aeruginosa progressed through an early phase characterized by a rapid growth in the initial 6 h, followed by an intermediate phase where gradual, slow growth was noted (up to 18 h). After the 18 h time point, P. aeruginosa biofilm growth reached stationary phase. Biofilm formation by S. marcescens followed different growth kinetics, with an initial latent phase of up to 2 h, followed by a rapid exponential growth phase (between 2 h to 6 h), reaching a plateau thereafter. In contrast, biofilm formation by S. aureus was characterized by a gradual increase in growth up to 24 h. Biofilms grown to 18 h were used in subsequent experiments, since although there were differences in kinetics, biofilms formed by all three species reached maturation phase by this time point.
Fig. 1.
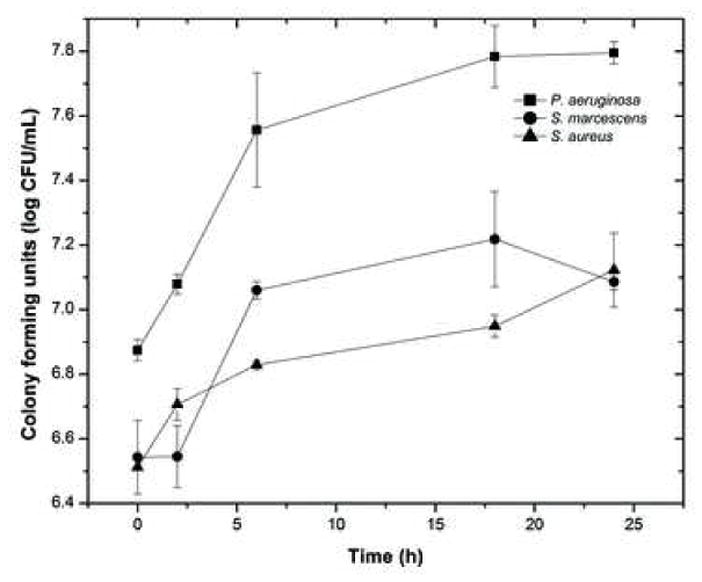
Growth kinetics of biofilms formed by P. aeruginosa, S. marcescens, and S. aureus on silicone hydrogel contact lens. Biofilm growth was monitored by determining the colony forming unit (CFU) count at each time point studied.
Multiple Strains of each Bacteria form Biofilm on Lotrafilcon A Lenses
To determine whether different bacterial species and strains can form biofilms on silicone hydrogel contact lenses, we cultured clinical and reference (ATCC) isolates of P. aeruginosa, S. marcescens, and S. aureus under biofilm-forming conditions on lotrafilcon A contact lenses, and measured growth by CFU. Figure 2 shows the colony forming units (CFUs) of biofilms formed by these isolates. Our data showed that all the isolates tested formed biofilms on contact lenses (approximately 5–7 log CFU/ml). No significant difference in the ability to form biofilms was observed between the clinical and reference isolates of P. aeruginosa. Among the S. marcescens strains, CFU from the reference isolate (ATCC 13880) was significantly lower than the three clinical isolates (MRL9195, 056SM and 094SM; P < .047). However, no significant difference in the ability of the clinical S. marcescens isolates to form biofilms was observed. Finally, among the S. aureus strains, the clinical isolate (094SA) formed significantly lower biofilm than the two reference isolates tested (ATCC 43300, ATCC 6538; P ≤ .01 for both comparisons). Since these data show that all bacterial species and strains tested were able to form biofilms on lotrafilcon A contact lenses, all subsequent analyses utilized the standard reference strains as recommended by the FDA and ISO guidelines, specifically P. aeruginosa (ATCC 9027), S. marcescens (ATCC 13880), and S. aureus (ATCC 6538).
Fig. 2.
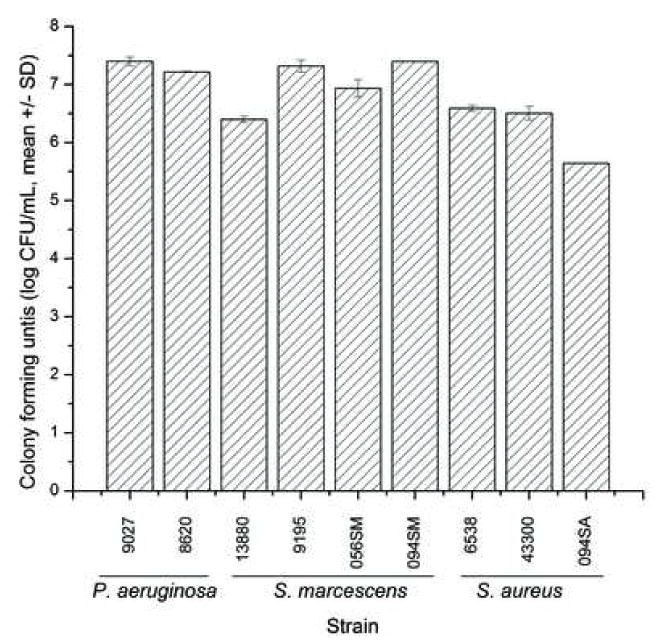
Comparison of the ability of clinical isolates and reference strains of P. aeruginosa, S. marcescens, and S. aureus to form biofilms on silicone hydrogel contact lenses, measured by determining the associated CFUs.
Ultrastructural/scanning electron microscopy analysis of Bacterial Biofilms formed on Lotrafilcon A lenses
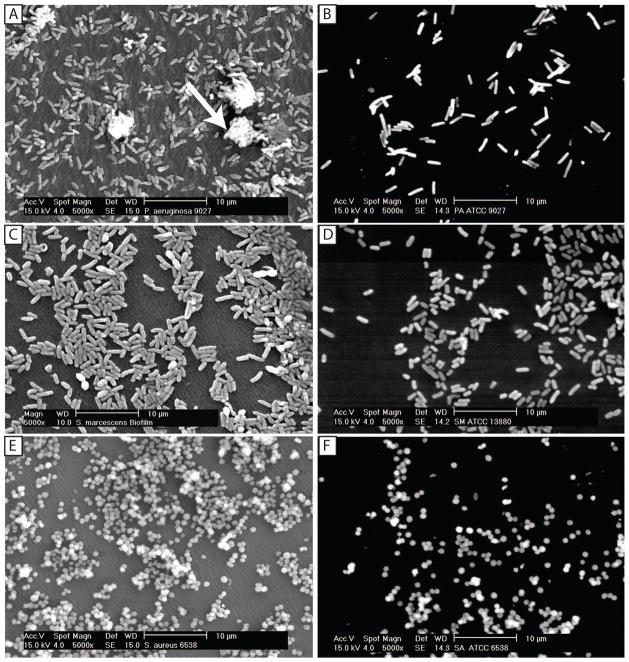
We used SEM to evaluate the gross morphology of bacterial biofilms formed on contact lenses. Our analyses revealed typical biofilm morphologies for the three bacterial isolates tested (Fig. 3). As expected, Pseudomonas biofilms exhibited a dense network of cells arranged in multiple layers, forming microcolonies, with clearly visible granular extracellular matrix (arrow, Fig. 3A). Similar topographies and microcolonies were observed in biofilms formed by S. marcescens and S. aureus (Fig. 3 C and E, respectively). Planktonically grown bacteria exhibited individual cells occurring mostly as monolayers, with no evidence of extracellular matrix (Fig. 3 B, D, and F).
Fig. 3.
Gross morphology and surface topography of biofilms formed by P. aeruginosa, S. marcescens and S. aureus, compared with the corresponding planktonically grown cells. Scanning electron microscopy was performed to evaluate gross morphology and surface topography of biofilms formed by (A) P. aeruginosa, (C) S. marcescens and (E) S. aureus after 18h incubation. Panels B, D, and F represent planktonically grown cells of these three bacteria for 18 h, respectively. Arrow indicates biofilm matrix. Magnification, x5000.
P. aeruginosa Formed Thicker Biofilms on Silicone Hydrogel Contact Lens than S. marcescens and S. aureus
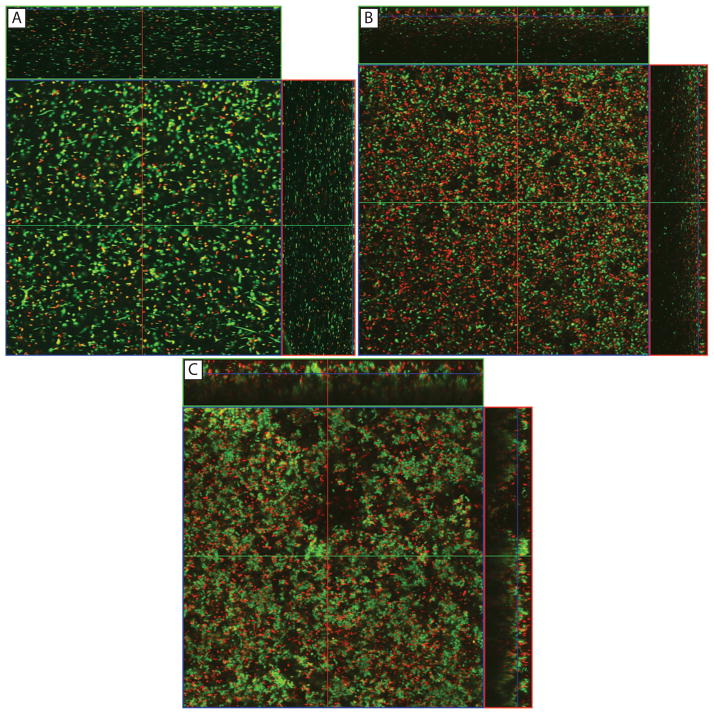
Confocal analyses revealed that biofilms formed by the three bacterial species exhibited heterogeneous architecture characterized by the presence of dense clusters of viable cells interspersed with dead cells (Fig. 4). Comparison of thickness of biofilms formed by these three species is shown in Table 3, and revealed that P. aeruginosa formed significantly thicker biofilm than S. marcescens and S. aureus (which had the thinnest biofilm; P < .02 for all comparisons). In contrast to biofilms, planktonically grown bacteria were present as single cells (data not shown).
Fig. 4.
Confocal analysis of the architecture of biofilms formed by P. aeruginosa, S. marcescens and S. aureus. Panels show orthogonal view of biofilms formed on silicone hydrogel contact lens by (A) P. aeruginosa, (B) S. marcescens, or (C) S. aureus. Magnification, x40.
Table 3.
Thickness of biofilms formed by bacterial species on silicone hydrogel contact lenses
| Species | Mean ± SD | 95% Confidence Interval for Mean | P – value (versus) | |||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | P. aeruginosa | S. marcescens | S. aureus | ||
| P. aeruginosa | 57.33 ± 3.05 | 49.74 | 64.92 | - | .002 | <.001 |
| S. marcescens | 43.33 ± 3.51 | 34.60 | 52.05 | .002 | - | .018 |
| S. aureus | 34.00 ± 1.00 | 31.51 | 36.48 | <.001 | .018 | - |
SD – Standard deviation.
Contact Lens Solutions are Active Against Planktonic but not Biofilm Forms of Bacteria
The ability of five multipurpose contact lens care solutions and the hydrogen peroxide-based Clear Care system to inhibit planktonic and biofilm growth of P. aeruginosa, S. marcescens and S. aureus was evaluated. As shown in Figure 5, all the multipurpose lens care solutions tested had at least a 6 log reduction of planktonically grown strains of P. aeruginosa, S. marcescens, and S. aureus. Incubation with the Clear Care solution led to a 6 log reduction of planktonically grown P. aeruginosa and S. marcescens, however, against S. aureus, it reduced CFU count by 5.07 logs which was not statistically different compared to the other products (P = .320). Importantly, all the care solutions met the ISO 14729 criteria of at least a 3-log reduction in CFUs for lens care solutions to be considered active against microbes.
Fig. 5.
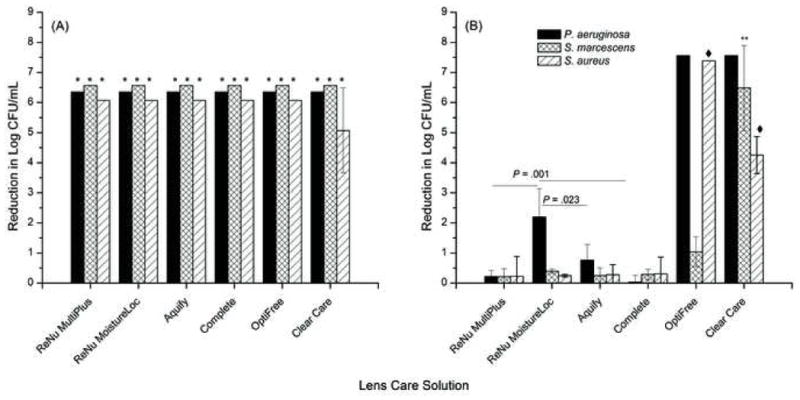
Activity of contact lens cleaning solutions against P. aeruginosa, S. marcescens, or S. aureus grown (A) as planktonic cells, or (B) as biofilms on lotrafilcon A lenses. The effect of contact lens care solutions against planktonically grown cells was evaluated according to International Organization for Standardization (ISO 14729) Stand Alone Procedure guidelines. The effect of contact lens care solutions against mature biofilm was assessed after soaking the biofilm coated lens in each manufacturer’s solution for the recommended soak time. Data represent reduction in log CFU (Mean ± Standard Deviation), for at least three replicates. *P ≤0.001 compared to untreated planktonic cells. Effects of Optifree (against P. aeruginosa and S. aureus) and Clear Care (against all three species) were significantly different (p<=0.02) from the biguanide preserved solutions. **P ≤ .001 when comparing effect of Clear Care solution against S. marcescens biofilms. ◆P ≤ .001 when comparing effect of OptiFree or Clear Care solutions against S. aureus biofilms.
In contrast to the activity of the lens care solutions against planktonically grown cells, four of the six solutions had little to no effect on the biofilm form of P. aeruginosa, S. marcescens, and S. aureus (Fig. 5B). Specifically, all the solutions containing biguanide preservative were completely ineffective against most biofilms formed by P. aeruginosa, S. marcescens, and S. aureus (no difference from the untreated control). The only exception was ReNu with MoistureLoc which significantly inhibited biofilm formation by P. aeruginosa (P = .001), however this approximately 2 log reduction was lower than that achieved against planktonic cells. Opti-Free Replenish solution had greater than a 7 log reduction of biofilms formed by P. aeruginosa and S. aureus (P < .0001) but was ineffective against biofilms formed by S. marcescens. In this regard, Opti-Free Replenish had a 1.04-log reduction in CFUs against S. marcescens biofilm which was not significantly different from the untreated control (P = .473). Clear Care, the only hydrogen peroxide system tested, was active against biofilms formed by all three bacterial species. In this regard, Clear Care exhibited a >7 log reduction against P. aeruginosa biofilm, a >6-log reduction of biofilm formed by S. marcescens, and a >4 log reduction of biofilm formed by S. aureus, respectively (all P < .0001 compared to untreated control and all biguanide preserved solutions). Compared to Opti-Free Replenish, Clear Care was more effective against S. marcescens biofilm (P < .0001), but less effective against S. aureus biofilm (P < .0001).
Taken together, our data show that these tested contact lens care solutions are active against planktonically grown P. aeruginosa, S. marcescens, and S. aureus cells. In general, hydrogen peroxide and polyquaternium preserved care solutions were more effective against contact lens associated biofilms compared to biguanide preserved solutions. However, the hydrogen peroxide system (Clear Care) was the only solution that was effective against biofilms formed by all three bacterial species.
DISCUSSION
In this study, we developed a reproducible in vitro model of bacterial biofilm formation on lotrafilcon A silicone hydrogel contact lenses and demonstrated that P. aeruginosa, S. marcescens, and S. aureus (major etiological agents of contact lens-associated infection and inflammation) can form biofilms on this contact lens polymer. We also showed that although all the tested lens care solutions were effective against planktonic bacterial growth, biguanide preserved care solutions were generally ineffective against bacterial biofilms in the developed in vitro model.
Several groups have investigated bacterial adhesion to contact lens surfaces and reported that this process is influenced by variables like temperature, culture media, lens material, surface coating, etc.37–42. Stapleton et al.43 showed that glycocalyx formation can occur as early as 30 minutes after adhesion of P. aeruginosa to low Dk soft lenses. However, there is a paucity of information on models of mature biofilms bound to soft contact lenses. The current study is the first description of a model that assesses antibacterial properties of lens care solutions against mature bacterial biofilms grown directly on silicone hydrogel contact lenses.
Bacterial adhesion has been shown to be higher on silicone hydrogel contact lens materials than on conventional hydrogels,40, 41, 44 which is likely to have clinical relevance because high levels of bacteria and silicone hydrogel lenses have been associated with some inflammatory events.6, 29, 45–48 In this regard, Selan et al.49 used a 24-well plate-based assay to demonstrate that in presence of BHI medium, phosphorylcholine-coated contact lenses were more resistant to bacterial adhesion and colonization compared to poly(2-hydroxyethyl methacrylate) (pHEMA) or silicone hydrogel lenses.
In our in vitro model, we found that biofilms formed by all three species reached mature phase by 18 h, although with distinct differences in their growth kinetics. For example, biofilm formation by P. aeruginosa progressed through early, intermediate and mature phases, while biofilm formation by S. marcescens exhibited a latent phase, followed by an exponential growth phase, reaching a plateau thereafter. Finally, S. aureus biofilms were characterized by a gradual increase in growth up to 24 h. Differential growth kinetics of biofilm formation have also been reported earlier for S. epidermidis and Enterococcus faecalis biofilms formed under varying conditions of hydrodynamic flow, and demonstrated latent, dynamic/accelerated, linear growth, and a mature phase during biofilm formation.50, 51 These results suggest that biofilm formation on contact lenses by different bacteria pass through distinct developmental phases.
We used SEM and CSLM to characterize the surface topography and three-dimensional architecture of bacterial biofilms. SEM analyses showed that biofilms exhibited a dense network of clumps of cells with visible granular extracellular matrix, while CSLM examination revealed that these biofilms exhibited heterogeneous architecture characterized by the presence of dense clusters of viable regions interspersed with nonviable regions. Earlier studies have also used SEM and CSLM to assess bacterial adherence and biofilms formed on contact lenses.10, 49, 52, 53 The clusters of microcolonies observed in our SEM analyses were similar to those reported earlier by McLaughlin-Borlace,10 who used SEM to evaluate biofilm formation on contact lenses and contact lens storage cases from patients with microbial keratitis. These investigators reported that biofilms were present more frequently (85%) on lens storage case surfaces compared with contact lens surfaces (55%). Moreover, these bacterial biofilms were comprised of clumps of cocci and sparse rods on contact lenses, while the lens cases had similar but more extensive biofilms. More recently, Selan et al.49 also used CSLM to show reduced adhesion and colonization of bacteria on phosphorylcholine-coated contact lenses compared to silicone-hydrogel and pHEMA lenses. The architecture of bacterial biofilms reported by these investigators was similar to those observed in this study.
One variable that can also impact the topography and architecture of bacterial biofilms formed on contact lenses is whether the lenses are unworn, or worn (which would be exposed to tear film during use). In the current study, we used fresh, unworn contact lenses, and thus were not exposed to tear film. The formation of a conditioning film comprised of tear proteins and other organic material has been shown to profoundly influence the physio-chemical properties of biomedical surfaces.38, 44 The effect of tear film deposition on contact lens biofilm formation is currently being investigated using the in vitro bacterial biofilm model described in this study.
Our studies revealed that all tested solutions exhibited potent activity against planktonically grown P. aeruginosa, S. marcescens, and S. aureus. When tested against biofilms, only the peroxide based care system (Clear Care) exhibited activity against all three bacterial species. The polymeric biguanide preserved solutions showed much less and variable activity against biofilms formed by these same organisms on lotrafilcon A lenses. Specifically, ReNu with MoistureLoc, MultiPlus, Aquify, and Complete MoisturePlus were ineffective against bacterial biofilms. Finally, a polyquaternium preserved MPDS (OPTI-Free Replenish) was very effective against biofilms formed by P. aeruginosa and S. aureus, but not those formed by S. marcescens. Hydrogen peroxide destabilizes cells and organic substrates via oxidation. OPTI-Free Replenish actually contains two biocides: polyquaternium-1, a quaternary ammonium compound, and myristamidopropyl dimethylamine. Polyquaternium-1 is predominantly antibacterial and myristamidopropyl dimethylamine is mainly antifungal and antiprotozoal.54 The exact mechanisms by which these substances are lethal to microorganisms are unknown, but it is believed they induce cytoplasmic membrane damage in P. aeruginosa, S. marcescens, and S. aureus.54, 55
Among the three species of biofilms tested in this study, S. marcescens biofilms were not susceptible to any of the multipurpose solutions while the planktonic form was always completely susceptible. Our findings are comparable to those reported earlier by Wilson et al.,21 who showed that biofilms formed by P. aeruginosa, S. marcescens, S. epidermidis, Streptococcus pyogenes, and C. albicans on wells of polyethylene contact lens cases retained viability to certain contact lens disinfectant solutions after exposure for the manufacturer’s minimum recommended disinfection times. These investigators also showed that biofilms formed by S. marcescens on lens cases were the most resistant to a number of disinfectant solutions. In a recent study, Vermeltfoort et al.20 showed that three different multipurpose care solutions (Opti-Free Express®, ReNu MultiPlus®, and SoloCare Aqua™) were not active against biofilms formed by P. aeruginosa and S. aureus on lens storage cases. However, Opti-Free was the most effective in reducing transfer of S. aureus bacterial cells from a biofilm laden case to a silicone hydrogel lens soaked for 8 hours within the case. These investigators evaluated the antimicrobial activity of disinfectant solutions against biofilms formed on contact lens cases, while we evaluated the susceptibility of biofilms formed on contact lenses. Nonetheless, our results are similar; suggesting that biocide-resistance of these biofilms is a broad phenotype.
We have intentionally not specified minimum criteria for defining efficacy of the care solutions against biofilm. The FDA criterion of a minimum 3 log reduction within the minimum recommended disinfection period applies only to the microbial challenge of planktonic bacteria inoculated during the stand alone test procedure. Just as the size of the microbial challenge recommended by ISO and FDA Stand Alone Procedure is not intended to be representative of the likely challenge in practice, the microbial challenge we have created in our contact lens-biofilm model is not intended to represent in vivo biofilms but to provide countable numbers from which estimation of the extent of viability loss can be determined.
The efficacy of multipurpose contact lens care solutions has come under scrutiny in the last two years.33, 34 Our study, like those of others,21, 56 reveals inadequacies in the testing procedures recommended by the FDA Premarket Notification 510(k) Guidance Document for Contact Lens Care Products.32 Although the FDA is seeking to revise its guidelines, currently, the disinfecting effect of contact lens care solutions for licensing purposes continues to be tested against planktonically grown microbial cells. Since biofilms are associated with contact lenses, their carrier cases, and adverse events, it is essential to incorporate testing of lens care solutions for activity against biofilms prior to launching a new product.
In conclusion, contact lens associated bacterial biofilms are more resistant than planktonic cells to antimicrobial agents in marketed contact lens solutions. These studies indicate an essential mechanism by which organisms may cause contact lens associated keratitis, and provide a model for understanding the biology, pathogenesis, and resistance mechanisms of contact lens-associated bacterial biofilms.
Acknowledgments
This work was supported by funds from the NIH (R01 DE017486-01A1, R01DE 13932-4, K23 EY015270-01 (LSF), EY18612 (EP), and P30 EY11373 (EP)), the Bristol Myers Squibb Freedom to Discover Award to MAG, the American Heart Association (Scientist Development Grant 0335313N) Award to PKM, Research to Prevent Blindness (RPB) Foundation, the Ohio Lions Eye Research Foundation, and the CLAO Education and Research Foundation (LSF). Assistance of the Confocal Scanning Laser Microscopy core facility (NCI grant P30CA43703-12) at Case Western Reserve University is gratefully acknowledged.
Footnotes
DISCLOSURES: This work was not funded by any corporate entity. However, LSF received research funding within the past 12 months from the following companies: Ciba Vision, Vistakon and CooperVision.
References
- 1.Woods CA, Jones DA, Jones LW, et al. A seven year survey of the contact lens prescribing habits of Canadian optometrists. Optom Vis Sci. 2007;84:505–510. doi: 10.1097/OPX.0b013e318073c318. [DOI] [PubMed] [Google Scholar]
- 2.Mack C. Contact Lenses 2007. Contact Lens Spectrum. 2008;23(1):26–34. [Google Scholar]
- 3.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–783. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- 4.Schein OD, McNally JJ, Katz J, et al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112:2172–2179. doi: 10.1016/j.ophtha.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Szczotka-Flynn L, Diaz-Insua M. Risk of corneal inflammatory events with silicone hydrogel and low Dk hydrogel extended contact lens wear: A Meta-analysis. Optometry & Vision Science. 2007;125:1–5. doi: 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- 7.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elder MJ, Stapleton F, Evans E, et al. Biofilm-related infections in ophthalmology. Eye. 1995;9 (Pt1):102–109. doi: 10.1038/eye.1995.16. [DOI] [PubMed] [Google Scholar]
- 9.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15:141–147. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin-Borlace L, Stapleton F, Matheson M, et al. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84:827–838. doi: 10.1046/j.1365-2672.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton F, Dart J. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br J Ophthalmol. 1995;79:864–865. doi: 10.1136/bjo.79.9.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zegans ME, Shanks RM, O’Toole GA. Bacterial biofilms and ocular infections. The ocular surface. 2005;3:73–80. doi: 10.1016/s1542-0124(12)70155-6. [DOI] [PubMed] [Google Scholar]
- 13.Zegans ME, Becker HI, Budzik J, et al. The role of bacterial biofilms in ocular infections. DNA and Cell Biology. 2002;21:415–420. doi: 10.1089/10445490260099700. [DOI] [PubMed] [Google Scholar]
- 14.Lattif AA, Chandra J, Chang J, et al. Proteomic and pathway analyses reveal phase-dependent over- expression of proteins associated with carbohydrate metabolic pathways in Candida albicans biofilms. The Open Proteomics Journal. 2008;1:5–26. [Google Scholar]
- 15.O’Toole GA, Pratt LA, Watnick PI, et al. Genetic approaches to study of biofilms. Methods Enzymol. 1999;310:91–109. doi: 10.1016/s0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 16.Yeater KM, Chandra J, Cheng G, et al. Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology. 2007;153:2373–2385. doi: 10.1099/mic.0.2007/006163-0. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Sanchez S, Aubert S, Iraqui I, et al. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukar Cell. 2004;3:536–545. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beenken KE, Dunman PM, McAleese F, et al. Global Gene Expression in Staphylococcus aureus Biofilms. The Journal of Bacteriology. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schembri MA, Kjaergaard K, Klemm P. Global gene expression in Escherichia coli biofilms. Mol Microbiol. 2003;48:253–267. doi: 10.1046/j.1365-2958.2003.03432.x. [DOI] [PubMed] [Google Scholar]
- 20.Vermeltfoort PB, Hooymans JM, Busscher HJ, et al. Bacterial transmission from lens storage cases to contact lenses-Effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008 doi: 10.1002/jbm.b.31102. [DOI] [PubMed] [Google Scholar]
- 21.Wilson LA, Sawant AD, Ahearn DG. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991;109:1155–1157. doi: 10.1001/archopht.1991.01080080115043. [DOI] [PubMed] [Google Scholar]
- 22.Evans DJ, Allison DG, Brown MR, et al. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 23.Imamura Y, Chandra J, Mukherjee PK, et al. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52:171–182. doi: 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfonso E, Mandelbaum S, Fox MJ, et al. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986;101:429–433. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- 25.Donnenfeld ED, Cohen EJ, Arentsen JJ, et al. Changing trends in contact lens associated corneal ulcers: an overview of 116 cases. CLAO J. 1986;12:145–149. [PubMed] [Google Scholar]
- 26.Hume EB, Stapleton F, Willcox MD. Evasion of cellular ocular defenses by contact lens isolates of Serratia marcescens. Eye Contact Lens. 2003;29:108–112. doi: 10.1097/01.ICL.0000062461.24391.7F. [DOI] [PubMed] [Google Scholar]
- 27.Hume EB, Willcox MD. Emergence of Serratia marcescens as an ocular surface pathogen. Arch Soc Esp Oftalmol. 2004;79:475–477. [PubMed] [Google Scholar]
- 28.Hume EB, Zhu H, Cole N, et al. Efficacy of contact lens multipurpose solutions against Serratia marcescens. Optom Vis Sci. 2007;84:316–320. doi: 10.1097/OPX.0b013e3180465543. [DOI] [PubMed] [Google Scholar]
- 29.Jalbert I, Willcox MD, Sweeney DF. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea. 2000;19:116–120. doi: 10.1097/00003226-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 31.Wu PZ, Thakur A, Stapleton F, et al. Staphylococcus aureus causes acute inflammatory episodes in the cornea during contact lens wear. Clin Experiment Ophthalmol. 2000;28:194–196. doi: 10.1046/j.1442-9071.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. Premarket notification [510(k)] guidance document for contact lens care products. Washington, D.C: Center for Devices and Radiological Health; 1997. [Google Scholar]
- 33.Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 34.Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144:169–180. doi: 10.1016/j.ajo.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans - development, architecture and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra J, McCormick TS, Imamura Y, et al. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun. 2007;75:2612–2620. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews CS, Denyer SP, Hall B, et al. A comparison of the use of an ATP-based bioluminescent assay and image analysis for the assessment of bacterial adhesion to standard HEMA and biomimetic soft contact lenses. Biomaterials. 2001;22:3225–3233. doi: 10.1016/s0142-9612(01)00160-0. [DOI] [PubMed] [Google Scholar]
- 38.Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–3224. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Saenz MC, Arias-Puente A, Fresnadillo-Martinez MJ, et al. Adherence of two strains of Staphylococcus epidermidis to contact lenses. Cornea. 2002;21:511–515. doi: 10.1097/00003226-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Henriques M, Sousa C, Lira M, et al. Adhesion of Pseudomonas aeruginosa and Staphylococcus epidermidis to silicone-hydrogel contact lenses. Optom Vis Sci. 2005;82:446–450. doi: 10.1097/01.opx.0000168585.53845.64. [DOI] [PubMed] [Google Scholar]
- 41.Kodjikian L, Casoli-Bergeron E, Malet F, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2007 doi: 10.1007/s00417-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 42.Schultz CL, Pezzutti MR, Silor D, et al. Bacterial adhesion measurements on soft contact lenses using a Modified Vortex Device and a Modified Robbins Device. J Ind Microbiol. 1995;15:243–247. doi: 10.1007/BF01569831. [DOI] [PubMed] [Google Scholar]
- 43.Stapleton F, Dart J, Matheson M. Woodward G Bacterial Adherence and Glycocalyx Formation on Unworn Hydrogel Lenses. Journal of the British Contact Lens Association. 1993;16:113–117. [Google Scholar]
- 44.Willcox MD, Harmis N, Cowell, et al. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials. 2001;22:3235–3247. doi: 10.1016/s0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- 45.Holden BA, La Hood D, Grant T, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 46.Sankaridurg PR, Willcox MD, Sharma S, et al. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–2431. doi: 10.1128/jcm.34.10.2426-2431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sankaridurg PR, Vuppala N, Sreedharan A, et al. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996;44:29–32. [PubMed] [Google Scholar]
- 48.Sankaridurg PR, Sharma S, Willcox M, et al. Colonization of hydrogel lenses with Streptococcus pneumoniae: risk of development of corneal infiltrates. Cornea. 1999;18:289–295. doi: 10.1097/00003226-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Selan L, Palma S, Scoarughi GL, et al. Phosphorylcholine Impairs Susceptibility to Biofilm Formation of Hydrogel Contact Lenses. Am J Ophthalmol. 2008 doi: 10.1016/j.ajo.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Baillif S, Casoli E, Marion K, et al. A novel in vitro model to study staphylococcal biofilm formation on intraocular lenses under hydrodynamic conditions. Invest Ophthalmol Vis Sci. 2006;47:3410–3416. doi: 10.1167/iovs.05-1070. [DOI] [PubMed] [Google Scholar]
- 51.Baillif S, Ecochard R, Casoli E, et al. Adherence and kinetics of biofilm formation of Staphylococcus epidermidis to different types of intraocular lenses under dynamic flow conditions. J Cataract Refract Surg. 2008;34:153–158. doi: 10.1016/j.jcrs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 52.Slusher MM, Myrvik QN, Lewis JC, et al. Extended-wear lenses, biofilm, and bacterial adhesion. Arch Ophthalmol. 1987;105:110–115. doi: 10.1001/archopht.1987.01060010116042. [DOI] [PubMed] [Google Scholar]
- 53.Farber BF, Hsieh HC, Donnenfeld ED, et al. A novel antibiofilm technology for contact lens solutions. Ophthalmology. 1995;102:831–836. doi: 10.1016/s0161-6420(95)30949-9. [DOI] [PubMed] [Google Scholar]
- 54.Codling CE, Maillard JY, Russell AD. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J Antimicrob Chemother. 2003;51:1153–1158. doi: 10.1093/jac/dkg228. [DOI] [PubMed] [Google Scholar]
- 55.Codling CE, Hann AC, Maillard JY, et al. An investigation into the antimicrobial mechanisms of action of two contact lens biocides using electron microscopy. Cont Lens Anterior Eye. 2005;28:163–168. doi: 10.1016/j.clae.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 56.May LL, Gabriel MM, Simmons RB, et al. Resistance of adhered bacteria to rigid gas permeable contact lens solutions. CLAO J. 1995;21:242–246. [PubMed] [Google Scholar]