Abstract
Abnormal death signaling in lymphocytes of systemic lupus erythematosus (SLE) patients has been associated with elevation of the mitochondrial transmembrane potential (Δψm) and increased production of reactive oxygen intermediates (ROI). The resultant ATP depletion sensitizes T cells for necrosis that may significantly contribute to inflammation in patients with SLE. In the present study, the role of mitochondrial signal processing in T cell activation was investigated. CD3/CD28 costimulation of PBL elicited transient mitochondrial hyperpolarization and intracellular pH (pHi) elevation, followed by increased ROI production. Baseline Δψm, ROI production, and pHi were elevated, while T cell activation-induced changes were blunted in 15 patients with SLE in comparison with 10 healthy donors and 10 rheumatoid arthritis patients. Similar to CD3/CD28 costimulation, treatment of control PBL with IL-3, IL-10, TGF-β1, and IFN-γ led to transient Δψm elevation. IL-10 had diametrically opposing effects on mitochondrial signaling in lupus and control donors. Unlike healthy or rheumatoid arthritis PBL, cells of lupus patients were resistant to IL-10-induced mitochondrial hyperpolarization. By contrast, IL-10 enhanced ROI production and cell death in lupus PBL without affecting ROI levels and survival of control PBL. Ab-mediated IL-10 blockade or stimulation with antagonistic lymphokine IL-12 normalized baseline and CD3/CD28-induced changes in ROI production and pHi with no impact on Δψm of lupus PBL. The results suggest that mitochondrial hyperpolarization, increased ROI production, and cytoplasmic alkalinization play crucial roles in altered IL-10 responsiveness in SLE.
Several lines of evidence suggest that abnormal T cell activation and cell death underlie the pathology of systemic lupus erythematosus (SLE)3 (1, 2). Potentially autoreactive T and B lymphocytes during development (3) and after completion of an immune response are removed by apoptosis, a physiological form of programmed cell death (4). Paradoxically, lupus T cells exhibit both enhanced spontaneous apoptosis and defective activation-induced cell death. Increased spontaneous apoptosis of PBL has been linked to chronic lymphopenia (5) and compartmentalized release of nuclear autoantigens in patients with SLE (6). By contrast, defective CD3-mediated cell death may be responsible for persistence of autoreactive cells (7).
Disruption of the mitochondrial transmembrane potential (Δψm) has been proposed as the point of no return in apoptotic signaling (8–11). The Δψm is dependent upon the electron transport chain transferring electrons from NADH to molecular oxygen and proton transport mediated by the F0F1-ATPase complex (12). The energy stored in the electrochemical gradient is used by F0F1-ATPase to convert ADP to ATP during oxidative phosphorylation. We have previously shown that elevation Δψm, i.e., mitochondrial hyperpolarization, occurs in the early phase of Fas-induced apoptosis of Jurkat human leukemia T cells and normal human PBL (13). Mitochondrial hyperpolarization precedes phosphatidylserine externalization and disruption of Δψm in Fas (13)- and H2O2-induced apoptosis (14). These observations were confirmed and extended to p53 (15), TNF-α (16), and staurosporin-induced apoptosis (17). Elevation of Δψm is independent from activation of caspases and represents an early event in apoptosis (13, 15). Recently, deviations in key mitochondrial checkpoints have been associated with abnormal apoptosis of SLE T cells: Δψm and mitochondrial reactive oxygen intermediate (ROI) production were elevated, whereas glutathione (GSH) levels were diminished compared with healthy or rheumatoid arthritis (RA) controls. Low GSH was consistent with increased ROI production due to use of reducing equivalents. The Δψm elevation was correlated with ATP depletion and predisposition of lupus T cells to necrosis, which in turn may significantly contribute to inflammation in patients with SLE (18).
With Δψm hyperpolarization and extrusion of H+ ions from the mitochondrial matrix, the cytochromes within the electron transport chain become more reduced, which favors generation of ROI (19). ROIs mediate signaling initiated via the CD3/CD28 receptors (20, 21). Moreover, T cell activation via CD3/CD28 receptors in duces mitochondrial hyperpolarization and ROI production in normal PBL (18). Abnormal T cell signaling has been related to an imbalance of cytokine production in SLE (22). ROIs influence activity of transcription factors AP-1 and NF-κB (23, 24), and, further downstream, may lead to the skewed expression of IL-2, TNF, and IL-10 (25, 26). Increased spontaneous apoptosis of lymphocytes has been linked to increased IL-10 production, release of Fas ligand, and overexpression of Fas receptor in SLE (27). Because increased ROI levels confer sensitivity to H2O2, NO, TNF, and Fas-induced cell death (13, 28), elevated baseline Δψm and ROI production may have key roles in altered activation and death of lupus T cells. In the present study, we show that mitochondrial hyperpolarization and increased ROI production are associated with intracellular alkalinization in lupus patients. IL-10, which is produced at elevated levels by lupus PBL, has a differential impact on Δψm, ROI production, and intracellular pH (pHi) in lymphocytes of lupus patients with respect to healthy and RA controls. IL-10 induced mitochondrial hyperpolarization of control PBL without influencing Δψm of lupus PBL. Moreover, IL-10 enhanced ROI production and cell death in lupus PBL without affecting ROI levels or survival of control PBL. Ab-mediated IL-10 blockade or stimulation with IL-12 normalized ROI production and pHi with no impact on Δψm of lupus PBL. The results suggest that mitochondrial hyperpolarization and increased ROI production play crucial roles in altered IL-10 responsiveness in SLE.
Materials and Methods
Human subjects
Fifteen patients with SLE were investigated. All patients satisfied the criteria for a definitive diagnosis (29). A total of 13 females (age 39.3 ± 5.3 years; range 18–63) and 2 males (age 44.8 ± 10.2 years; range 25–55) was studied. As controls, 10 age- and sex-matched healthy subjects and 10 patients with RA (8 females, age 51.3 ± 6.7 years; 2 males, age 54.0 ± 0.0 years) (30) were studied. RA patients were treated with methotrexate, sulfasalazine, cyclosporin A, leflunomide, or etanercept. Of the 15 SLE patients, 11 were receiving prednisone (5–50 mg/day) and 13 were treated with immunosuppressive drugs, including hydroxychloroquine (200–400 mg/day) and azathioprine (50 mg/day) or methotrexate (7.5 mg/wk). Disease activity was assessed by the SLE disease activity index (SLEDAI) score (31). Twelve patients had a SLEDAI ≤ 10 and were considered relatively inactive. The remaining 3 patients with SLEDAI >10 were considered active. The study has been approved by the Institutional Review Board for the Protection of Human Subjects.
Cell culture, T cell activation, and cytokine treatments
PBMCs were isolated from heparinized venous blood on Ficoll-Hypaque gradient. PBL were separated after the removal of monocytes by adherence to autologous serum-coated petri dishes (32). PBL were resuspended at 106 cells/ml in RPMI 1640 medium, supplemented with 10% FCS, 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml gentamicin in 12-well plates at 37°C in a humidified atmosphere with 5% CO2. Cross-linking of the CD3 Ag was performed by addition of PBL to plates precoated with 1 μg/ml/well OKT3 mAb (CRL 8001 from American Type Culture Collection, Manassas, VA) for 1 h at 37°C. CD28 costimulation was performed by addition of 500 ng/ml mAb CD28.2 (BD PharMingen, San Diego, CA). Proliferative responses were assessed by [3H]TdR incorporation assay (32). Following 72-h incubation and subsequent pulsing of 105 PBL with 0.4 μCi [3H]TdR per microtiter plate well, [3H]TdR incorporation by CD3/CD28-costimulated cells (12,129 ± 978 cpm) was enhanced with respect to unstimulated control PBL (90 ± 24 cpm; p < 0.0001). In comparison with control donors, CD3/CD28-induced [3H]TdR incorporation was diminished in lupus PBL (7,888 ± 1,016; p < 0.0001). Cells were also cultured in the presence or absence of human recombinant cytokines IL-2 (50–500 U/ml), IL-3 (10–100 ng/ml), IL-4 (10–100 ng/ml), IL-6 (5–50 ng/ml), IL-7 (5–50 ng/ml), IL-10 (10–100 ng/ml), IL-12 (5–50 ng/ml), IL-15 (50–500 ng/ml), TNF-α (20 ng/ml), TGF-β1 (5–50 ng/ml), and IFN-γ (500 U/ml). All cytokines were obtained from PeproTech (Rocky Hill, NJ). Polyclonal goat anti-human IL-10-neutralizing Ab was obtained from R&D Systems (Minneapolis, MN).
Cell viability assays
Apoptosis was monitored by observing cell shrinkage and nuclear fragmentation, and quantified by flow cytometry after concurrent staining with fluorescein-conjugated annexin V (annexin V-FITC; FL-1; R&D Systems) and propidium iodide (PI; FL-2), as described earlier (13, 28, 33, 34). Staining with PE-conjugated annexin V (annexin V-PE; R&D Systems) was used to monitor phosphatidylserine externalization (FL-2) in parallel with measurement of ROI levels and Δψm (see below). Apoptosis rates were expressed as percentage of annexin V-positive/PI-negative cells. Necrosis was assessed by observing cellular and nuclear swelling (3). Swollen nuclei of necrotic cells were observed by staining with PI (50 μg/ml). Necrotic cells were enumerated by direct PI staining using flow cytometry and fluorescence microscopy (18). Necrosis rates were expressed as percentage of PI-positive population within annexin-positive cells. As described earlier (28, 35), live or apoptotic cells did not stain directly with PI and required permeabilization with 0.1% Triton X-100. When using hydroethidine (HE; FL-2) for ROI measurement, cells were costained with annexin V-FITC (FL-1; R&D Systems). Thus, annexin V-PE or annexin V-FITC was matched with emission spectra of potentiometric and oxidation-sensitive fluorescent probes. Specific combinations are described in each figure legend. Staining with annexin V alone or in combination with dihydrorhodamine 123 (DHR), HE, 3,3′-dihexyloxacarbocyanine iodide (DiOC6), chloromethyl-X-rhosamine (CMXRos), or tetramethylrhodamine methyl esther (TMRM) (see below) was conducted in 10 mM HEPES, pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2.
Flow cytometric analysis of ROI production and Δψm
Production of ROI was assessed fluorometrically using oxidation-sensitive fluorescent probes 5,6-carboxy-2′,7′-dichlorofluorescein-diacetate (DCFH-DA), DHR, and HE (Molecular Probes, Eugene, OR), as described earlier (28). Following apoptosis assay, cells were washed three times in 5 mM HEPES-buffered saline, pH 7.4; incubated in HEPES-buffered saline with 0.1 μM DHR for 2 min, 1 μM DCFH-DA for 15 min, or 1 μM HE for 15 min; and samples were analyzed using a FACStarPlus flow cytometer (BD Biosciences, Palo Alto, CA) equipped with tunable argon ion laser delivering 200 mW power at 488 nm. Fluorescence emission from 5,6-carboxy-2′,7′-dichlorofluorescein (DCF; green) or DHR (green) was detected at a wavelength of 530 ± 30 nm. Fluorescence emission from oxidized HE, ethidium (red), was detected at a wavelength of 605 nm. Dead cells and debris were excluded from the analysis by electronic gating of forward and side scatter measurements. Although R123, the fluorescent product of DHR oxidation, binds selectively to the inner mitochondrial membrane, ethidium and DCF remain in the cytosol of living cells. DHR and HE were more sensitive than DCF for measurement of ROI production. Δψm was estimated by staining with 20 nm DiOC6 (Molecular Probes), a cationic lipophilic dye (8, 36, 37), for 15 min at 37°C in the dark before flow cytometry (excitation, 488 nm; emission, 525 nm recorded in FL-1). Fluorescence of DiOC6 is oxidation independent and correlates with Δψm (37). The Δψm was also quantitated using a potential-dependent J-aggregate-forming lipophilic cation, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) (38). JC-1 selectively incorporates into mitochondria, where it forms monomers (fluorescence in green, 527 nm) or aggregates, at high transmembrane potentials (fluorescence in red, 590 nm) (38, 39). Cells were incubated with 0.5 μM JC-1 for 15 min at 37°C before flow cytometry. Δψm changes were also confirmed by staining with 1 μM CMXRos (excitation, 579 nm; emission, 599 nm recorded in FL-2) and 1 μM TMRM (excitation, 543 nm; emission, 567 nm recorded in FL-2; all from Molecular Probes). Cotreatment with a protonophore, 5 μM carbonyl cyanide m-chlorophenylhydrazone (Sigma-Aldrich, St. Louis, MO) for 15 min at 37°C resulted in decreased DHR, DiOC6, CMXRos, TMRM, and JC-1 fluorescence, and served as a positive control for disruption of Δψm (13). Each measurement was conducted on 10,000 cells.
ATP measurement
Intracellular ATP levels were determined using the luciferin-luciferase method (40). A total of 5 × 106 PBL that had been cultured in vitro for 16 h was collected by centrifugation and washed in PBS. The pellet was resus-pended in 50 μl of PBS and mixed with equal volumes of 2.5% trichloroacetic acid. Such extracts were stored at −20°C. The total protein content of each sample was determined using the Lowry assay (41). ATP content of PBL from SLE patients and control donors was assayed in parallel. The bioluminescence assay was performed in an AutoLumat LB953 automated luminometer (Berthold, Wildbad, Germany) using an ATP determination kit (Molecular Probes), according to the manufacturer's instructions. ATP standard curves were established in each experiment and were linear in the 5–5000 nM range. To eliminate the impact of nonspecific inhibitors in the cellular extracts, standard amounts of ATP were added to the reaction mixtures as controls, and ATP levels were remeasured (42). The sample volume added to the reaction mixtures was less than 2% of the total assay volume. ATP/ADP ratio and ADP levels were assessed with the ApoGlow kit (Lumitech, Nottingham, U.K.).
Intracellular pH measurements
The pHi measurements were conducted with flow cytometry using the pH-sensitive dye carboxy seminaphthorhodafluor-1 (SNARF-1)-acetoxymethyl ester acetate (SNARF-1; Molecular Probes), as described earlier (43). SNARF-1 enters cells passively as a nonpolar ester. It is then hydrolyzed by intracellular esterases into a polar compound unable to leave membrane-intact cells. The emission spectrum of SNARF-1 undergoes a pH-dependent wavelength shift. To assess pHi, PBL were centrifuged at 1000 rpm for 10 min and resuspended in PBS. SNARF-1 was added to the cells at 5 μg/ml, and the samples were then incubated for 30 min at 37°C. Fresh 0.5 mg/ml stock solutions of SNARF-1 were made in DMSO for each experiment. After incubation, cells were washed in PBS and analyzed by flow cytometry. The dye was excited with 200 mW of the 488 nm argon laser, and fluorescence was collected in two wavelengths (FL2, 580 nm, and FL3, 650 nm) in the pulse-processing mode. The pHi was calculated from the FL3/FL2 ratio. A standard calibration curve was generated for each experiment by staining the cells in high K+ buffers of varying pH values (120 mM KCl, 30 mM NaCl, 0.5 mM MgSO4, 1 mM CaCl2, 1 mM NaHPO4, 5 mM glucose, and 10 mM HEPES) and in the presence of 5 μg/ml nigericin (Sigma-Aldrich; diluted from a stock solution of 500 μg/ml in ethanol) to equilibrate the intracellular/extracellular pH.
IL-10 production
IL-10 levels were measured in supernatants of PBL after 5-day culture in vitro using an ELISA kit (Endogen, Woburn, MA).
Statistics
Results were analyzed by Student's t test or Mann-Whitney rank sum test for nonparametric data. Correlation was measured using Pearson's correlation coefficient. Changes were considered significant at p < 0.05.
Results
Coordinate changes of Δψm, ROI production, and pHi in peripheral blood T cells
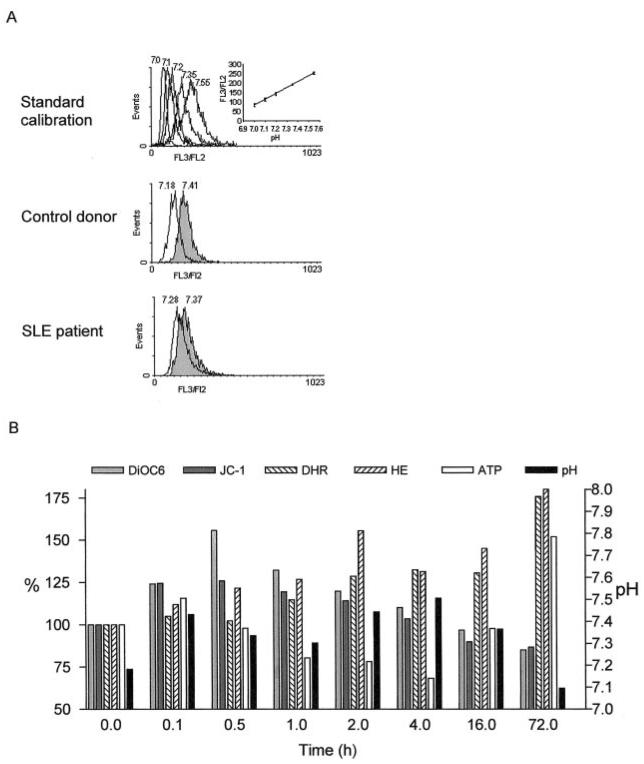
PBL of patients with SLE exhibit increased spontaneous (5) and diminished activation-induced apoptosis (7). Previous studies suggested that an increase of Δψm or mitochondrial hyperpolarization was an early event in apoptosis and T cell activation (13, 18). Apoptosis has been generally characterized by cellular acidification that is required for activity of caspases (44). However, growth factor withdrawal-induced apoptosis of hemopoetic cells has recently been associated with initial mitochondrial hyperpolarization and cytoplasmic alkalinization (45). T cell activation by mitogenic lectins or stimulation of CD3 or CD2 receptors also leads to cytoplasmic alkalinization (46, 47). Therefore, changes in pH may be relevant for signaling abnormalities in SLE. The pHi was determined based on the FL3/FL2 ratio of SNARF-1 fluorescence. A standard calibration curve was generated for each experiment by staining the cells in high K+ buffers of pH values varying between 7.0 and 7.55 (Fig. 1A). CD3/CD28 costimulation of normal PBL was accompanied by an increase of pHi, i.e., alkalinization, as early as 6 min and lasted up to 16 h following T cell activation (Fig. 1B). By 72 h, pHi returned to baseline. Induction of apoptosis by serum withdrawal or treatment with 1 μg/ml Fas Ab or 50 μM H2O2 caused a transient elevation of pHi lasting for up to 3 h, followed by a drop below baseline (not shown). These results were consistent with intracellular acidification at later stages of cell death in correlation with previous findings (44). In parallel, Δψm was assessed by the potentiometric dyes, monitoring DiOC6 fluorescence in annexin V negative and red fluorescence of JC-1 (FL-2), as described earlier (13, 14, 18). CD3/CD28 costimulation led to elevation of Δψm, beginning as early as 6 min and peaking ~30 min to 1 h after treatment (Fig. 1B), as noted by both DiOC6 and JC-1 measurements. CD3/CD28-induced mitochondrial hyperpolarization was followed by depletion of intracellular ATP 30–60 min later. ATP depletion lasted up to 4 h, then returned to baseline levels (Fig. 1B). ROI production, as monitored by DHR and HE fluorescence, increased gradually and became maximal 72 h after CD3/CD28 costimulation (Fig. 1B).
FIGURE 1.
A, Measurement of pHi based on the FL3/FL2 ratio of SNARF-1 fluorescence. A standard calibration curve was recorded for each experiment by SNARF-1 staining of PBL incubated in high K+ buffers of pH values preset between 7.0 and 7.55. Histograms from control donor and a representative lupus patient show SNARF-1 fluorescence recorded without (open curves) and with CD3/CD28 costimulation for 1 h (shaded curves). Values over histograms indicate pHi based of standard calibration. B, Effect of CD3/CD28 costimulation on the ΔΨm, ROI production, ATP content, and pHi of normal PBL. The ΔΨm was measured by DiOC6 and JC-1 fluorescence, while ROI production was assessed by DHR and HE fluorescence of annexin V-negative cells. Data represent mean of three independent experiments. DiOC6, JC-1, DHR, and HE fluorescence as well as ATP levels are shown along the left y-axis relative to baseline (0 h) values normalized at 100.0%. Absolute pHi values are represented along the right y-axis.
Baseline Δψm, ROI production, and pHi are elevated, while T cell activation-induced changes are blunted in patients with SLE
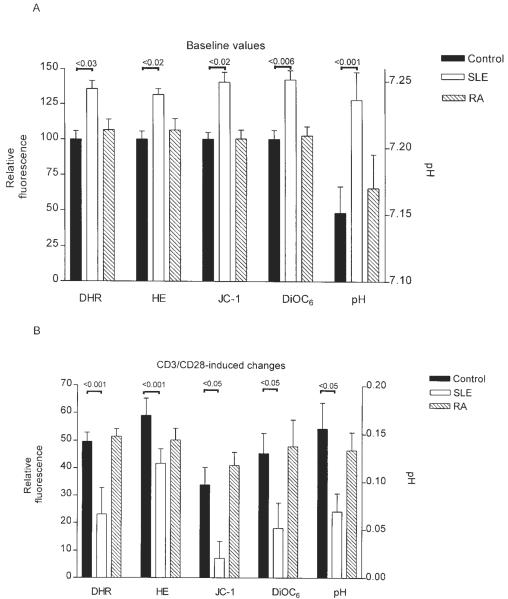
Because pHi can affect processing of activation and apoptosis signals, it was assessed in PBL of lupus patients and healthy as well as RA controls. As shown in Figs. 1A and 2A, baseline pHi was increased in patients with SLE (7.236 ± 0.021) with respect to healthy (7.151 ± 0.019; p = 0.013) or RA controls (7.17 ± 0.02; p = 0.03). A pH calibration curve was generated in each experiment (Fig. 1A). In accordance with previous findings (18), elevation of Δψm and ROI production were noted in lupus patients with respect to healthy and RA controls. By contrast, CD3/CD28-induced mitochondrial hyperpolarization, ROI production, and elevation of pHi were reduced in lupus patients (ΔpHi: +0.069 ± 0.019) as compared with healthy controls (ΔpHi: +0.155 ± 0.026; p = 0.005). No significant differences were noted between healthy and RA donors (ΔpHi: +0.133 ± 0.019) studied in parallel (Fig. 2B).
FIGURE 2.
A, Increased ROI production, mitochondrial hyperpolarization, and cytoplasmic alkalinization in PBL of lupus patients with respect to healthy and RA controls. Before measurements, PBL from 10 healthy controls, 15 patients with SLE, and 10 patients with RA were cultured in vitro for 16 h. The Δψm was measured by DiOC6 and JC-1 fluorescence, while ROI production was assessed by DHR and HE fluorescence of annexin V-negative cells and displayed on the left y-axis with respect to mean of controls normalized at 100.0%; pHi values are represented along the right y-axis. B, Effect of CD3/CD28 costimulation on ROI levels (DHR and HE fluorescence), Δψm (DiOC6 and JC-1 fluorescence), and pHi. ROI levels were assessed 6 h, while Δψm and pHi were determined 1 h after CD3/CD28 costimulation. Columns and error bars represent mean ± SEM.
Differential regulation of Δψm and ROI production by IL-10 in normal and lupus lymphocyte PBL
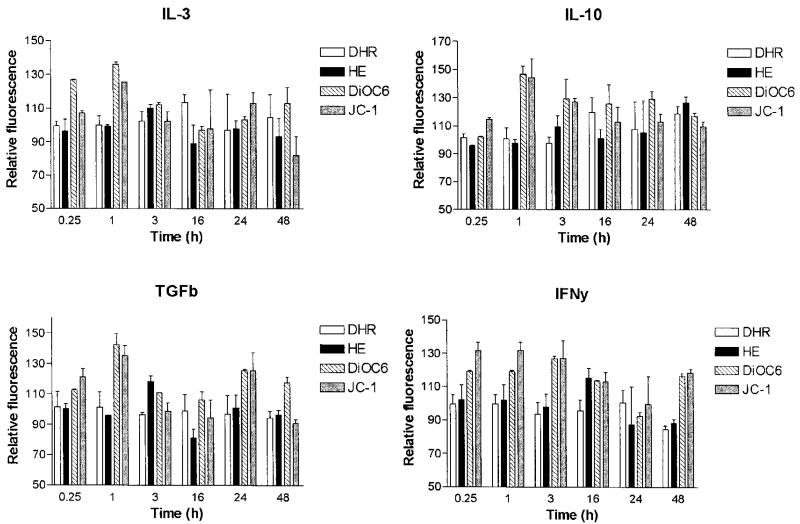
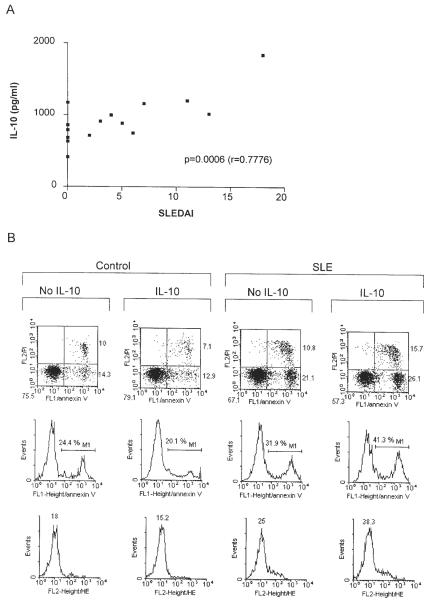
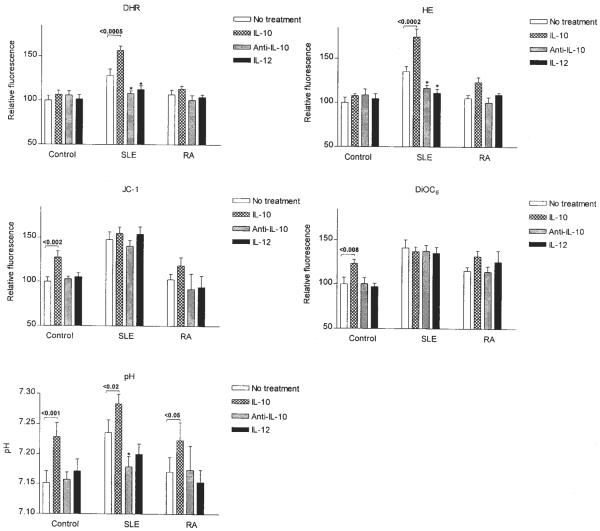
Aberrant T cell activation has been associated with altered lymphokine milieu in SLE (22). Moreover, cytokines also influence susceptibility of T cells to apoptosis (48). Therefore, we investigated the effect of IL-2 (50–500 U/ml), IL-3 (10–100 ng/ml), IL-4 (10–100 ng/ml), IL-6 (5–50 ng/ml), IL-7 (5–50 ng/ml), IL-10 (10–100 ng/ml), IL-12 (5–50 ng/ml), IL-15 (50–500 ng/ml), TNF-α (20 ng/ml), TGF-β1 (5–50 ng/ml), and IFN-γ (500 U/ml) on Δψm. As shown in Fig. 3, IL-3 (10 ng/ml), IL-10 (10 ng/ml), TGF-β1 (5 ng/ml), and IFN-γ (500 U/ml) significantly augmented Δψm after incubation for 1–16 h in normal PBL. With regard to the lymphokines causing mitochondrial hyperpolarization, increased production of IL-10 (26, 49), while diminished levels of IFN-γ (50) and TGF-β1 (51) were noted in patients with SLE. Following 5-day incubation, IL-10 production, ROI levels, Δψm, and pH were determined in lupus and control PBL. Indeed, IL-10 production was increased in PBL of lupus patients (934.7 ± 85.8 pg/ml) in comparison with healthy controls (566.1 ± 58.9 pg/ml; p = 0.014). IL-10 production by lupus PBL correlated with SLEDAI (Fig. 4, p = 0.0006). As described earlier, Δψm did not correlate with disease activity or medications (18). Because increased production of IL-10 may account, at least in part, for mitochondrial dysfunction, effect of IL-10 in control and lupus PBL was comparatively analyzed. Treatment with IL-10 increased DiOC6 (+45.5 ± 7.4%; p = 0.008) and JC-1 fluorescence (+33.9 ± 6.4%; p = 0.0019), i.e., elicited mitochondrial hyperpolarization (Fig. 5), while it did not affect ROI production (Fig. 5) or apoptosis of control PBL (Fig. 4). By contrast, IL-10 enhanced ROI production, as measured by increased DHR (+28.8 ± 5.5%; p = 0.0005) and HE fluorescence (+38.4 ± 6.4%; p = 0.0002) without influencing Δψm of lupus PBL (Fig. 5). IL-10 induced apoptosis and necrosis of lupus PBL (Fig. 4). Selective induction of cell death by IL-10 in lupus vs control PBL was consistent with previous findings (27). IL-10 increased pHi in control (+0.156 ± 0.026; p = 0.0011) and lupus cells as well (+0.069 ± 0.018; p = 0.02; Fig. 5).
FIGURE 3.
Effect of IL-3 (10 ng/ml), IL-10 (10 ng/ml), TGF-β1 (5 ng/ml), and IFN-γ (500 U/ml) on Δψm (DiOC6 and JC-1 fluorescence) and ROI productions (DHR and HE fluorescence) of control PBL. Significant elevation of Δψm was noted after 1-h exposure to each cytokine (p < 0.01). Relative fluorescence values are shown along the y-axis with respect to baseline (0 h) values normalized at 100.0%. Data represent mean ± SEM of four experiments.
FIGURE 4.
A, Correlation of IL-10 production and SLEDAI in patients with SLE. IL-10 production was measured in supernatant of PBL cultured in vitro for 5 days. Correlation between IL-10 levels and SLEDAI was calculated using Pearson's correlation coefficient. B, Effect of IL-10 on cell survival in control and lupus PBL. After 16-h treatment with 10 ng/ml IL-10, cell survival was analyzed by flow cytometry following staining with annexin V-FITC and PI. Dot plots (row 1) show percentage of apoptotic (annexin V-positive and PI-negative, lower right quadrants) and necrotic (annexin V-positive and PI-positive, upper right quadrants) cells. Histograms in row 2 indicate the percentage of annexin V-positive population, representing total cell death (M1). ROI production was monitored in parallel by HE and annexin V-FITC staining. Values over histograms (row 3) show mean channel HE fluorescence (FL2) gated on annexin V-FITC-negative (FL1) cells.
FIGURE 5.
Effect of treatment with IL-10 (10 ng/ml), Ab to IL-10 (25 μg/ml anti-IL-10), and IL-12 (10 ng/ml) on Δψm (DiOC6 and JC-1 fluorescence), ROI production (DHR and HE fluorescence), and pHi in PBL from healthy controls and patients with SLE or RA. Values of p indicate significant effects by IL-10; *, p values < 0.05 for effects by anti-IL-10 and IL-12.
Anti-IL-10 and IL-12 normalize ROI production and pHi without affecting mitochondrial hyperpolarization of lupus PBL
Because IL-10 levels were elevated in patients with SLE and IL-10 increased Δψm of normal PBL (Fig. 3), we investigated the effect of Ab-mediated blockade of IL-10 and stimulation by the IL-10 antagonist lymphokine IL-12 on mitochondrial dysfunction of lupus T cells. As shown in Fig. 5, pretreatment of lupus PBL with 25 μg/ml neutralizing IL-10 Ab for 16 h significantly diminished ROI production, as measured by DHR (−19.9 ± 6.9%; p = 0.017) and HE fluorescence (−19.0 ± 4.0%; p = 0.0011). Pretreatment with 10 ng/ml IL-12 also reduced DHR (−15.4 ± 6.8%; p = 0.02) and HE fluorescence (−24.2 ± 5.4%; p = 0.005) in lupus cells. Neither Ab to IL-10 nor IL-12 affected ROI production by normal or RA PBL (Fig. 5). Ab to IL-10 (p = 0.03), but not IL-12 (p = 0.056), reduced pHi of lupus PBL (Fig. 5). Baseline Δψm of normal or RA PBL or mitochondrial hyperpolarization of lupus PBL was not influenced by IL-10 Ab or IL-12. TGF-β1 did not affect ROI production, pHi, or mitochondrial hyperpolarization of lupus PBL (data not shown).
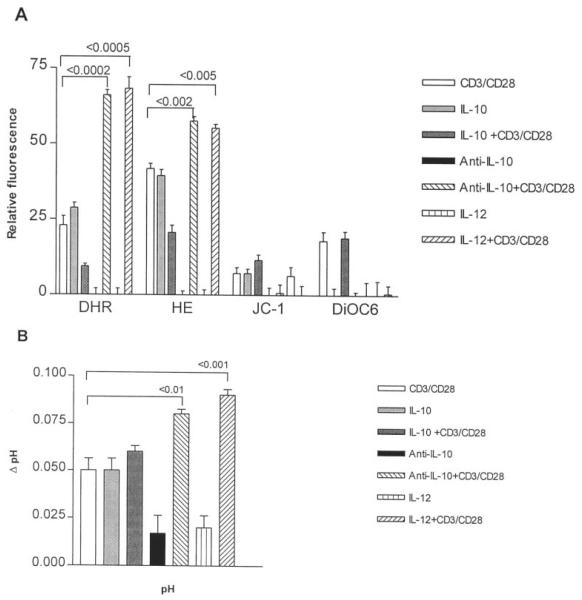
The effect of IL-10, Ab to IL-10, IL-12, and TGF-β1 on CD3/CD28-induced mitochondrial hyperpolarization, ROI production, and cytoplasmic alkalinization was also investigated in lupus patients and healthy as well as RA controls. Pretreatment for 16 h with Ab to IL-10 and IL-12 increased CD3/CD28-induced ROI production in lupus PBL comparable with those in normal and RA controls (Fig. 6A). Ab to IL-10 and IL-12 also enhanced CD3/CD28-induced cytoplasmic alkalinization in lupus PBL (Fig. 6B). Neither Ab to IL-10 nor IL-12 normalized CD3/CD28-induced Δψm elevation in lupus PBL. TGF-β1 had no significant effect on CD3/CD28-induced changes in Δψm, ROI production, or pHi (data not shown).
FIGURE 6.
Effect of IL-10 (10 ng/ml), anti-IL-10 (25 μg/ml), and IL-12 (10 ng/ml) on CD3/CD28 induced changes in Δψm (DiOC6 and JC-1 fluorescence, A), ROI production (DHR and HE fluorescence, A), and pHi (B). PBL from 10 patients with SLE were pretreated for 16 h with IL-10 (10 ng/ml), anti-IL-10 (25 μg/ml Ab), or IL-12 (10 ng/ml), then washed and stimulated with CD3 and CD28 mAbs. The Δψm and pHi were assessed 1 h, while ROI production was determined 6 h after CD3/CD28 costimulation. Values of p reflect significant enhancement of CD3/CD28-induced ROI production and elevation of pHi following pretreatment with anti-IL-10 and IL-12.
Discussion
The Δψm (negative inside and positive outside) is of key importance for ATP synthesis and ROI production, thus regulating cellular activation, survival, and death signals. Although disruption of the mitochondrial membrane potential has been proposed as the point of no return in apoptotic signaling (8–10), recent data from this laboratory indicate that elevation of Δψm, i.e., mitochondrial hyperpolarization, occurs in the early phase of Fas- and H2O2-induced apoptosis of Jurkat human leukemia T cells and normal human PBL (13, 14). Abnormal apoptosis of SLE T cells has been associated with deviations in key mitochondrial checkpoints: Δψm and mitochondrial ROI production were elevated, whereas GSH levels were diminished compared with healthy or RA controls (18). Intracellular ATP content and ATP/ADP ratio were reduced and correlated with Δψm elevation in lupus. Mitochondrial hyperpolarization can be induced by T cell activation via CD3/CD28 costimulation or inhibition of F0F1-ATPase by oligomycin. The resultant ATP depletion sensitizes T cells for necrosis, which may significantly contribute to inflammation in patients with SLE. This was consistent with a role for intracellular ATP concentration in the decision of the cell to die via apoptosis or necrosis (52). With Δψm hyperpolarization and extrusion of H+ ions from the mitochondrial matrix, the cytochromes within the electron transport chain become more reduced, which favors generation of ROI (19). Thus, mitochondrial hyperpolarization is a likely cause of increased ROI production, and may be ultimately responsible for increased spontaneous cell death in patients with SLE. Mitochon drial hyperpolarization also occurred during T cell activation, which indicated that this event represents an early and reversible step in apoptosis. In parallel with mitochondrial hyperpolarization, T cell activation through CD3/CD28 costimulation also elicited cytoplasmic alkalinization. In comparison with healthy and RA controls, lymphocytes of lupus patients exhibited cytoplasmic alkalinization, while CD3/CD28-induced changes in Δψm, ROI production, and pHi were diminished in SLE. Because caspase-mediated apoptosis requires a disruption of Δψm and cellular acidification, baseline mitochondrial hyperpolarization and cytoplasmic alkalinization represent an altered state of T cell activation rather than ongoing apoptosis in patients with SLE.
The present data reveal that, similar to CD3/CD28 costimulation, certain lymphokines, such as IL-3, IL-10, TGF-β1, and IFN-γ, can also elicit a transient elevation of Δψm. Among these lymphokines, production of IL-10 was increased in patients with SLE and correlated with disease activity. IL-10 elicited cell death of lupus PBL, while it did not influence viability of normal PBL, in accordance with previous observations (27). IL-10 had diametrically opposing effects on Δψm and ROI production in lupus patients on the one hand and healthy as well as RA controls in contrast. Although Δψm was increased by IL-10 in control and RA PBL, it remained unchanged after IL-10 stimulation of lupus PBL. Maximal baseline hyperpolarization may account for the inability of IL-10 or CD3/CD28 costimulation to further augment Δψm in SLE. In contrast, ROI production was selectively increased by IL-10 in lupus PBL. Of note, ROI were shown to increase expression of IL-10 mRNA (25) and IL-10 secretion (53), suggesting that increased IL-10 levels and ROI production represent interacting components of a positive feedback loop, amplifying cell death signaling in patients with SLE (18). IL-10 blockade or stimulation with antagonistic lymphokine IL-12 reduced ROI production and pHi without affecting baseline Δψm in SLE. This suggested that increased IL-10 production was not responsible for mitochondrial hyperpolarization; however, it may have contributed to ROI production and alkalinization. Moreover, IL-10 blockade or IL-12 normalized CD3/CD28-induced ROI production and cytoplasmic alkalinization without affecting Δψm of lupus PBL. These results, in agreement with previous studies (27, 54), indicate that increased IL-10 production may partially interfere with CD3/CD28-induced T cell activation in SLE. Alternatively, TGF-β1 had no significant effect on baseline or CD3/CD28-induced changes in Δψm, ROI production, and pHi, arguing against its role in aberrant T cell activation. Indeed, diminished production of TGF-β1 may be important in augmented Ig production by lupus B cells (51).
The Δψm reflects the energy stored in the electrochemical gradient across the inner mitochondrial membrane, which, in turn, is used by F0F1-ATPase to convert ADP to ATP during oxidative phosphorylation. Both T cell activation and apoptosis require the energy provided by ATP (55). Mitochondrial hyperpolarization also occurs during T cell activation, which indicates that this event represents an early and reversible step in apoptosis. In this study, we showed that stimulation of normal T cells through the CD3 and CD28 receptors or incubation with IL-3, IL-10, TGF-β1, and IFN-γ caused a transient elevation of Δψm. Thus, repetitive T cell activation in vivo could be responsible for prolonged mitochondrial hyperpolarization. However, repeated T cell activation through CD3/CD28 costimulation, Fas stimulation, or IL-10 treatment at 1- to 6-day intervals did not elicit persistent Δψm elevation, but led to a disruption of Δψm, followed by activation-induced cell death (data not shown), as described earlier (18, 56). The lack of correlation between IL-10 levels and Δψm as well as the failure of IL-10 blockade or IL-12 treatment to reduce Δψm argue against a role for IL-10 in mitochondrial hyperpolarization in SLE. Incubation of lupus PBL up to 5 days in vitro failed to affect the extent of Δψm elevation with respect to control PBL. Persistent mitochondrial hyperpolarization may originate from an intramitochondrial block affecting reversal of commonly arising activation signals. There are a number of potential mechanisms underlying Δψm elevation (11). Lupus patients exhibit deficiency of protein kinase A (57, 58) that may favor intramitochondrial translocation of Bad, a Bcl-2 family protein (59, 60). Other mitochondrial membrane ion transporters, OH−/Pi antiporter, K+/H+ antiporter, Na+/H+ exchanger, H+/Pi and H+/pyruvate symotransporters, and/or electrogenic K+ uniporter (12), may also be involved. Diminished ADP import through the voltage-dependent anion channel of the outer membrane and/or the adenine nucleotide translocator of the inner membrane could interfere with use of the electrochemical gradients and result in elevation of Δψm. The latter mechanism is favored by a relative increase of the ADP/ATP ratio in cytosol of lupus PBL (18).
Mitochondrial hyperpolarization predisposes for increased ROI production (19). Oxidative stress affects activity of transcription factors AP-1 and NF-κB (23, 24), and, further downstream, may lead to the skewed expression of IL-2, TNF, and IL-10 (25). Increased spontaneous apoptosis of lymphocytes has been linked to increased IL-10 production, release of Fas ligand, and overexpression of Fas receptor in SLE (27). Because increased ROI levels confer sensitivity to H2O2, NO, TNF, and Fas-induced cell death (13, 28), elevated baseline Δψm, ROI production, and pHi may have key roles in altered activation and death of lupus T cells. Although mitochondrial hyperpolarization was not affected, IL-10 Ab or IL-12 normalized ROI production and intracellular alkalinization in lupus PBL. Therefore, while the mechanism of mitochondrial hyperpolarization is further investigated, the present study supports a role for IL-10 antagonists in correcting signaling dysfunction and treatment of patients with SLE.
Footnotes
This work was supported in part by Grants RO1 DK 49221 and RO1 AI 48079 from the National Institutes of Health and the Central New York Community Foundation.
Abbreviations used in this paper: SLE, systemic lupus erythematosus; Δψm, mitochondrial transmembrane potential; CMXRos, chloromethyl-X-rosamine; DCF, 5,6-carboxy-2′,7′-dichlorofluorescein; DCFH-DA, DCF-diacetate; DHR, dihydrorhodamine 123; DiOC6, 3,3′-dihexyloxacarbocyanine iodide; GSH, reduced glutathione; HE, hydroethidine; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide; pHi, intracellular pH; PI, propidium iodide; ROI, reactive oxygen intermediate; SLEDAI, SLE disease activity index; SNARF, seminaphthorhodafluor; TMRM, tetramethylrhodamine methyl ester.
References
- 1.Elkon KB. Apoptosis in SLE: too little or too much? Clin. Exp. Rheumatol. 1994;12:553. [PubMed] [Google Scholar]
- 2.Perl A, Banki K. Molecular mimicry, altered apoptosis, and immuno-modulation as mechanisms of viral pathogenesis in systemic lupus erythematosus. In: Kammer GM, Tsokos GC, editors. Lupus: Molecular and Cellular Pathogenesis. Humana Press; Totowa: 1999. p. 43. [Google Scholar]
- 3.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 1992;10:267. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 5.Emlen W, Niebur JA, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J. Immunol. 1994;152:3685. [PubMed] [Google Scholar]
- 6.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-α. Clin. Immunol. Immunopathol. 1996;81:293. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
- 8.Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA. 1996;93:14559. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susin SA, Zamzami N, Castedo M, Daugas E, Wang H, Geley S, Fassy F, Reed RC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/Apo-1/CD95- and ceramide-induced apoptosis. J. Exp. Med. 1997;186:25. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vander Heiden M, Chandel NS, Williamson EK, Schumaker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 11.Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid. Redox Signal. 2000;2:551. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- 12.Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death or organelles, cells, and organisms. Mol. Aspects Med. 1999;20:139. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 13.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J. Immunol. 1999;162:1466. [PMC free article] [PubMed] [Google Scholar]
- 14.Puskas F, Gergely P, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by bcl-2. EMBO J. 1999;18:6027. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb E, Vander Heiden MG, Thompson CG. Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor α-induced apoptosis. Mol. Cell. Biol. 2000;20:5680. doi: 10.1128/mcb.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku H, Murphy MP. Changes in mitochondrial membrane potential during staurosporin-induced apoptosis in Jurkat cells. FEBS Lett. 2000;475:267. doi: 10.1016/s0014-5793(00)01681-1. [DOI] [PubMed] [Google Scholar]
- 18.Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, Perl A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stryer L. Biochemistry. Freeman; New York: 1988. [Google Scholar]
- 20.Los M, Schenk H, Hexel K, Baeuerle PA, Droge W, Schulze-Osthoff K. IL-2 gene expression and NF-κB activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14:3731. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 22.Handwerger BS, Luzian I, Da Silva L, Storrer CE, Via CS. Cytokines in the immunopathogenesis of lupus. In: G. M.; Tsokos GC, editor. Lupus: Molecular and Cellular Pathogenesis. Humana; Totowa: 1999. p. 321. [Google Scholar]
- 23.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Karin N. Is NF-κB the sensor of oxidative stress? FASEB J. 1999;13:1137. [PubMed] [Google Scholar]
- 25.Le Moine O, Louis H, Stordeur P, Collet JM, Goldman M, Deviere J. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology. 1997;113:1701. doi: 10.1053/gast.1997.v113.pm9352875. [DOI] [PubMed] [Google Scholar]
- 26.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J. Exp. Med. 1995;181:839. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J. Clin. Invest. 1997;100:2622. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J. Biol. Chem. 1996;271:32994. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 29.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 30.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 31.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Committee on Prognosis Studies in SLE Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 32.Perl A, Gonzalez-Cabello R, Lang I, Gergely P. Effector activity of OKT4+ and OKT8+ T-cell subsets in lectin-dependent cell-mediated cytotoxicity against adherent HEp-2 cells. Cell. Immunol. 1984;84:185. doi: 10.1016/0008-8749(84)90089-3. [DOI] [PubMed] [Google Scholar]
- 33.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 34.Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie CAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and Abl. J. Exp. Med. 1995;182:1545. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 36.Petit PX, O'Connor JE, Grunwald D, Brown SC. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur. J. Biochem. 1990;194:389. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanner MK, Wellhausen SR, Klein JB. Flow cytometric analysis of altered mononuclear cell transmembrane potential induced by cyclosporin. Cytometry. 1993;14:59. doi: 10.1002/cyto.990140111. [DOI] [PubMed] [Google Scholar]
- 38.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr., Bo Chen L. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming cation JC-1. Proc. Natl. Acad. Sci. USA. 1991;88:3671. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cossarizza A, Franceschi C, Monti D, Salvioli S, Bellesia E, Rivabene R, Biondo L, Rainaldi G, Tinari A, Malorni W. Protective effect of N-acetylcysteine in tumor necrosis factor-α-induced apoptosis in U937 cells: the role of mitochondria. Exp. Eye Res. 1995;220:232. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- 40.Lundin A. Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Methods Enzymol. 2000;305:346. doi: 10.1016/s0076-6879(00)05499-9. [DOI] [PubMed] [Google Scholar]
- 41.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- 42.Lundin A. ATP extractants neutralized by cyclodextrins. In: Campbell AK, Kricka LJ, Stanley PE, editors. Bioluminescence and Chemiluminescence. Wiley; Chichester: 1994. p. 399. [Google Scholar]
- 43.Wieder ED, Hang H, Fox MH. Measurement of intracellular pH using flow cytometry with carboxy-SNARF-1. Cytometry. 1993;14:916. doi: 10.1002/cyto.990140810. [DOI] [PubMed] [Google Scholar]
- 44.Meisenholder GW, Martin SJ, Green DR, Nordberg J, Babior BM, Gottlieb RA. Events in apoptosis: acidification is downstream of protease activation and BCL-2 protection. J. Biol. Chem. 1996;271:16260. doi: 10.1074/jbc.271.27.16260. [DOI] [PubMed] [Google Scholar]
- 45.Khaled AR, Reynolds DA, Young HA, Thompson CA, Muegge K, Durum SK. IL-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the bcl-2 family: roles of intracellular pH, ADP transport, and F0F1-ATPase. J. Biol. Chem. 2001;276:6453. doi: 10.1074/jbc.M006391200. [DOI] [PubMed] [Google Scholar]
- 46.Mills GB, Cheung RK, Cragoe EJ, Jr., Grinstein S, Gelfand EW. Activation of the Na+/H+ antiport is not required for lectin-induced proliferation of human T lymphocytes. J. Immunol. 1986;136:1150. [PubMed] [Google Scholar]
- 47.Fischer GF, Holter W, Majdic O, Cragoe EJ, Jr., Knapp W. T cell stimulation via CD2 molecules is regularly accompanied by an increase in cytoplasmic pH: different effects of lectins and CD3 antibodies. J. Immunol. 1988;141:404. [PubMed] [Google Scholar]
- 48.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 49.Llorente L, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Maillot MC, Durand-Gasselin I, Fourrier BM, Galanaud P, Emilie D. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur. Cytokine Network. 1993;4:421. [PubMed] [Google Scholar]
- 50.Mishra N, Brown DR, Olorenshaw IM, Kammer GM. Trichostatin A reverses skewed expression of CD154, interleukin-10, and interferon-γ gene and protein expression in lupus T cells. Proc. Natl. Acad. Sci. USA. 2001;98:2628. doi: 10.1073/pnas.051507098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. Decreased production of TGF-β by lymphocytes from patients with systemic lupus erythematosus. J. Immunol. 1998;160:2539. [PubMed] [Google Scholar]
- 52.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997;185:1481. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virdee K, Parone PA, Tolkovsky AM. Phosphorylation of the pro-apoptotic protein BAD on serine 155, a novel site, contributes to cell survival. Curr. Biol. 2002;10:1151. doi: 10.1016/s0960-9822(00)00702-8. [DOI] [PubMed] [Google Scholar]
- 54.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Interleukin-10 blockade corrects impaired in vitro cellular immune responses of systemic lupus erythematosus patients. Arthritis Rheum. 2001;43:1976. doi: 10.1002/1529-0131(200009)43:9<1976::AID-ANR8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 55.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 56.Tsokos GC, Liossis SC. Immune cell signaling defects in lupus: activation, anergy and death. Immunol. Today. 1999;20:119. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]
- 57.Kammer GM. High prevalence of T cell type I protein kinase A deficiency in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1458. doi: 10.1002/1529-0131(199907)42:7<1458::AID-ANR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Mishra N, Khan IU, Tsokos GC, Kammer GM. Association of deficient type II protein kinase A activity with aberrant nuclear translocation of the RII β subunit in systemic lupus erythematosus T lymphocytes. J. Immunol. 2002;165:2830. doi: 10.4049/jimmunol.165.5.2830. [DOI] [PubMed] [Google Scholar]
- 59.Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 2002;349:547. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J. Biol. Chem. 2002;275:25046. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]