Abstract
Objective
Peripheral blood lymphocytes (PBLs) from systemic lupus erythematosus (SLE) patients exhibit increased spontaneous and diminished activation-induced apoptosis. We tested the hypothesis that key biochemical checkpoints, the mitochondrial transmembrane potential (ΔΨm) and production of reactive oxygen intermediates (ROIs), mediate the imbalance of apoptosis in SLE.
Methods
We assessed the ΔΨm with potentiometric dyes, measured ROI production with oxidation-sensitive fluorochromes, and monitored cell death by annexin V and propidium iodide staining of lymphocytes, using flow cytometry. Intracellular glutathione levels were measured by high-performance liquid chromatography, while ATP and ADP levels were assessed by the luciferin–luciferase assay.
Results
Both ΔΨm and ROI production were elevated in the 25 SLE patients compared with the 25 healthy subjects and the 10 rheumatoid arthritis patients. Intracellular glutathione contents were diminished, suggesting increased utilization of reducing equivalents in SLE. H2O2, a precursor of ROIs, increased ΔΨm and caused apoptosis in normal PBLs. In contrast, H2O2-induced apoptosis and ΔΨm elevation were diminished, particularly in T cells, and the rate of necrotic cell death was increased in patients with SLE. The intracellular ATP content and the ATP:ADP ratio were reduced and correlated with the ΔΨm elevation in lupus. CD3:CD28 costimulation led to transient elevation of the ΔΨm, followed by ATP depletion, and sensitization of normal PBLs to H2O2-induced necrosis. Depletion of ATP by oligomycin, an inhibitor of F0F1–ATPase, had similar effects.
Conclusion
T cell activation and apoptosis are mediated by ΔΨm elevation and increased ROI production. Mitochondrial hyperpolarization and the resultant ATP depletion sensitize T cells for necrosis, which may significantly contribute to inflammation in patients with SLE.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease accompanied by signaling abnormalities in T and B lymphocytes and production of antinuclear autoantibodies. Several lines of evidence suggest that abnormalities of programmed cell death, or apoptosis, underlie the pathology of SLE (1,2). Apoptosis represents a physiologic mechanism for the elimination of potentially autoreactive lymphocytes during development (3). After completion of an immune response, excess T or B cells are also removed by apoptosis (4).
Defects in apoptotic signaling through the Fas pathway contribute to autoimmunity in lpr and gld mice (5,6). While mutations of Fas receptor (FasR) or Fas ligand (FasL) have been associated with a lupus-like autoimmune syndrome in mice with the lpr or gld background (5) and FasR defects have been documented in a rare form of lymphoproliferative disease in humans (7,8), Fas-mediated signaling appears to be intact in patients with SLE (9–11). Defective CD3-mediated cell death in lupus patients, possibly related to decreased intracellular synthesis of tumor necrosis factor α (TNFα), has been linked to the persistence of autoreactive cells (10). In contrast, increased spontaneous apoptosis of peripheral blood lymphocytes (PBLs) has been linked to chronic lymphopenia (12) and compartmentalized release of nuclear autoantigens in patients with SLE (13).
Reactive oxygen intermediates (ROIs) have long been considered to be toxic byproducts of aerobic existence; however, evidence is now accumulating that controlled levels of ROIs modulate various aspects of cellular function and are necessary for signal transduction pathways, including those mediating T cell activation (14–16) and apoptosis (17–20). Increased production of ROIs was demonstrated in studies of TNF-(21–23) and Fas-mediated cell death (20,24–29). Disruption of the mitochondrial membrane potential has been proposed as the point of no return in apoptotic signaling (24,30,31). Recent data from this laboratory indicate that elevation of the mitochondrial transmembrane potential (ΔΨm), i.e., mitochondrial hyperpolarization, occurs in the early phase of Fas-induced apoptosis of Jurkat human leukemia T cells and normal human PBLs (29). Mitochondrial hyperpolarization precedes phosphatidylserine (PS) externalization and a disruption of ΔΨm in Fas- (29) and H2O2-induced apoptosis (32). These observations were confirmed and extended to p53 (33), TNFα (34), and staurosporine-induced apoptosis (35). Elevation of the ΔΨm occurs independently of activation of caspases and represents an early event in apoptosis (29,33).
The precise mechanism underlying increased spontaneous and decreased activation-induced apoptosis in lymphocytes from SLE patients is yet to be elucidated. To address this important issue, we investigated key biochemical checkpoints, ΔΨm and ROI production, in spontaneous and H2O2-induced apoptosis. Depending on ΔΨm, H2O2 is transformed into ROIs in mitochondria (36,37). The ΔΨm is the result of an electrochemical gradient maintained by 2 transport systems, the electron-transport chain and the F0F1–ATPase complex (37). The electron-transport chain catalyzes the flow of electrons from NADH to molecular oxygen and the translocation of protons across the inner mitochondrial membrane, thus creating a voltage gradient with negative charges inside the mitochondrial matrix (38). F0F1–ATPase complexes play crucial roles in oxidative phosphorylation, such as the conversion of ADP to ATP at the expense of the electrochemical gradient during oxidative phosphorylation (38). Using the energy of ATP hydrolysis, F0F1–ATPase can pump protons out of the mitochondrial matrix into the intermembrane space, thus causing an elevation of ΔΨm.
The findings of the present study reveal a key role for coordinate mitochondrial hyperpolarization and ATP depletion in abnormal T cell death in patients with SLE.
PATIENTS AND METHODS
Study subjects
Twenty-five patients with SLE were evaluated. All of them satisfied the American College of Rheumatology (ACR) criteria for a definitive diagnosis of SLE (39). Twenty-three women with a mean ± SD age of 39.3 ± 5.3 years (range 18–63 years) and 2 men with a mean ± SD age of 44.8 ± 10.2 years (ages 25 and 55 years, respectively) were studied. As controls, 25 age- and sex-matched healthy subjects and 10 patients with rheumatoid arthritis (RA) (8 women with a mean ± SD age of 51.3 ± 6.7 years; 2 men with a mean ± SD age of 54.0 ± 0.0 years) diagnosed according to the ACR criteria (40) were studied.
The RA patients were receiving methotrexate, sulfasalazine, cyclosporin A, leflunomide, or etanercept. Fifteen of the SLE patients were receiving prednisone (5–50 mg/day) and 17 were receiving immunosuppressive drugs, including hydroxychloroquine (200–400 mg/day) and either azathioprine (50 mg/day) or methotrexate (7.5 mg/week).
Disease activity in the SLE patients was assessed by the SLE Disease Activity Index (SLEDAI) (41). Eleven patients had SLEDAI scores of ≤10 and were considered to have relatively inactive disease. The remaining 14 patients had SLEDAI scores of >10 and were considered to have active disease. The study was approved by the Institutional Review Board for the Protection of Human Subjects.
Cell culture and viability assays
Peripheral blood mononuclear cells were isolated from heparinized venous blood on a Ficoll-Hypaque gradient. PBLs were separated after the removal of monocytes by adherence to autologous serum–coated petri dishes (42). PBLs were resuspended at 106 cells/ml in RPMI 1640 medium, supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 IU/ml of penicillin, and 100 μg/ml of gentamicin in 12-well plates at 37°C in a humidified atmosphere with 5% CO2. The rate of apoptosis was assessed after incubation for 20 minutes and for 1, 2, 3, 16, 24, and 72 hours in the presence or absence of 50 μM H2O2.
Stimulation of CD3 antigen was performed by adding PBLs to plates that had been precoated with 1 μg/ml/well of OKT3 monoclonal antibody (mAb) (CRL 8001; American Type Culture Collection, Rockville, MD) for 1 hour at 37°C. CD28 costimulation was performed by addition of 500 ng/ml of mAb CD28.2 (PharMingen, San Diego, CA). Proliferative responses were assessed by 3H-thymidine incorporation assay (42).
Apoptosis was monitored by observing cell shrinkage and nuclear fragmentation, and was quantified by flow cytometry after concurrent staining with fluorescein isothiocyanate (FITC)–conjugated annexin V (fluorescence channel 1 [FL-1]; R&D Systems, Minneapolis, MN) and propidium iodide (PI) (FL-2) as described previously (20,29,43,44). Staining with phycoerythrin (PE)–conjugated annexin V (R&D Systems) was used to monitor PS externalization (FL-2) in parallel with measurement of ROI levels and ΔΨm (see below). Apoptosis rates were expressed as the percentage of annexin V–positive/PI-negative cells.
Necrosis was assessed by observing cellular and nuclear swelling (3). Swollen nuclei of necrotic cells were observed by staining with PI (50 μg/ml). Necrotic cells were enumerated by direct PI staining using flow cytometry and fluorescence microscopy. Necrosis rates were expressed as the percentage of the PI-positive population within annexin-positive cells. As described previously (20,45), neither live nor apoptotic cells stained directly with PI and required permeabilization with 0.1% Triton X-100. When using hydroethidine (HE) (FL-2) for ROI measurement, cells were costained with annexin V–FITC. Thus, annexin V–PE and annexin V–FITC were matched with the emission spectra of potentiometric and oxidation-sensitive fluorescent probes. Specific combinations are described in the figure legends.
Staining with annexin V alone or in combination with dihydrorhodamine (DHR) or 3,3′-dihexyloxacarbocyanine iodide (DiOC6) was carried out in 10 mM HEPES, pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2. Using 3-color fluorescence, mitochondrial ROI levels, ΔΨm, and PS externalization within T cell subsets were concurrently analyzed by parallel staining with DHR or DiOC6 (FL-1), annexin V–PE (FL-2), and Quantum Red/Cy5-conjugated mAb to CD3, CD4, and CD8 (FL-3; Sigma, St. Louis, MO), as well as CD45RA and CD45RO (PharMingen). Quantum Red contains 2 covalently linked fluorochromes, PE and Cy5. PE absorbs light energy at 488 nm and emits at 670 nm, in the excitation range of Cy5, which acts as an acceptor dye. For fluorescence microscopy, cells were photographed using a Nikon Eclipse E800 camera (Nikon, Tokyo, Japan). Green and red fluorescent images were digitally superimposed using SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Flow cytometric analysis of ROI production and ΔΨm
Production of ROIs was assessed fluorometrically using oxidation-sensitive fluorescent probes 5,6-carboxy-2′,7′-dichlorofluorescein diacetate (DCFH-DA), DHR, and HE (Molecular Probes, Eugene, OR) as described elsewhere (20). Following the apoptosis assay, cells were washed 3 times in 5 mM HEPES buffered saline, pH 7.4, incubated in HEPES buffered saline with either 0.1 μM DHR for 2 minutes, 1 μM DCFH-DA for 15 minutes, or 1 μM HE for 15 minutes, and the samples were analyzed using a FACStar Plus flow cytometer (Becton Dickinson, Mountain View, CA) equipped with an argon ion laser delivering 200 mW of power at 488 nm. Fluorescence emission from 5,6-carboxy-2′,7′-dichlorofluorescein (DCF; green) or DHR (green) was detected at a wavelength of 530 ± 30 nm (mean ± SEM). Fluorescence emission from oxidized HE, ethidium (red), was detected at a wavelength of 605 nm. Dead cells and debris were excluded from the analysis by electronic gating of forward and side scatter measurements. While R123, the fluorescent product of DHR oxidation, binds selectively to the inner mitochondrial membrane, ethidium and DCF remain in the cytosol of living cells. DHR and HE were more sensitive than DCF for measuring ROI production.
The ΔΨm was estimated by staining with 20 nm DiOC6 (Molecular Probes), a cationic lipophilic dye (24,46,47), for 15 minutes at 37°C in the dark before flow cytometry (excitation 488 nm, emission 525 nm, recorded in FL-1). The fluorescence of DiOC6 is oxidation-independent and correlates with ΔΨm (47). We also quantitated ΔΨm with a potential-dependent J-aggregate-forming lipophilic cation, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) (48). JC-1 selectively incorporates into mitochondria, where it forms monomers (green fluorescence at 527 nm) or aggregates, at high transmembrane potentials (red fluorescence at 590 nm) (48,49). Cells were incubated with 0.5 μM JC-1 for 15 minutes at 37°C before flow cytometry. Cotreatment with a protonophore, 5 μM carbonyl cyanide m-chlorophenylhydrazone (Sigma) for 15 minutes at 37°C resulted in decreased DHR, DiOC6, and JC-1 fluorescence and served as a positive control for disruption of ΔΨm (29). Each measurement was performed on 10,000 cells.
Measurement of ATP
Intracellular ATP levels were determined using the luciferin-luciferase method (50). PBLs (5 × 106) that had been cultured in vitro for 16 hours were collected by centrifugation and washed in phosphate buffered saline. The pellet was resuspended in 50 μl of phosphate buffered saline and mixed with equal volumes of 2.5% trichloroacetic acid. These extracts were stored at −20°C.
The total protein content of each sample was determined using the Lowry assay (51). The ATP levels in PBLs from SLE patients and control donors were assayed in parallel. The bioluminescence assay was performed in an AutoLumat LB953 automated luminometer (Berthold, Wildbad, Germany) using an ATP determination kit (Molecular Probes) according to the manufacturer's instructions. ATP standard curves were established in each experiment and were linear in the 5–5,000 nM range. To eliminate the impact of nonspecific inhibitors in the cellular extracts, standard amounts of ATP were added to the reaction mixtures as controls and ATP levels were remeasured (52). The sample volume added to the reaction mixtures was <2% of the total assay volume. The ATP:ADP ratio and ADP levels were assessed with the ApoGlow kit (Lumitech, Nottingham, UK).
Measurement of glutathione levels
The total glutathione content was determined by the enzymatic recycling procedure essentially as described by Tietze (53). Cells (106) were resuspended in 50 μl of 4.5% 5-sulfosalicylic acid. The acid-precipitated protein was pelleted by centrifugation at 4°C for 10 minutes at 15,000g. The total protein content of each sample was determined using the Lowry assay (51). The reduced glutathione (GSH) content of the aliquot assayed was determined in comparison with reference curves generated with known amounts of GSH (20).
GSH and oxidized glutathione (GSSG) were measured by reverse-phase ion-exchange high-performance liquid chromatography (HPLC) using ultraviolet light detection at 365 nm (54). Briefly, 106 cells were deproteinized in the presence of 10% perchloric acid and 1 mM bathophenanthrolinedisulfonic acid. After repeated freezing and thawing, samples were centrifuged at 15,000g for 3 minutes. Then, 50 μl of 100 mM monoiodoacetic acid in 0.2 mM m-cresol purple was added to 500 μl of supernatant. Samples were neutralized by the addition of 480 μl of 2M KOH and 2.4M KHCO3 and incubated in the dark at room temperature for 10 minutes. Then, 1 ml of 1% fluorodinitrobenzene was added, and the samples were incubated in the dark at 4°C overnight. After centrifugation and filtering, 100 μl of the supernatants was injected into the HPLC equipped with a photodiode array detector (Waters Alliance System, Milford, MA) and a Waters Spherisorb NH2 column (4.6 × 250 mm; 10 μm).
Statistical analysis
Alterations in cell survival, ROI production, ΔΨm, GSH, and ATP levels were analyzed by Student's t-test or Mann-Whitney rank sum test for nonparametric data. Correlation was analyzed using Pearson's correlation coefficient. Changes were considered significant at P < 0.05.
RESULTS
Mitochondrial hyperpolarization and increased ROI levels in peripheral blood T lymphocytes from SLE patients
Spontaneous apoptosis was assessed after 16 hours' incubation of PBLs from patients with SLE in parallel with PBLs from control donors, based on binding of fluorochrome-conjugated annexin V to externalized PS on the surface of apoptotic cells (43,44). The rate of cell death was increased in PBLs from SLE patients compared with those from the 25 healthy donors as well as those from the 10 patients with RA (Table 1). Apoptosis was higher among SLE patients with SLEDAI scores >10, as compared with patients with SLEDAI scores ≤10 (Table 1), which is consistent with previously reported findings (12,55). Apoptosis rates were similar in patients taking and those not taking corticosteroids (Table 1). Similarly, apoptosis rates in SLE patients treated with immunosuppressive agents did not differ from those in patients who were not treated with these medications (Table 1).
Table 1.
Spontaneous apoptosis, mitochondrial transmembrane potential, and production of reactive oxygen intermediates in peripheral blood lymphocytes from patients with SLE*
| Study group | No. of subjects | % annexin V+† | ΔΨm‡ |
ROI production‡ |
||
|---|---|---|---|---|---|---|
| DiOC6 | JC-1 | DHR | HE | |||
| All SLE patients | 25 | 8.82 ± 0.69 | 132.8 ± 6.9 | 128.1 ± 5.9 | 140.1 ± 7.2 | 143.1 ± 7.3 |
| Control subjects | 25 | 6.08 ± 0.34 (0.009) | 100.0 ± 5.5 (0.0005) | 100.0 ± 4.1 (0.003) | 100.0 ± 5.1 (0.001) | 100.0 ± 5.8 (0.0001) |
| RA patients | 10 | 6.29 ± 0.40 (0.032) | 102.5 ± 6.5 (0.014) | 100.4 ± 6.3 (0.01) | 106.7 ± 7.3 (0.01) | 106.5 ± 7.9 (0.01) |
| SLE patient subgroups | ||||||
| SLEDAI | ||||||
| ≤10 | 11 | 7.01 ± 1.08 | 128.8 ± 11.9 | 130.3 ± 9.5 | 132.7 ± 11.2 | 129.6 ± 12.4 |
| >10 | 14 | 10.25 ± 0.72 (0.016) | 136.0 ± 8.4 | 126.4 ± 7.8 | 145.9 ± 9.4 | 153.6 ± 7.9 |
| Prednisone | ||||||
| Not taking | 10 | 9.84 ± 1.3 | 134.4 ± 13.3 | 127.8 ± 9.5 | 134.7 ± 12.5 | 133.5 ± 12.5 |
| Taking | 15 | 8.14 ± 0.75 | 131.8 ± 7.7 | 128.3 ± 7.8 | 143.7 ± 8.8 | 149.4 ± 8.8 |
| Immunosuppressants | ||||||
| Not taking | 8 | 9.9 ± 0.94 | 128.0 ± 11.8 | 121.6 ± 13.7 | 140.9 ± 14.5 | 145.7 ± 14.4 |
| Taking | 17 | 8.31 ± 0.91 | 135.1 ± 8.7 | 131.1 ± 6.1 | 139.7 ± 8.4 | 141.8 ± 8.6 |
Experiments were performed as described in Patients and Methods. Controls were analyzed in parallel with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients in each experiment. Values are the mean ± SM. P values less than 0.05 are shown in parentheses. Immunosuppressive drugs consisted of either hydroxychloroquine, azathioprine, cyclosporine, methotrexate, or cyclophosphamide. SLEDAI = SLE Disease Activity Index.
Before analysis, peripheral blood lymphocytes were preincubated in medium for 16 hours. The percentage of cells undergoing apoptosis was assessed by determining annexin V positivity.
The mitochondrial transmembrane potential (ΔΨm) was measured by 3,3′-dihexyloxacarbocyanine iodide (DiOC6) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) fluorescence. The production of reactive oxygen intermediates (ROIs) was assessed by dihydrorhodamine 123 (DHR) and hydroethidine (HE) fluorescence. Values are the mean ± SEM channel fluorescence of annexin V–negative cells from SLE and RA patients relative to that of cells from control donors processed in parallel. The mean of the controls was normalized to 100.0.
Elevations of ΔΨm and ROI levels are key early checkpoints in several apoptosis pathways (29,32,33,35). To monitor the timing of PS externalization with respect to ΔΨm and mitochondrial ROI production, cells undergoing apoptosis were analyzed by staining with annexin V–PE (FL-2) or annexin V–FITC (FL-1), which were matched with the emission spectra of concurrently utilized potentiometric (DiOC6, FL-1; JC-1, FL-2) and oxidation-sensitive (DHR, FL-1; HE, FL-2) fluorescent probes, respectively, as described previously (29). The ΔΨm and ROI levels were elevated in annexin V–negative cells from SLE patients compared with those from healthy or RA donors (Table 1). The ΔΨm and ROI levels did not correlate with SLEDAI scores or with corticosteroid or immunosuppressive drug treatment in lupus patients (Table 1).
As shown in Figure 1, the rate of spontaneous apoptosis (annexin V–PE staining, red fluorescence) was increased and ΔΨm was elevated (as indicated by more intense green/DiOC6 fluorescence) in annexin V–PE–negative nonapoptotic cells in lupus PBLs. This was consistent with previous findings that elevation of ΔΨm precedes PE externalization in apoptosis signaling (29,32,33,35).
Figure 1.

Fluorescence microscopy (top panels) and flow cytometry (bottom panels) of the mitochondrial transmembrane potential (ΔΨm) in peripheral blood lymphocytes (PBLs) from a healthy control subject and a patient with systemic lupus erythematosus (SLE). Cells were cultured for 16 hours in vitro and stained with annexin V–phycoerythrin (PE) (red) and with 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (green) and visualized by fluorescence microscopy (original magnification × 200). The ΔΨm (green fluorescence; fluorescence channel 1 [FL-1]) was increased in nonapoptotic cells of SLE PBLs, and the frequency of apoptotic cells (red fluorescence; FL-2) was increased in SLE PBLs. Numbers in the upper right corner of the dot-plots show the percentage of annexin V–positive cells; x mean = mean channel fluorescence of DiOC6 (FL-1) in annexin V–negative cells; numbers at the top of the histograms show the mean channel fluorescence of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1; FL-2) gated on live cells, based on forward/side scatter analysis.
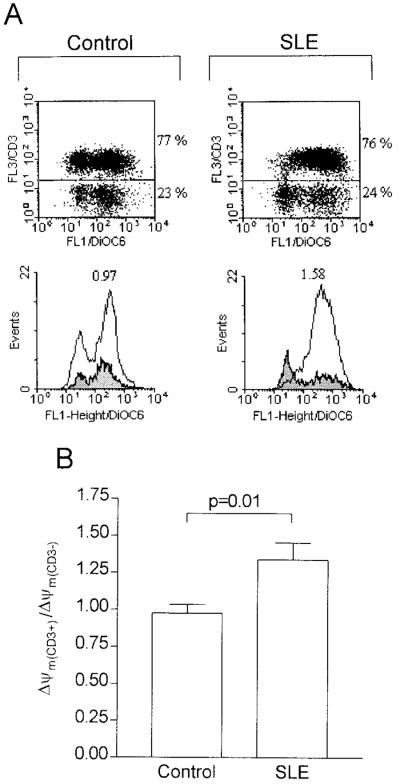
The ratio of ΔΨm(CD3+) to ΔΨm(CD3–) was 0.97 ± 0.06 (mean ± SEM) in control donors and 1.34 ± 0.12 in lupus patients (P = 0.01) (Figure 2). Thus, mitochondrial hyperpolarization was confined to CD3+ T lymphocytes (Figure 2), affecting CD4, CD8, CD45RO, and CD45RA cell subsets in lupus patients (data not shown).
Figure 2.
Prominence of mitochondrial hyperpolarization in T cells from SLE patients. A, PBLs were cultured in vitro for 16 hours, and the ΔΨm was measured by DiOC6 fluorescence (FL-1). T cells were detected by staining with Cy5-conjugated anti-CD3 monoclonal antibody (FL-3 in the dot-plots). Annexin V–positive cells were electronically gated out. Histograms showing the ΔΨm (DiOC6 fluorescence; FL-1) of CD3-positive cells (open) are overlaid on those showing the ΔΨm of CD3-negative cells (shaded). Numbers at the top of the histograms show the ΔΨm ratio of CD3-positive to CD3-negative cells. B, The ΔΨm ratio of CD3-positive to CD3-negative cells in 25 patients with SLE and 25 healthy donors. See Figure 1 for definitions.
Diminished levels of GSH in SLE patients
A normal reducing environment, which is required for cellular integrity, is provided by GSH, which protects cells from damage by ROIs (56). GSH and its oxidized form, GSSG, were measured in freshly isolated PBLs by HPLC. GSH levels were diminished in patients with SLE (mean ± SEM 3.60 ± 0.30 ng/μg of protein) compared with those of normal donors (5.11 ± 0.50 ng/μg of protein) (P = 0.016). No difference in GSSG content was found between lupus patients (1.17 ± 0.09 ng/μg of protein) and control donors (1.13 ± 0.10 ng/μg of protein).
Induction of necrosis rather than apoptosis by H2O2 in SLE patients
ROIs modulate cellular function and are necessary for signal transduction pathways, including those mediating apoptosis (17,19,20,57,58) and T cell activation through the CD3–T cell receptor (TCR) complex (15,16). Treatment with 50 μM H2O2, a precursor of ROIs, induced apoptosis of PBLs, a finding consistent with previous reports (20,32). Interestingly, a greater proportion of lupus PBLs showed morphologic changes, cellular and nuclear swelling, and trypan blue staining, which is consistent with necrosis (3). The proportion of cellular debris was also increased in SLE patients upon H2O2 treatment.
The swollen nuclei of necrotic cells were visualized by staining with PI (Figure 3A). Necrotic cells were enumerated by PI staining using flow cytometry. PI staining was dramatically increased among annexin V–positive cells from lupus patients upon H2O2 treatment (Figure 3B). While rates of spontaneous apoptosis were higher in lupus PBLs (Table 1), rates of increased apoptosis (annexin V–positive/PI-negative) 16 hours after exposure to H2O2 were diminished (P < 0.0001) in SLE patients (10.3 ± 1.1%) compared with control donors (20.5 ± 0.8%) (Figure 3C). In response to treatment with H2O2, 4.5 ± 0.8% of normal PBLs underwent necrosis, while the rate of necrosis was 19.0 ± 1.2% in lupus PBLs (P < 0.0001) (Figure 3D). The rate of necrosis in response to H2O2 treatment in patients with RA (5.2 ± 1.3%) was similar to that in healthy controls. The rate of necrosis was increased in lupus patients (19.0 ± 1.2%; P < 0.0001) with respect to both control and RA donors.
Figure 3.
Analysis of cell death in SLE patients and healthy controls in response to stimulation with H2O2. PBLs from 15 SLE patients and 15 controls were cultured in the presence and absence of 50 μM H2O2 for 16 hours and analyzed by fluorescence microscopy and flow cytometry. A, Fluorescence micrographs of H2O2-stimulated PBLs from representative control and SLE patient donors. Cells were stained with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI). After 16 hours' incubation in the absence of H2O2 (no treatment), the frequency of annexin V–FITC–staining apoptotic cells was increased in SLE PBLs. After 16 hours of treatment with H2O2, the frequency of apoptotic cells was lower in SLE PBLs, while that of necrotic cells with swollen nuclei directly staining with PI was elevated (original magnification × 400). B, Flow cytometric analysis of apoptotic (annexin V–positive and PI-negative; lower right quadrant) and necrotic (annexin V–positive and PI-positive; upper right quadrant) cells after stimulation with 50 μM H2O2 for 16 hours. PBLs were from different donors than those used in A. C, Apoptosis rates were determined in 15 healthy donors and 15 SLE patients, based on the percentage of annexin V–phycoerythrin (PE)–positive/PI-negative cells. D, After H2O2 treatment, necrosis was prominent in SLE patients compared with healthy controls. Data points correspond to the percentage of PI-positive cells within the annexin V–positive population induced by H2O2 treatment. See Figure 1 for other definitions.
Blunting of the H2O2-induced elevation of ΔΨm and ROI levels in T cells from SLE patients
H2O2 induces mitochondrial ROI production, which, in turn, leads to apoptosis (32,36). As expected, treatment with H2O2 increased the ROI content of annexin V–negative cells (Figure 4A). The extent of H2O2-induced changes in ROI levels, as monitored by DHR and HE fluorescence, correlated with increases in annexin V positivity both in healthy and SLE donors (Figure 4B). H2O2-induced elevations in ROI levels were diminished in patients with SLE (Figure 4C).
Figure 4.
Effect of H2O2 treatment on the production of reactive oxygen intermediates (ROIs) in SLE patients. A, ROI levels were monitored in live cells by flow cytometry after staining with annexin V–phycoerythrin (PE; FL-2) and dihydrorhodamine (DHR; FL-1) (dot-plots). Numbers in the upper right corner of the dot-plots show the percentage of annexin V–positive cells; numbers at the top of the first row of histograms show the mean channel fluorescence of DHR (FL-1) in annexin V–negative cells. Histograms of H2O2-treated cells (shaded) are overlaid on those of untreated control cells (open). ROI production was also monitored by hydroethidine (HE) staining. Numbers at the top of the second row of histograms show the mean channel fluorescence of HE-positive cells (FL-2) gated on annexin V–FITC-negative cells (FL-1). B, Correlation between H2O2-induced ROI production and apoptosis, as determined by Pearson's correlation coefficient. Increases in apoptosis and ROI production were estimated by annexin V positivity (Δannexin V) and DHR fluorescence (ΔDHR), respectively. C, H2O2-induced ROI production in SLE patients and controls. Bars show the mean ± SEM of H2O2-induced changes in DHR and HE fluorescence in PBLs from SLE patients and controls. See Figure 1 for other definitions.
To further assess mitochondrial function, ΔΨm was measured by DiOC6 and JC-1 fluorescence. As shown in Figure 5A, ΔΨm was increased as early as 20 minutes after exposure of normal PBLs to H2O2. In contrast, H2O2 treatment failed to shift ΔΨm in lupus PBLs (Figures 5A and B). Both in control and in lupus PBLs, ΔΨm dropped below baseline levels 16 hours after addition of H2O2 (Figure 5A), in accordance with disruption of ΔΨm at later stages of the apoptotic process (29). Similar results were obtained with DiOC6 and JC-1 probes (Figures 5A and B).
Figure 5.
H2O2-induced changes in ΔΨm in SLE patients and healthy controls. PBLs from 15 control donors and 15 SLE patients were cultured in media for 16 hours in the presence and absence of 50 μM H2O2. A, The ΔΨm was monitored 20 minutes and 16 hours after exposure to H2O2 by concurrent staining with annexin V–phycoerythrin (PE) (FL-2) and DiOC6 (FL-1) (dot-plots and first row of histograms) or staining with JC-1 alone (second row of histograms). Numbers in the upper right corner of the dot-plots show the percentage of annexin V–positive cells; numbers at the top of the first row of histograms show the mean channel fluorescence of DiOC6 (FL-1) of annexin V–negative cells. Histograms of H2O2-treated cells (shaded) are overlaid on those of untreated control cells (open). The FL-2 fluorescence of JC-1 aggregates is shown in the second row of histograms. Numbers at the top of the second row of histograms show the mean channel fluorescence of JC-1–positive cells (FL-2). B, H2O2-induced changes in ΔΨm in SLE and control donors. Bars show the mean ± SEM of changes in ΔΨm measured by DiOC6 and JC-1 fluorescence after 20 minutes of H2O2 treatment. See Figure 1 for other definitions.
In control PBLs, the ratio of CD3-positive cells increased within the annexin V–positive compartment following H2O2 exposure (Figure 6A). In contrast, lupus T cells were relatively resistant to H2O2-induced apoptosis (Figures 6A and B). No differences in the rates of apoptosis were noted between CD4 and CD8 cells in controls or SLE patients (data not shown).
Figure 6.
Cell surface phenotyping of T cells from SLE patients and controls during H2O2-induced apoptosis. A, Representative dot-plots of PBLs costained with annexin V–phycoerythrin (PE; FL-2) and Cy5-conjugated anti-CD3 monoclonal antibody (FL-3), after treatment with H2O2 for 16 hours. Numbers in the upper right corner show the percentage of CD3-positive cells within the annexin V–positive compartment. B, After treatment with H2O2, the percentage of CD3-positive cells within the annexin V–positive compartment was diminished in patients with SLE. See Figure 1 for other definitions.
Diminished levels of intracellular ATP in SLE patients
Hyperpolarization of ΔΨm in lupus T cells and their inability to raise ΔΨm after H2O2 treatment indicate a fundamental mitochondrial dysfunction in SLE. The ΔΨm is the result of an electrochemical gradient maintained by 2 transport systems, the electron transport chain and the F0F1–ATPase complex (37). The F0F1–ATPase complex can synthesize or hydrolyze ATP, depending on the presence or absence of an electro-chemical proton gradient and substrate availability (37). The intracellular ATP concentration is also a key factor in the decision between apoptosis and necrosis in Jurkat human T cells (59). In yeast, hyperpolarization of ΔΨm and ROI production were found to be mediated by the proton-pumping function of the F0F1–ATPase complex coupled with ATP depletion (60). Therefore, we examined whether elevated baseline ΔΨm (mitochondrial hyperpolarization) and predisposition for H2O2-induced necrosis were due to ATP hydrolysis in SLE.
As shown in Figure 7A, intracellular ATP concentrations were diminished (P = 0.002), while ADP levels were normal in lupus patients. ATP levels were independent of medication or disease activity (data not shown). The ATP levels in RA patients were similar to those in control donors and were higher than those in SLE patients (P = 0.0147). Of note, ATP concentrations correlated inversely with the ΔΨm in SLE patients (Figure 7B). This suggested that hyperpolarization of ΔΨm was related to an increased ATPase/proton-pumping activity of the F0F1–ATPase rather than diminished ATP synthesis due to limiting ADP pools.
Figure 7.
A, Intracellular ATP and ADP content in PBLs from 24 SLE patients, 10 rheumatoid arthritis (RA) patients, and 17 healthy donors. Cells were cultured for 16 hours in vitro and assayed for ATP and ADP content. Values are the mean ± SEM. B, In parallel, cell aliquots were stained with DiOC6 and JC-1, and the ΔΨm was measured by flow cytometry. The correlation between intracellular ATP levels and the ΔΨm in patients with SLE was calculated using Pearson's correlation coefficient. See Figure 1 for other definitions.
To assess whether ATP depletion and hyperpolarization of ΔΨm are mediated by the F0F1 complex, its activity was blocked with oligomycin (59). Because oligomycin selectively inhibits F0F1-mediated generation of ATP in mitochondria, we cultured normal PBLs in medium with a high concentration of glucose to maintain ATP levels within 80% of baseline through anaerobic glycolysis (59). Pretreatment of PBLs with oligomycin for 30 minutes lowered the intracellular concentration of ATP by a mean ± SEM 22.8 ± 4.3% (P < 0.01) and led to an elevation of the ΔΨm (Figure 8A).
Figure 8.
A, Effect of CD3/CD28 stimulation and oligomycin treatment on intracellular ATP content and the ΔΨm (DiOC6 fluorescence) in normal PBLs. PBLs from 4 healthy donors were pretreated with CD3/CD28 antibodies or 2.5 μM oligomycin. Values are the mean ± SEM percentage of control cells incubated in parallel (n = 4 independent experiments). B, Effect of pretreatment with CD3/CD28 (1 hour) or oligomycin (2.5 μM for 30 minutes) on H2O2-induced elevation of the ΔΨm in PBLs from 4 healthy donors. Values are the percentage elevation of the ΔΨm elicited by exposure to H2O2 (50 μM for 20 minutes) in CD3/CD28- or oligomycin-pretreated cells compared with control cells. C, Effect of pretreatment with CD3/CD28 (1 hour) or oligomycin (2.5 μM for 30 minutes) on H2O2-induced necrosis of PBLs from healthy donors. Untreated (control), oligomycin-pretreated, and CD3/CD28-pretreated PBLs were exposed to 50 μM H2O2 for 16 hours, and necrosis was assessed by the ratio of propidium iodide–positive/annexin V–positive cells. Values are the mean ± SEM of 6 independent experiments. See Figure 1 for definitions.
Induction of ΔΨm elevation and ATP depletion and sensitization for H2O2-induced necrosis by CD3/CD28 costimulation of control PBLs
Defective signaling through the CD3–TCR complex is thought to play a central role in the pathogenesis of SLE (61,62). Pre-stimulation with mitogenic lectins (29) or anti-CD3 antibody for 5 days is required to sensitize peripheral blood T lymphocytes to apoptotic signaling through the FasR (9,63,64). Incubation of PBLs for as little as 20 minutes on ice with 1 μg/ml of Fas antibody or concanavalin A increased the ΔΨm (29). These observations suggested that elevation of the ΔΨm is required for both T cell activation and apoptosis.
To assess this possibility, we examined the effect of CD3/CD28 costimulation on ΔΨm and ROI levels. PBLs were added to plates that had been precoated with 1 μg/ml/well of OKT3 mAb and costimulated with 500 ng/ml of CD28 mAb for up to 3 days. CD3/CD28 costimulation resulted in a transient elevation of ΔΨm, which began as early as 5 minutes and peaked ~1 hour after treatment, as noted by both DiOC6 and JC-1 measurements (Figure 8A). CD3/CD28-induced mitochondrial hyperpolarization was followed by depletion of intracellular ATP 30–60 minutes later. ATP depletion lasted up to 4 hours, then returned to baseline levels (Figure 8A).
CD3/CD28 prestimulation or oligomycin pre-treatment of normal PBLs prevented H2O2-induced elevation of ΔΨm beyond that caused by CD3/CD28 or oligomycin alone (Figure 8B). CD3/CD28 prestimulation for 1 hour facilitated necrosis of normal lymphocytes upon exposure to H2O2. Likewise, ATP depletion by oligomycin pretreatment enhanced H2O2-induced necrosis (Figure 8C).
DISCUSSION
The present study identifies deviations in key biochemical checkpoints that mediate abnormal apoptosis of lupus T cells. We found that ΔΨm and ROI levels were elevated in SLE patients compared with healthy controls and with RA controls. GSH levels were diminished in freshly isolated PBLs from SLE patients, which was consistent with ongoing oxidative stress in vivo.
Increased ROI production may lead to skewed expression of redox-sensitive lymphokines and surface receptors. For example, ROIs regulate gene transcription and the release of TNFα and interleukin-10 (IL-10) (65), both of which are elevated in sera (66,67) and freshly isolated PBLs (68,69) from SLE patients. Cell surface expression of FasR (70–72) and FasL is also redox sensitive (73). Along the same line, increased spontaneous apoptosis of lymphocytes has been linked to increased IL-10 production, release of FasL, and overexpression of FasR (55,74,75). Elevated nitric oxide production may also contribute to increased spontaneous apoptosis (76,77). Mitochondrial ROI production and ΔΨm are early checkpoints in Fas- (29) and H2O2-induced apoptosis (32). Increased ROI levels confer sensitivity to H2O2, nitric oxide, TNFα, and Fas-induced cell death (20). Therefore, elevated baseline levels of ROI and ΔΨm may play key roles in the enhanced spontaneous death of PBLs in patients with SLE.
Glucocorticoid hormones, cytotoxic drugs, and hydroxychloroquine can induce apoptosis of thymocytes and accelerate cell death of mature lymphocytes (3,78). Most of our SLE and RA patients received a combination of corticosteroids, hydroxychloroquine, and other immunosuppressive medications. Spontaneous apoptosis, ΔΨm, and ROI levels in PBLs from SLE patients were elevated in comparison with those in PBLs from healthy controls and RA patients. Consistent with previous findings (12), spontaneous apoptosis was not accelerated in patients with RA in comparison with healthy controls. Furthermore, enhanced spontaneous death, mitochondrial hyperpolarization, or increased ROI production by PBLs from patients with SLE was not associated with the administration of steroids, hydroxychloroquine, or cytotoxic drugs. Therefore, mitochondrial hyperpolarization, increased ROI production, and enhanced spontaneous apoptosis of lupus PBLs can be attributed primarily to disease pathogenesis.
Complement deficiency affects clearance of immune complexes and could also influence the elimination of apoptotic bodies, thus evoking an autoimmune response (79). However, mitochondrial hyperpolarization affects nonapoptotic annexin V–negative cells, and it is unlikely to be a result of complement deficiency and diminished clearance of apoptotic bodies. Indeed, we found no correlation between complement deficiency and hyperpolarization in our SLE patients. This is consistent with an association between complement deficiency and disease activity and a lack of association between hyperpolarization and disease activity. Previously, Georgescu et al (55) found increased apoptosis in 3 patients with vasculitis, 2 with Wegener's granulomatosis, and 1 with Takayasu arteritis. Systemic vasculitis can also be a manifestation of lupus. Within the group of 25 patients with SLE, the extent of mitochondrial hyperpolarization or ATP depletion in the 6 patients who had vasculitis was similar to that in the 19 patients who did not have vasculitis. Eleven of the 25 SLE patients had a SLEDAI score ≤10, of whom 10 had a SLEDAI score <5. The 10 patients with a SLEDAI score <5 also showed mitochondrial hyperpolarization and ATP depletion; thus, these biochemical changes are not due to a systemic inflammatory response.
ROIs mediate signaling through the CD3/CD28 receptors. In response to treatment with exogenous H2O2, a precursor or ROI, lupus T cells failed to undergo apoptosis, and cell death preferentially occurred via necrosis. Endogenous H2O2 is generated by superoxide dismutase from ROIs, O2−, or OH−, in mitochondria (36). In turn, H2O2 is scavenged by catalase and glutathione peroxidase (56). While H2O2 is freely diffusible, it has no unpaired electrons and, by itself, is not an ROI (36). Induction of apoptosis by H2O2 requires mitochondrial transformation into an ROI, e.g., OH−, through the Fenton reaction (36,37). As previously noted (32), H2O2 triggered a rapid increase in ΔΨm and ROI production that was followed by apoptosis of PBLs in healthy subjects. In contrast, H2O2 failed to elevate ΔΨm, ROI production, and apoptosis, but rather, elicited necrosis in lupus patients. Both CD3/CD28-induced H2O2 production and H2O2-induced apoptosis require mitochondrial ROI production. Therefore, diminished CD3/CD28-induced H2O2 production and H2O2-induced apoptosis together with deficient elevation of ΔΨm and ROI levels reveal deviations of key biochemical checkpoints in the mitochondria of patients with SLE.
Baseline mitochondrial hyperpolarization and ROI levels correlated with diminished GSH levels in patients with SLE, suggesting increased utilization of reducing equivalents. It is presently unclear whether synthesis of GSH or regeneration of GSH from its oxidized form is altered in lupus patients. GSH is also required for IL-2-dependent T cell proliferation (14) as well as CD2- and CD3-mediated T cell activation (80). Thus, a low GSH content may also inhibit CD3-induced H2O2 production. Nevertheless, GSH deficiency predisposes cells for ROI-induced cell death (20,32,81). Diminished H2O2-induced apoptosis of cells with low baseline GSH levels indicates a severe dysfunction of redox signaling in patients with SLE.
The ΔΨm (negative inside and positive outside) is dependent upon the electron-transport chain transferring electrons from NADH to molecular oxygen and on proton transport mediated by the F0F1–ATPase complex (37). During oxidative phosphorylation, the F0F1–ATPase converts ADP to ATP, utilizing the energy stored in the electrochemical gradient. Alternatively, using the energy of ATP hydrolysis, F0F1–ATPase can pump protons out of the mitochondrial matrix into the intermembrane space, causing ΔΨm hyperpolarization.
Thus, mitochondrial hyperpolarization may occur by 1 of 2 ways. First, deficiency of cellular ADP could cause diminished utilization of the electrochemical gradient, ATP depletion, and hyperpolarization. However, ADP levels were not diminished, but were slightly elevated, in lupus PBLs. This suggested that ATP depletion and ΔΨm hyperpolarization were not due to a lack of ADP in patients with SLE. Second, inhibition of the enzymatic activity of F0F1–ATPase would decrease utilization of the electrochemical gradient and cause ΔΨm hyperpolarization, ATP depletion, and ADP accumulation. Since blocking of F0F1–ATPase by oligomycin led to ΔΨm hyperpolarization and elevated ROI production, prevented H2O2- or CD3/CD28-induced elevation of ΔΨm in normal PBLs, and sensitized for H2O2- induced necrosis, a similar mechanism may also be operational in patients with SLE. Indeed, we found an inverse correlation, i.e., an association of diminished apoptosis with increased necrosis, upon H2O2 treatment of PBLs from patients with SLE (P = 0.0038). Moreover, the rate of H2O2-induced necrosis correlated inversely with ATP levels in lupus patients (P = 0.0026). These findings are consistent with a requirement of ATP for apoptosis and predisposition by ATP depletion for necrosis (59).
With ΔΨm hyperpolarization and extrusion of H+ ions from the mitochondrial matrix, the cytochromes within the electron transport chain become more reduced, which favors the generation of ROIs (38). Thus, mitochondrial hyperpolarization is a likely cause of increased ROI production and may be ultimately responsible for increased spontaneous apoptosis in patients with SLE. Mitochondrial hyperpolarization also occurs during T cell activation, which indicates that this event represents an early and reversible step in apoptosis. CD3/CD28 stimulation caused a transient elevation of ΔΨm and ATP depletion and sensitized cells to H2O2-induced necrosis. Thus, repetitive T cell activation in vivo could be responsible for prolonged mitochondrial hyperpolarization and ATP depletion in SLE. A 28–32% increase in the −200 mV ΔΨm may have a tremendous impact on mitochondrial energy coupling and ATP synthesis (37). Both T cell activation and apoptosis require the energy provided by ATP (82). The intracellular ATP concentration is a key switch in the cell's decision to die via apoptosis or necrosis (59), and therefore, depletion of ATP may be responsible for defective H2O2-induced apoptosis and a shift to necrosis in patients with SLE.
Apoptosis is a physiologic process that results in nuclear condensation and break-up of the cell into membrane-enclosed apoptotic bodies suitable for phagocytosis by macrophages, thus preventing inflammation. In contrast, necrosis is a pathologic process that results in cellular swelling, followed by lysis and release of proteases, oxidizing molecules, and other proinflammatory and chemotactic factors, resulting in tissue damage and increased availability of free autoantigens (3). Indeed, lymphocyte necrosis occurs in the bone marrow (83) and lymph nodes of lupus patients (84) and may significantly contribute to the inflammatory process (85). Therefore, ATP depletion and mitochondrial hyperpolarization not only play key roles in abnormal T cell activation and cell death in patients with SLE, but also represent novel targets of pharmacologic intervention.
ACKNOWLEDGMENT
We thank Dr. Richard Cross for helpful discussions and review of the manuscript.
Supported in part by grants from the NIH (DK-49221 and AI-48079) and the Central New York Community Foundation.
Footnotes
Presented in part at the Annual Meeting of the American Association of Immunologists, Seattle, WA, May 2000, and at the 64th Annual Scientific Meeting of the American College of Rheumatology, Philadelphia, PA, November 2000.
REFERENCES
- 1.Elkon KB. Apoptosis in SLE: too little or too much? Clin Exp Rheumatol. 1994;12:553–9. [PubMed] [Google Scholar]
- 2.Perl A, Banki K. Molecular mimicry, altered apoptosis, and immunomodulation as mechanisms of viral pathogenesis in systemic lupus erythematosus. In: Kammer GM, Tsokos GC, editors. Lupus: molecular and cellular pathogenesis. Humana Press; Totowa (NJ): 1999. pp. 43–64. [Google Scholar]
- 3.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–93. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 7.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–46. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 8.Drappa J, Vaishnaw AK, Sullivan KE, Chu J, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996;335:1643–9. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 9.Mysler E, Bini P, Drappa J, Ramos P, Friedman SM, Krammer PH, et al. The apoptosis-1/Fas protein in human systemic lupus erythematosus. J Clin Invest. 1994;93:1029–34. doi: 10.1172/JCI117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-α. Clin Immunol Immunopathol. 1996;81:293–302. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
- 11.Caricchio R, Cohen PL. Spontaneous and induced apoptosis in systemic lupus erythematosus: multiple assays fail to reveal consistent abnormalities. Cell Immunol. 1999;198:54–60. doi: 10.1006/cimm.1999.1576. [DOI] [PubMed] [Google Scholar]
- 12.Emlen W, Niebur JA, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–92. [PubMed] [Google Scholar]
- 13.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilos DL, Wedner HJ. The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol. 1985;135:2740–7. [PubMed] [Google Scholar]
- 15.Los M, Droge W, Stricker K, Bauerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25:159–65. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 16.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–44. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 17.Lipton SA, Choi Y, Pan Z, Lei SZ, Chen HV, Sucher NJ, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–31. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 18.Korsmeyer SJ. Regulators of cell death. Trends Genet. 1995;11:101–5. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 19.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 20.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J Biol Chem. 1996;271:32994–3001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 21.Meier B, Radeke HH, Selle S, Younes M, Sies H, Resch K, et al. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumor necrosis factor-α. Biochem J. 1989;263:539–45. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennet T, Richter C, Peterhans E. Tumor necrosis factor-α induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–92. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze-Osthoff K, Krammer PH, Droge W. Divergent signaling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994;13:4587–96. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci U S A. 1996;93:14559–63. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasahara Y, Iwai K, Yachie A, Ohta K, Konno A, Seki H, et al. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–53. [PubMed] [Google Scholar]
- 26.Williams MS, Henkart PA. Role of reactive oxygen intermediates in TCR-induced death of T cell blasts and hybridomas. J Immunol. 1996;157:2395–402. [PubMed] [Google Scholar]
- 27.Gulbins E, Brenner B, Schlottmann K, Welsch J, Heinle H, Koppenhoefer UL, et al. Fas-induced programmed cell death is mediated by a Ras-regulated O2− synthesis. Immunology. 1996;89:205–12. doi: 10.1046/j.1365-2567.1996.d01-743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–77. [PubMed] [Google Scholar]
- 29.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–79. [PMC free article] [PubMed] [Google Scholar]
- 30.Susin SA, Zamzami N, Castedo M, Daugas E, Wang H, Geley S, et al. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/Apo-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vander Heiden M, Chandel NS, Williamson EK, Schumaker PT, Thompson CB. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 32.Puskas F, Gergely P, Jr, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–61. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by bcl-2. EMBO J. 1999;18:6027–36. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottlieb E, Vander Heiden MG, Thompson CG. Bcl-XL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor α-induced apoptosis. Mol Cell Biol. 2000;20:5680–9. doi: 10.1128/mcb.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku H, Murphy MP. Changes in mitochondrial membrane potential during staurosporin-induced apoptosis in Jurkat cells. FEBS Lett. 2000;475:267–72. doi: 10.1016/s0014-5793(00)01681-1. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 37.Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells, and organisms. Mol Aspects Med. 1999;20:139–184. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 38.Stryer L. Biochemistry. 3rd edition WH Freeman; New York: 1988. [Google Scholar]
- 39.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 40.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 41.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang DH, The Committee on Prognosis Studies in SLE Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 42.Perl A, Gonzalez-Cabello R, Lang I, Gergely P. Effector activity of OKT4+ and OKT8+ T-cell subsets in lectin-dependent cell-mediated cytotoxicity against adherent HEp-2 cells. Cell Immunol. 1984;84:185–93. doi: 10.1016/0008-8749(84)90089-3. [DOI] [PubMed] [Google Scholar]
- 43.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 44.Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie CAA, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 46.Petit PX, O'Connor JE, Grunwald D, Brown SC. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990;194:389–97. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanner MK, Wellhausen SR, Klein JB. Flow cytometric analysis of altered mononuclear cell transmembrane potential induced by cyclosporin. Cytometry. 1993;14:59–69. doi: 10.1002/cyto.990140111. [DOI] [PubMed] [Google Scholar]
- 48.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming cation JC-1. Proc Natl Acad Sci U S A. 1991;88:3671–5. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cossarizza A, Franceschi C, Monti D, Salvioli S, Bellesia E, Rivabene R, et al. Protective effect of N-acetylcysteine in tumor necrosis factor-α-induced apoptosis in U937 cells: the role of mitochondria. Exp Eye Res. 1995;220:232–40. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- 50.Lundin A. Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Methods Enzymol. 2000;305:346–70. doi: 10.1016/s0076-6879(00)05499-9. [DOI] [PubMed] [Google Scholar]
- 51.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 52.Lundin A. ATP extractants neutralised by cyclodextrins. In: Campbell AK, Kricka LJ, Stanley PE, editors. Bioluminescence and Chemiluminescence. John Wiley & Sons; Chichester: 1994. pp. 399–402. [Google Scholar]
- 53.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 54.Fariss MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–9. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- 55.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–33. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayes P. The pentose phosphate pathway and other pathways of hexose metabolism. In: Murray R, Granner D, Mayes P, Rodwell V, editors. Harper's Biochemistry. Appleton & Lange; Norwalk (CT): 1993. pp. 201–11. [Google Scholar]
- 57.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, et al. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–7. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 58.Hockenberry DM, Oltvai ZN, Yin X, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 59.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machida K, Tanaka T. Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization mediated by F(0)F(1)-ATPase in yeast. FEBS Lett. 1999;462:108–12. doi: 10.1016/s0014-5793(99)01506-9. [DOI] [PubMed] [Google Scholar]
- 61.Dayal AK, Kammer GM. The T cell enigma in lupus. Arthritis Rheum. 1996;39:23–33. doi: 10.1002/art.1780390104. [DOI] [PubMed] [Google Scholar]
- 62.Tsokos GC, Liossis SC. Immune cell signaling defects in lupus: activation, anergy and death. Immunol Today. 1999;20:119–24. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]
- 63.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, et al. Differential expression of apoptosis related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–8. [PubMed] [Google Scholar]
- 64.Alderson MK, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, et al. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178:2231–5. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Moine O, Louis H, Stordeur P, Collet JM, Goldman M, Deviere J. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology. 1997;113:1701–6. doi: 10.1053/gast.1997.v113.pm9352875. [DOI] [PubMed] [Google Scholar]
- 66.Al-Janadi M, al-Balla S, al-Dalaan A, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- 67.Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor α and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067–4. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- 68.Swaak AJ, van den Brink HG, Aarden LA. Cytokine production (IL-6 and TNFα) in whole blood cell cultures of patients with systemic lupus erythematosus. Scand J Rheumatol. 1996;25:233–8. doi: 10.3109/03009749609069992. [DOI] [PubMed] [Google Scholar]
- 69.Handwerger BS, Luzian I, Da Silva L, Storrer CE, Via CS. Cytokines in the immunopathogenesis of lupus. In: Kammer GM, Tsokos GC, editors. Lupus: molecular and cellular pathogenesis. Humana Press; Totowa (NJ): 1999. pp. 321–40. [Google Scholar]
- 70.Li D, Yang B, Mehta JL. Ox-LDL induces apoptosis in human coronary artery endothelial cells: role of PKC, PTK, bcl-2, and Fas. Am J Physiol. 1998;275:H568–76. doi: 10.1152/ajpheart.1998.275.2.H568. [DOI] [PubMed] [Google Scholar]
- 71.Orlinick JR, Vaishnaw A, Elkon KB, Chao MV. Requirement of cysteine-rich repeats of the Fas receptor for binding of the Fas ligand. J Biol Chem. 1997;272:28889–94. doi: 10.1074/jbc.272.46.28889. [DOI] [PubMed] [Google Scholar]
- 72.Bennett M, Macdonald K, Chan S, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-induced apoptosis. Science. 1998;282:290–3. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 73.Kasibhatla S, Genestier L, Green DR. Regulation of Fas ligand expression during activation-induced cell death in T lymphocytes. J Biol Chem. 1999;274:987–92. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- 74.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–11. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorenz H-M, Grünke M, Hieronymus T, Herrmann M, Kühnel A, Manger B, et al. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. 1997;40:306–17. doi: 10.1002/art.1780400216. [DOI] [PubMed] [Google Scholar]
- 76.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc Assoc Am Physicians. 1999;111:611–21. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 77.Cooper GS, Dooley MA, Treadwell EL, St. Clair EW, Parks CG, Gilkeson GS. Hormonal, environmental, and infectious risk factors for developing systemic lupus erythematosus. Arthritis Rheum. 1998;41:1714–24. doi: 10.1002/1529-0131(199810)41:10<1714::AID-ART3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 78.Meng XW, Feller JM, Ziegler JB, Pittman SM, Ireland CM. Induction of apoptosis in peripheral blood lymphocytes following treatment in vitro with hydroxychloroquine. Arthritis Rheum. 1997;40:927–35. doi: 10.1002/art.1780400522. [DOI] [PubMed] [Google Scholar]
- 79.Walport MJ. Advances in immunology: complement. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 80.Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990;87:3343–7. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvemini F, Franze A, Iervolino A, Filosa S, Salzano S, Ursini MV. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J Biol Chem. 1999;274:2750–7. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- 82.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–30. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 83.Lorand-Metze I, Carvalho MA, Costallat LT. Morphology of bone marrow in systemic lupus erythematosus [German] Pathologe. 1994;15:292–6. doi: 10.1007/s002920050057. [DOI] [PubMed] [Google Scholar]
- 84.Ko YH, Dal Lee J. Fine needle aspiration cytology in lupus lymphadenopathy: a case report. Acta Cytol. 1992;36:748–51. [PubMed] [Google Scholar]
- 85.Eisner MD, Amory J, Mullaney B, Tierney L, Jr, Browner WS. Necrotizing lymphadenitis associated with systemic lupus erythematosus. Semin Arthritis Rheum. 1996;26:477–82. doi: 10.1016/s0049-0172(96)80028-x. [DOI] [PubMed] [Google Scholar]