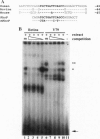
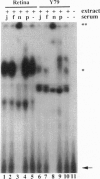
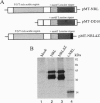
Abstract
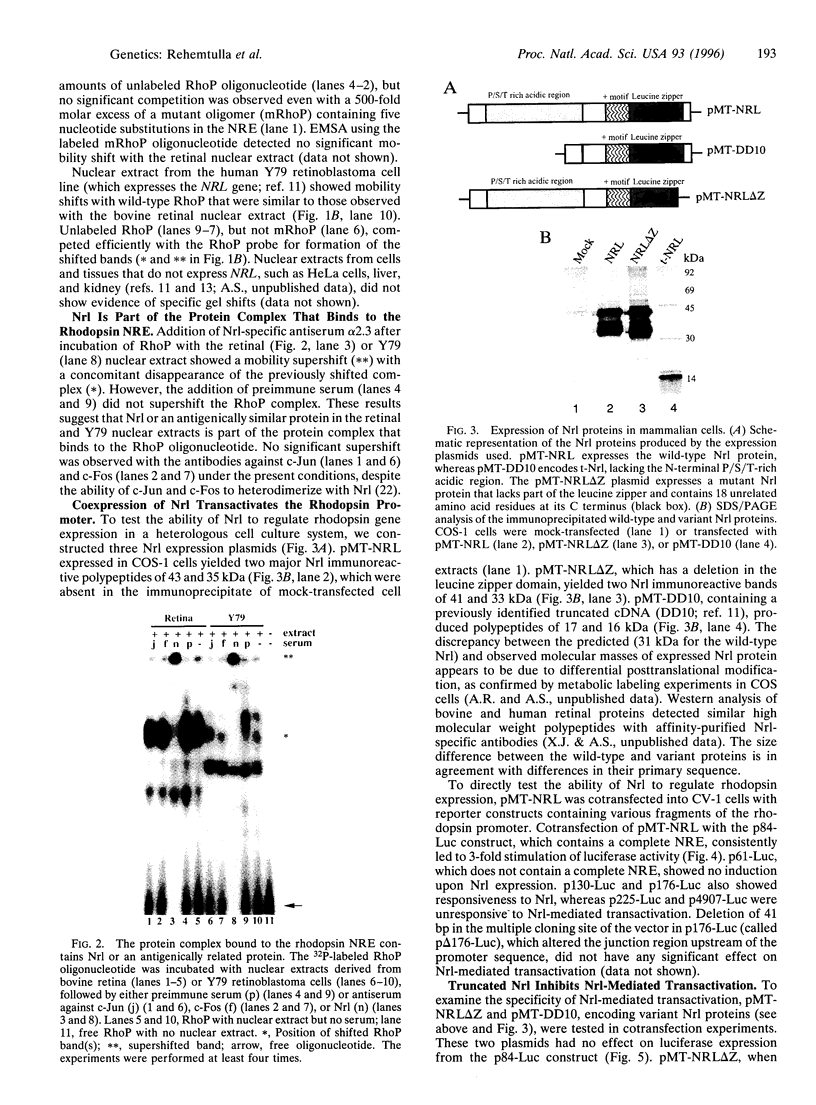
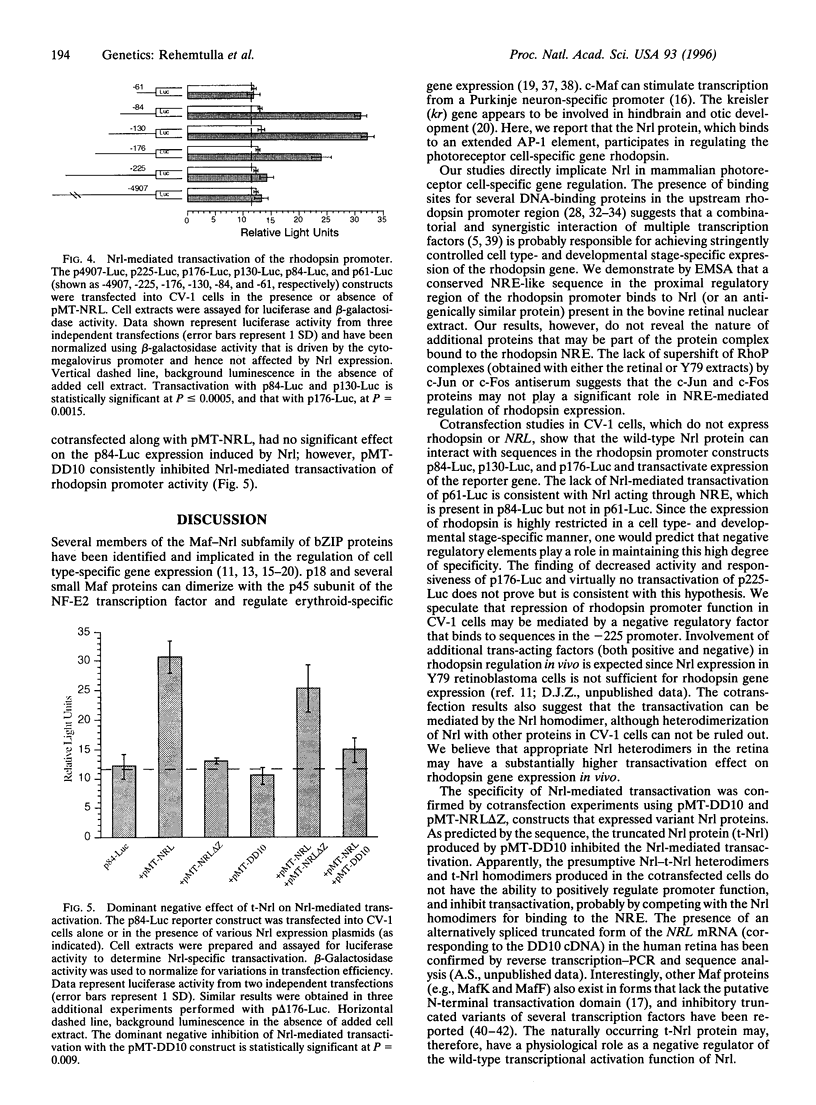
The retinal protein Nrl belongs to a distinct subfamily of basic motif-leucine zipper DNA-binding proteins and has been shown to bind extended AP-1-like sequence elements as a homo- or heterodimer. Here, we demonstrate that Nrl can positively regulate the expression of the photoreceptor cell-specific gene rhodopsin. Electrophoretic mobility-shift analysis reveals that a protein(s) in nuclear extracts from bovine retina and the Y79 human retinoblastoma cell line binds to a conserved Nrl response element (NRE) in the upstream promoter region of the rhodopsin gene. Nrl or an antigenically similar protein is shown to be part of the bound protein complex by supershift experiments using Nrl-specific antiserum. Cotransfection studies using an Nrl-expression plasmid and a luciferase reporter gene demonstrate that interaction of the Nrl protein with the -61 to -84 region of the rhodopsin promoter (which includes the NRE) stimulates expression of the reporter gene in CV-1 monkey kidney cells. This Nrl-mediated transactivation is specifically inhibited by coexpression of a naturally occurring truncated form of Nrl (dominant negative effect). Involvement of Nrl in photoreceptor gene regulation and its continued high levels of expression in the adult retina suggest that Nrl plays a significant role in controlling retinal function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. Determination of cellular types in the retina. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1677–1682. [PubMed] [Google Scholar]
- Andrews N. C., Kotkow K. J., Ney P. A., Erdjument-Bromage H., Tempst P., Orkin S. H. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J. A molecular view of vertebrate retinal development. Mol Neurobiol. 1987 Spring-Summer;1(1-2):9–46. doi: 10.1007/BF02935263. [DOI] [PubMed] [Google Scholar]
- Beebe D. C. Homeobox genes and vertebrate eye development. Invest Ophthalmol Vis Sci. 1994 Jun;35(7):2897–2900. [PubMed] [Google Scholar]
- Buettner R., Kannan P., Imhof A., Bauer R., Yim S. O., Glockshuber R., Van Dyke M. W., Tainsky M. A. An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol Cell Biol. 1993 Jul;13(7):4174–4185. doi: 10.1128/mcb.13.7.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes S. P., Barsh G. S. The mouse segmentation gene kr encodes a novel basic domain-leucine zipper transcription factor. Cell. 1994 Dec 16;79(6):1025–1034. doi: 10.1016/0092-8674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Engel J. D. Meticulous AP-1 factors. Nature. 1994 Feb 10;367(6463):516–517. doi: 10.1038/367516a0. [DOI] [PubMed] [Google Scholar]
- Farjo Q., Jackson A. U., Xu J., Gryzenia M., Skolnick C., Agarwal N., Swaroop A. Molecular characterization of the murine neural retina leucine zipper gene, Nrl. Genomics. 1993 Nov;18(2):216–222. doi: 10.1006/geno.1993.1458. [DOI] [PubMed] [Google Scholar]
- Fujiwara K. T., Kataoka K., Nishizawa M. Two new members of the maf oncogene family, mafK and mafF, encode nuclear b-Zip proteins lacking putative trans-activator domain. Oncogene. 1993 Sep;8(9):2371–2380. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrímsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul 30;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kataoka K., Itoh K., Hayashi N., Nishizawa M., Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994 Feb 10;367(6463):568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Fujiwara K. T., Noda M., Nishizawa M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol. 1994 Nov;14(11):7581–7591. doi: 10.1128/mcb.14.11.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Noda M., Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994 Jan;14(1):700–712. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. A conserved region adjacent to the basic domain is required for recognition of an extended DNA binding site by Maf/Nrl family proteins. Oncogene. 1994 Nov;9(11):3149–3158. [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun. Oncogene. 1994 Mar;9(3):675–684. [PubMed] [Google Scholar]
- Kurschner C., Morgan J. I. The maf proto-oncogene stimulates transcription from multiple sites in a promoter that directs Purkinje neuron-specific gene expression. Mol Cell Biol. 1995 Jan;15(1):246–254. doi: 10.1128/mcb.15.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lem J., Applebury M. L., Falk J. D., Flannery J. G., Simon M. I. Tissue-specific and developmental regulation of rod opsin chimeric genes in transgenic mice. Neuron. 1991 Feb;6(2):201–210. doi: 10.1016/0896-6273(91)90356-5. [DOI] [PubMed] [Google Scholar]
- Liu I. S., Chen J. D., Ploder L., Vidgen D., van der Kooy D., Kalnins V. I., McInnes R. R. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994 Aug;13(2):377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Morabito M. A., Yu X., Barnstable C. J. Characterization of developmentally regulated and retina-specific nuclear protein binding to a site in the upstream region of the rat opsin gene. J Biol Chem. 1991 May 25;266(15):9667–9672. [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991 Feb 22;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. In the eye of the beholder: visual pigments and inherited variation in human vision. Cell. 1994 Aug 12;78(3):357–360. doi: 10.1016/0092-8674(94)90414-6. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Kataoka K., Goto N., Fujiwara K. T., Kawai S. v-maf, a viral oncogene that encodes a "leucine zipper" motif. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A., Kaufman R. J. Preferred sequence requirements for cleavage of pro-von Willebrand factor by propeptide-processing enzymes. Blood. 1992 May 1;79(9):2349–2355. [PubMed] [Google Scholar]
- Sheshberadaran H., Takahashi J. S. Characterization of the chicken rhodopsin promoter: identification of retina-specific and glass-like protein binding domains. Mol Cell Neurosci. 1994 Aug;5(4):309–318. doi: 10.1006/mcne.1994.1037. [DOI] [PubMed] [Google Scholar]
- Struhl K. Mechanisms for diversity in gene expression patterns. Neuron. 1991 Aug;7(2):177–181. doi: 10.1016/0896-6273(91)90256-y. [DOI] [PubMed] [Google Scholar]
- Swaroop A., Xu J. Z., Pawar H., Jackson A., Skolnick C., Agarwal N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Adler R. A developmentally regulated basic-leucine zipper-like gene and its expression in embryonic retina and lens. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1351–1355. doi: 10.1073/pnas.91.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J., Barr P. J., Wong P. A., Kiefer M. C., Brake A. J., Kaufman R. J. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M., Zhou L., Peng Y. W., Eddy R. L., Shows T. B., Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993 Oct;11(4):689–701. doi: 10.1016/0896-6273(93)90079-7. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Swaroop A. Neural retina-specific leucine zipper gene NRL (D14S46E) maps to human chromosome 14q11.1-q11.2. Genomics. 1992 Oct;14(2):491–492. doi: 10.1016/s0888-7543(05)80248-4. [DOI] [PubMed] [Google Scholar]
- Yen J., Wisdom R. M., Tratner I., Verma I. M. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Chung M., Morabito M. A., Barnstable C. J. Shared nuclear protein binding sites in the upstream region of the rat opsin gene. Biochem Biophys Res Commun. 1993 Feb 26;191(1):76–82. doi: 10.1006/bbrc.1993.1186. [DOI] [PubMed] [Google Scholar]
- Zack D. J., Bennett J., Wang Y., Davenport C., Klaunberg B., Gearhart J., Nathans J. Unusual topography of bovine rhodopsin promoter-lacZ fusion gene expression in transgenic mouse retinas. Neuron. 1991 Feb;6(2):187–199. doi: 10.1016/0896-6273(91)90355-4. [DOI] [PubMed] [Google Scholar]