Abstract
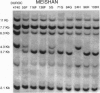
Identification of individual major genes affecting quantitative traits in livestock species has been limited to date. By using a candidate gene approach and a divergent breed cross involving the Chinese Meishan pig, we have shown that a specific allele of the estrogen receptor (ER) locus is associated with increased litter size. Female pigs from synthetic lines with a 50% Meishan background that were homozygous for this beneficial allele produced 2.3 more pigs in first parities and 1.5 more pigs averaged over all parities than females from the same synthetic lines and homozygous for the undesirable allele. This beneficial ER allele was also found in pigs with Large White breed ancestory. Analysis of females with Large White breed background showed an advantage for females homozygous for the beneficial allele as compared to females homozygous for the other allele of more than 1 total pig born. Analyses of growth performance test records detected no significant unfavorable associations of the beneficial allele with growth and developmental traits. Mapping of the ER gene demonstrated that the closest known genes or markers were 3 centimorgans from ER. To our knowledge, one of these, superoxide dismutase gene (SOD2), was mapped for the first time in the pig. Analysis of ER and these linked markers indicated that ER is the best predictor of litter size differences. Introgression of the beneficial allele into commercial pig breeding lines, in which the allele was not present, and marker-assisted selection for the beneficial allele in lines with Meishan and Large White background have begun.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L., Haley C. S., Ellegren H., Knott S. A., Johansson M., Andersson K., Andersson-Eklund L., Edfors-Lilja I., Fredholm M., Hansson I. Genetic mapping of quantitative trait loci for growth and fatness in pigs. Science. 1994 Mar 25;263(5154):1771–1774. doi: 10.1126/science.8134840. [DOI] [PubMed] [Google Scholar]
- Archibald A. L., Haley C. S., Brown J. F., Couperwhite S., McQueen H. A., Nicholson D., Coppieters W., Van de Weghe A., Stratil A., Winterø A. K. The PiGMaP consortium linkage map of the pig (Sus scrofa). Mamm Genome. 1995 Mar;6(3):157–175. doi: 10.1007/BF00293008. [DOI] [PubMed] [Google Scholar]
- Collins F. S. Positional cloning moves from perditional to traditional. Nat Genet. 1995 Apr;9(4):347–350. doi: 10.1038/ng0495-347. [DOI] [PubMed] [Google Scholar]
- Davis G. H., McEwan J. C., Fennessy P. F., Dodds K. G., Farquhar P. A. Evidence for the presence of a major gene influencing ovulation rate on the X chromosome of sheep. Biol Reprod. 1991 Apr;44(4):620–624. doi: 10.1095/biolreprod44.4.620. [DOI] [PubMed] [Google Scholar]
- Ellegren H., Chowdhary B. P., Johansson M., Marklund L., Fredholm M., Gustavsson I., Andersson L. A primary linkage map of the porcine genome reveals a low rate of genetic recombination. Genetics. 1994 Aug;137(4):1089–1100. doi: 10.1093/genetics/137.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm M., Winterø A. K., Christensen K., Kristensen B., Nielsen P. B., Davies W., Archibald A. Characterization of 24 porcine (dA-dC)n-(dT-dG)n microsatellites: genotyping of unrelated animals from four breeds and linkage studies. Mamm Genome. 1993;4(4):187–192. doi: 10.1007/BF00417561. [DOI] [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V. K., Weiler J. E., O'Brien P. J., MacLennan D. H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991 Jul 26;253(5018):448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Korach K. S. Insights from the study of animals lacking functional estrogen receptor. Science. 1994 Dec 2;266(5190):1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberson W. R., Johnson R. K., Zimmerman D. R., Long T. E. Direct responses to selection for increased litter size, decreased age at puberty, or random selection following selection for ovulation rate in swine. J Anim Sci. 1991 Aug;69(8):3129–3143. doi: 10.2527/1991.6983129x. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Schork N. J. Genetic dissection of complex traits. Science. 1994 Sep 30;265(5181):2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Lehrer S., Sanchez M., Song H. K., Dalton J., Levine E., Savoretti P., Thung S. N., Schachter B. Oestrogen receptor B-region polymorphism and spontaneous abortion in women with breast cancer. Lancet. 1990 Mar 17;335(8690):622–624. doi: 10.1016/0140-6736(90)90410-7. [DOI] [PubMed] [Google Scholar]
- Montgomery G. W., Lord E. A., Penty J. M., Dodds K. G., Broad T. E., Cambridge L., Sunden S. L., Stone R. T., Crawford A. M. The Booroola fecundity (FecB) gene maps to sheep chromosome 6. Genomics. 1994 Jul 1;22(1):148–153. doi: 10.1006/geno.1994.1355. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Pakdel F., Le Guellec C., Vaillant C., Le Roux M. G., Valotaire Y. Identification and estrogen induction of two estrogen receptors (ER) messenger ribonucleic acids in the rainbow trout liver: sequence homology with other ERs. Mol Endocrinol. 1989 Jan;3(1):44–51. doi: 10.1210/mend-3-1-44. [DOI] [PubMed] [Google Scholar]
- Paterson A. H., Lander E. S., Hewitt J. D., Peterson S., Lincoln S. E., Tanksley S. D. Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature. 1988 Oct 20;335(6192):721–726. doi: 10.1038/335721a0. [DOI] [PubMed] [Google Scholar]
- Rohrer G. A., Alexander L. J., Keele J. W., Smith T. P., Beattie C. W. A microsatellite linkage map of the porcine genome. Genetics. 1994 Jan;136(1):231–245. doi: 10.1093/genetics/136.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild M. F., Larson R. G., Jacobson C., Pearson P. PvuII polymorphisms at the porcine oestrogen receptor locus (ESR). Anim Genet. 1991;22(5):448–448. doi: 10.1111/j.1365-2052.1991.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Stuber C. W., Lincoln S. E., Wolff D. W., Helentjaris T., Lander E. S. Identification of genetic factors contributing to heterosis in a hybrid from two elite maize inbred lines using molecular markers. Genetics. 1992 Nov;132(3):823–839. doi: 10.1093/genetics/132.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaske D. A., Larson R. G., Liu H. C., Warner C. M., Rothschild M. F. Rapid communication: restriction fragment length polymorphism at the porcine superoxide dismutase locus. J Anim Sci. 1995 Mar;73(3):921–921. doi: 10.2527/1995.733921x. [DOI] [PubMed] [Google Scholar]
- Womack J. E. Veterinary medicine. Molecular genetics arrives on the farm. Nature. 1992 Nov 12;360(6400):108–109. doi: 10.1038/360108a0. [DOI] [PubMed] [Google Scholar]