Abstract
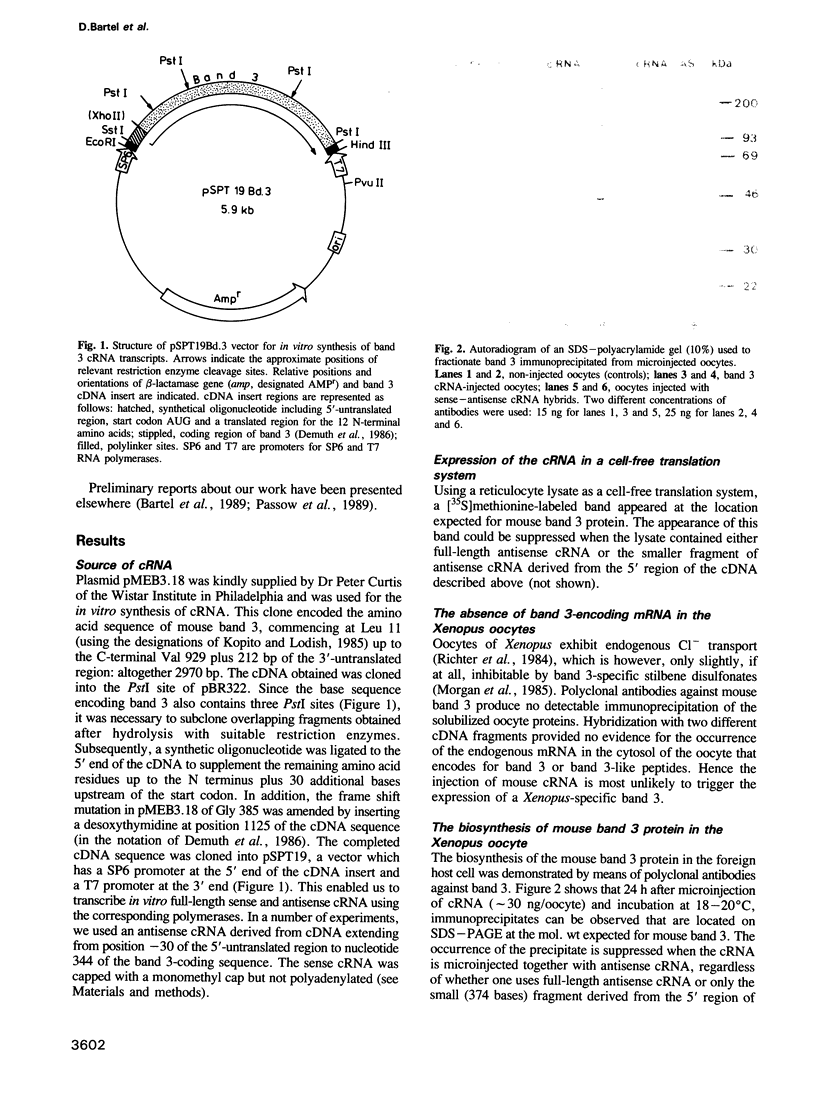
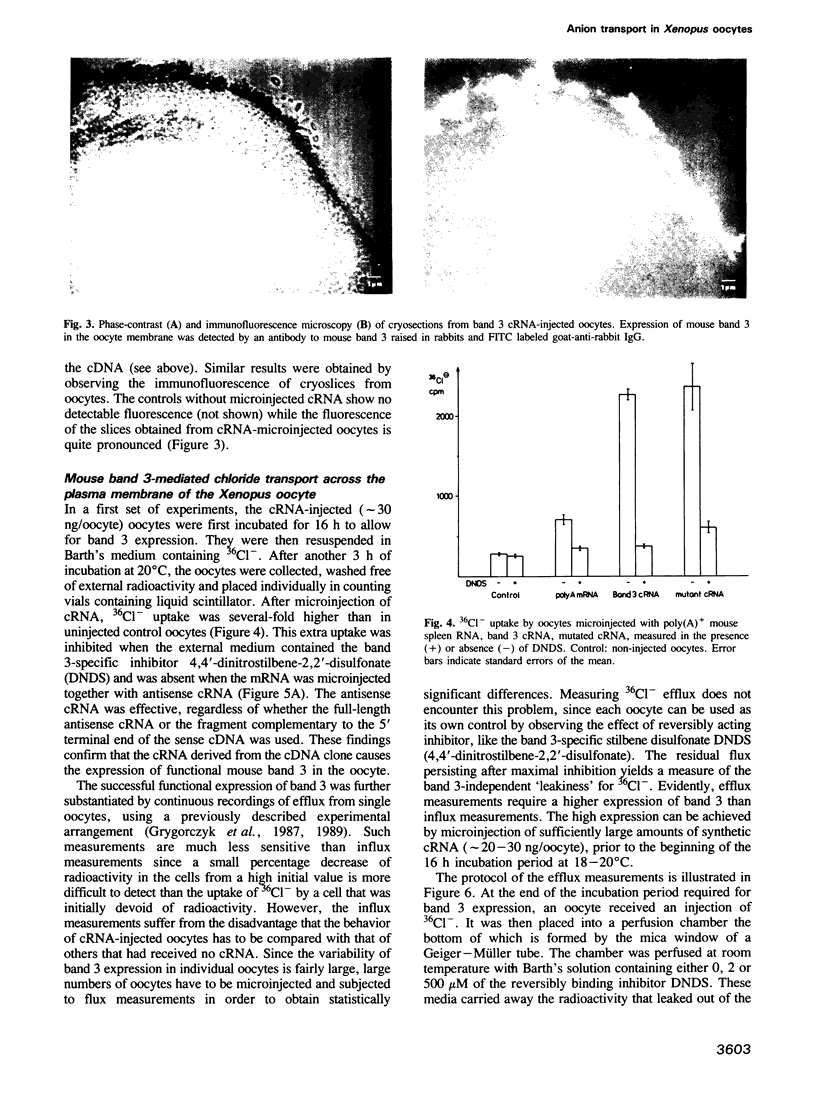
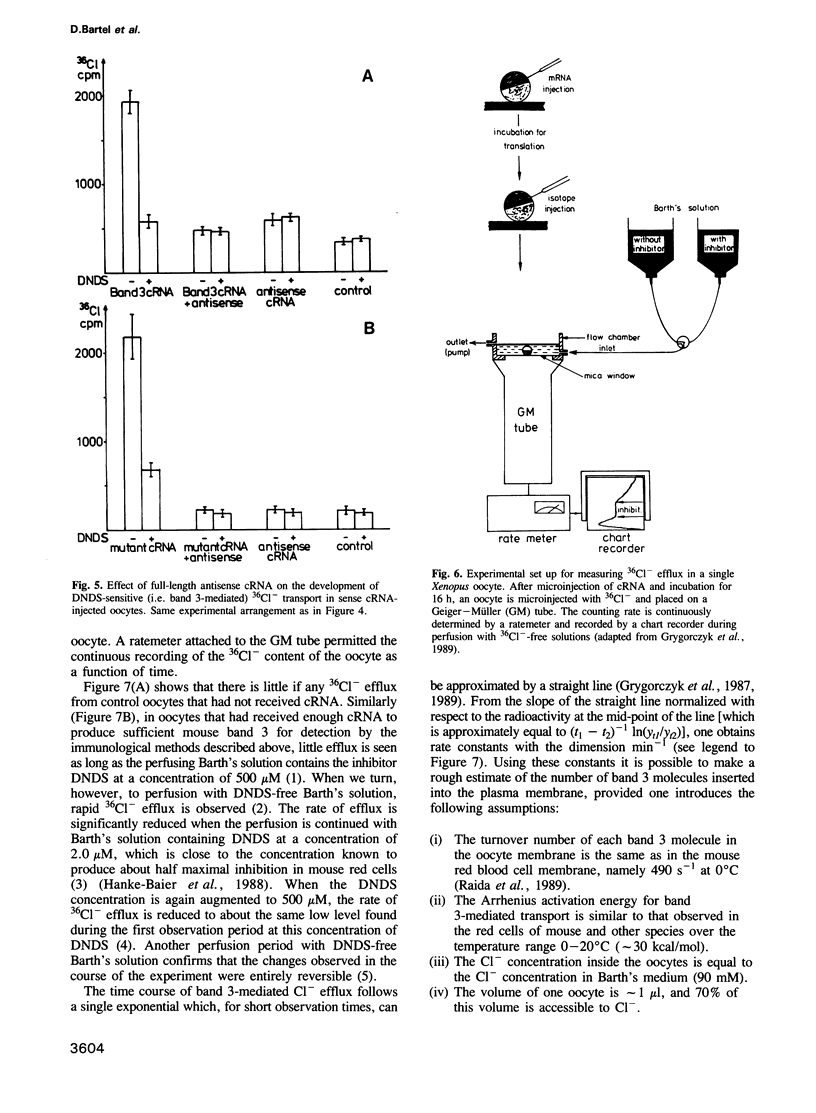
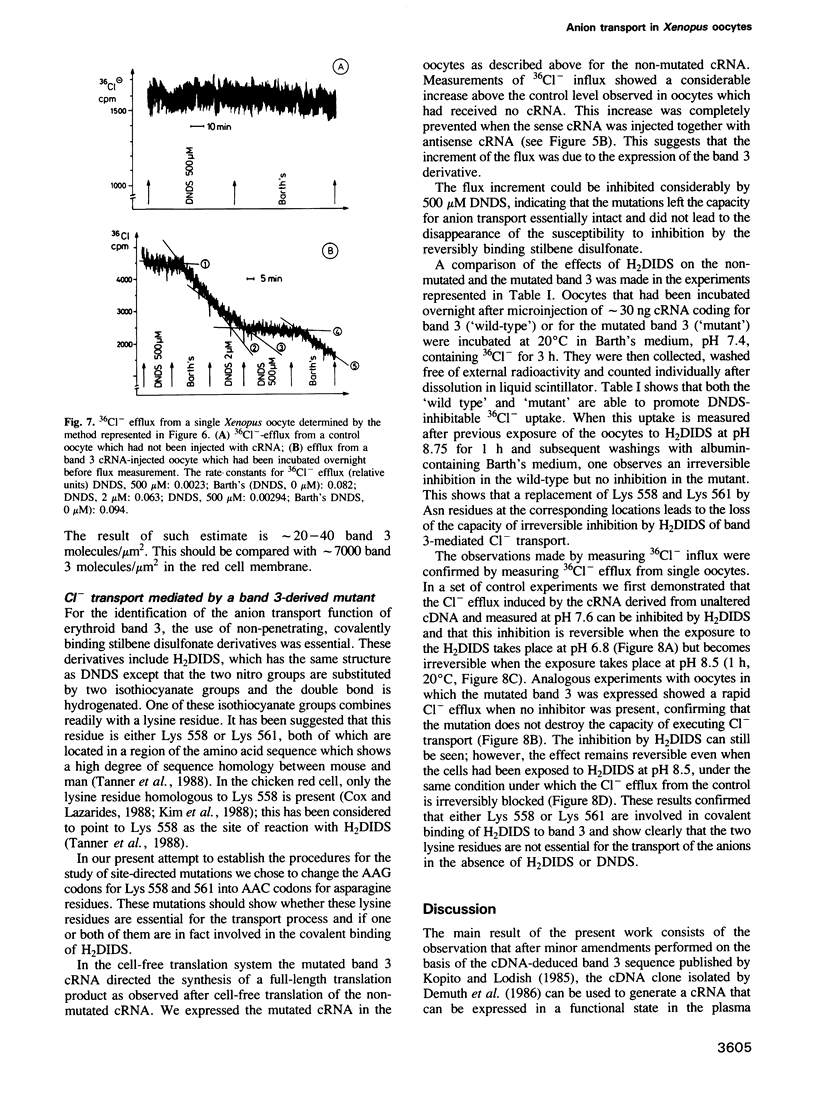
A vector was constructed containing a cDNA for mouse band 3 obtained from Demuth et al. (1986, EMBO J., 5, 1205-1214), a synthetic linker (containing 5'-non-translated region, start codon and a coding region for the first 12 N-terminal amino acids), and RNA polymerase promoters suitable for in vitro transcription of cRNA. After injection of the cRNA into the cytoplasm of Xenopus oocytes and incubation for 16 h, expression of mouse band 3 was demonstrated by immunoprecipitation, immunohistochemical methods and influx or efflux measurements with 36Cl-. Antisense cRNA inhibits the expression. Lysines 558 and 561 were replaced by asparagines using oligonucleotide-directed mutagenesis. Like the original band 3, the mutant shows stilbene disulfonate-inhibitable anion exchange. However, in contrast to the original band 3, inhibition by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate (H2DIDS) is no longer irreversible. This indicates that thiourea bond formation between H2DIDS and band 3 involves one of the two modified lysine residues. It also shows that the two lysine residues are not essential for the execution of the anion transport function of band 3. The results described suggest that the cDNA clone of Demuth et al. (1986) encodes a protein with properties that are representative for the properties of the bulk of the band 3 protein in the plasma membrane of the red cell of the mouse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Kopito R. R., Libresco S. M., Lodish H. F. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem. 1988 Nov 15;263(32):17092–17099. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay M., Cabantchik Z. I. Anion transport in red blood cells. II. Kinetics of reversible inhibition by nitroaromatic sulfonic acids. Membr Biochem. 1979;2(2):255–281. doi: 10.3109/09687687909063867. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Biosynthesis of the erythrocyte anion transport protein. J Biol Chem. 1981 Nov 10;256(21):11337–11344. [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Schlüter K., Allen D. P., Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985 Dec 13;230(4731):1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., Armstrong J., Colman A. The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res. 1985 Oct 25;13(20):7375–7394. doi: 10.1093/nar/13.20.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Pace R. J., Chan S. I. Chloride binding to the anion transport binding sites of band 3. A 35Cl NMR study. J Biol Chem. 1984 May 25;259(10):6472–6480. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R., Hanke-Baier P., Schwarz W., Passow H. Measurement of erythroid band 3 protein-mediated anion transport in mRNA-injected oocytes of Xenopus laevis. Methods Enzymol. 1989;173:453–466. doi: 10.1016/s0076-6879(89)73032-9. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R., Schwarz W., Passow H. Potential dependence of the "electrically silent" anion exchange across the plasma membrane of Xenopus oocytes mediated by the band-3 protein of mouse red blood cells. J Membr Biol. 1987;99(2):127–136. doi: 10.1007/BF01871232. [DOI] [PubMed] [Google Scholar]
- Hanke-Baier P., Raida M., Passow H. Comparison of murine band 3 protein-mediated Cl- transport as measured in mouse red blood cells and in oocytes of Xenopus laevis. Biochim Biophys Acta. 1988 May 9;940(1):136–140. doi: 10.1016/0005-2736(88)90017-x. [DOI] [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Ideguchi H., Matsuyama H., Hamasaki N. Heterogeneity of human erythrocyte band 3 analyzed by two-dimensional gel electrophoresis. Eur J Biochem. 1982 Jul;125(3):665–671. doi: 10.1111/j.1432-1033.1982.tb06734.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Kinetics and mechanism of anion transport in red blood cells. Annu Rev Physiol. 1985;47:519–533. doi: 10.1146/annurev.ph.47.030185.002511. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Tracey C. M., Goodman J. R., Cone J. C., Bassel P. S. Polypeptides immunologically related to band 3 are present in nucleated somatic cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6882–6886. doi: 10.1073/pnas.80.22.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. R., Yew N. S., Ansorge W., Voss H., Schwager C., Vennström B., Zenke M., Engel J. D. Two different mRNAs are transcribed from a single genomic locus encoding the chicken erythrocyte anion transport proteins (band 3). Mol Cell Biol. 1988 Oct;8(10):4416–4424. doi: 10.1128/mcb.8.10.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Marchesi V. T. The carboxyl-terminal domain of human erythrocyte band 3. Description, isolation, and location in the bilayer. J Biol Chem. 1981 Jun 25;256(12):6463–6468. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M., Hanke P., Grygorczyk R., Tintschl A., Fasold H., Passow H. Mediation of anion transport in oocytes of Xenopus laevis by biosynthetically inserted band 3 protein from mouse spleen erythroid cells. EMBO J. 1985 Aug;4(8):1927–1931. doi: 10.1002/j.1460-2075.1985.tb03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T. J., Li Y. T., Morrison M. Effect of endo-beta-galactosidase on intact human erythrocytes. J Biol Chem. 1979 Sep 10;254(17):8103–8106. [PubMed] [Google Scholar]
- Pappert G., Schubert D. The state of association of band 3 protein of the human erythrocyte membrane in solutions of nonionic detergents. Biochim Biophys Acta. 1983 Apr 21;730(1):32–40. doi: 10.1016/0005-2736(83)90313-9. [DOI] [PubMed] [Google Scholar]
- Passow H., Fasold H., Gärtner E. M., Legrum B., Ruffing W., Zaki L. Anion transport across the red blood cell membrane and the conformation of the protein in Band 3. Ann N Y Acad Sci. 1980;341:361–383. doi: 10.1111/j.1749-6632.1980.tb47184.x. [DOI] [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:61–203. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Richter H. P., Jung D., Passow H. Regulatory changes of membrane transport and ouabain binding during progesterone-induced maturation of Xenopus oocytes. J Membr Biol. 1984;79(3):203–210. doi: 10.1007/BF01871059. [DOI] [PubMed] [Google Scholar]
- Sabban E., Marchesi V., Adesnik M., Sabatini D. D. Erythrocyte membrane protein band 3: its biosynthesis and incorporation into membranes. J Cell Biol. 1981 Dec;91(3 Pt 1):637–646. doi: 10.1083/jcb.91.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ship S., Shami Y., Breuer W., Rothstein A. Synthesis of tritiated 4,4'-diisothiocyano-2,2'-stilbene disulfonic acid ([3H]DIDS) and its covalent reaction with sites related to anion transport in human red blood cells. J Membr Biol. 1977 May 12;33(3-4):311–323. doi: 10.1007/BF01869522. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The band 3 protein of the human red cell membrane: a review. J Supramol Struct. 1978;8(3):311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. L., Keast R. K., Jennings M. L., Pessin J. E. Heterogeneity in the human erythrocyte band 3 anion-transporter revealed by Triton X-114 phase partitioning. Biochem J. 1988 Oct 1;255(1):229–234. [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis R. H., Rubin H. Calcium protects DNase I from proteinase K: a new method for the removal of contaminating RNase from DNase I. Anal Biochem. 1980 Sep 1;107(1):260–264. doi: 10.1016/0003-2697(80)90519-9. [DOI] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O., Bjerrum P. J. Titration of transport and modifier sites in the red cell anion transport system. J Gen Physiol. 1982 Feb;79(2):253–282. doi: 10.1085/jgp.79.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaki L., Fasold H., Schuhmann B., Passow H. Chemical modification of membrane proteins in relation to inhibition of anion exchange in human red blood cells. J Cell Physiol. 1975 Dec;86(3 Pt 1):471–494. doi: 10.1002/jcp.1040860305. [DOI] [PubMed] [Google Scholar]
- Zaki L. Inhibition of anion transport across red blood cells with 1,2-cyclohexanedione. Biochem Biophys Res Commun. 1981 Mar 16;99(1):243–251. doi: 10.1016/0006-291x(81)91738-1. [DOI] [PubMed] [Google Scholar]




