Abstract
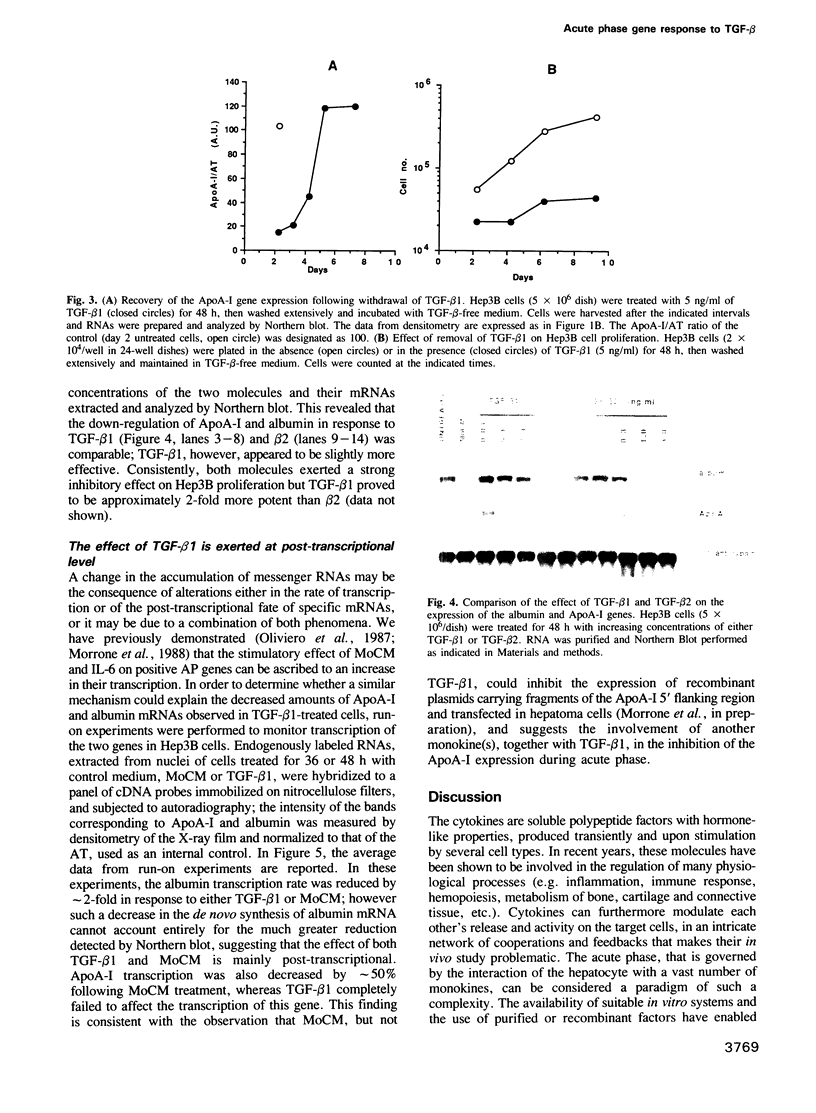
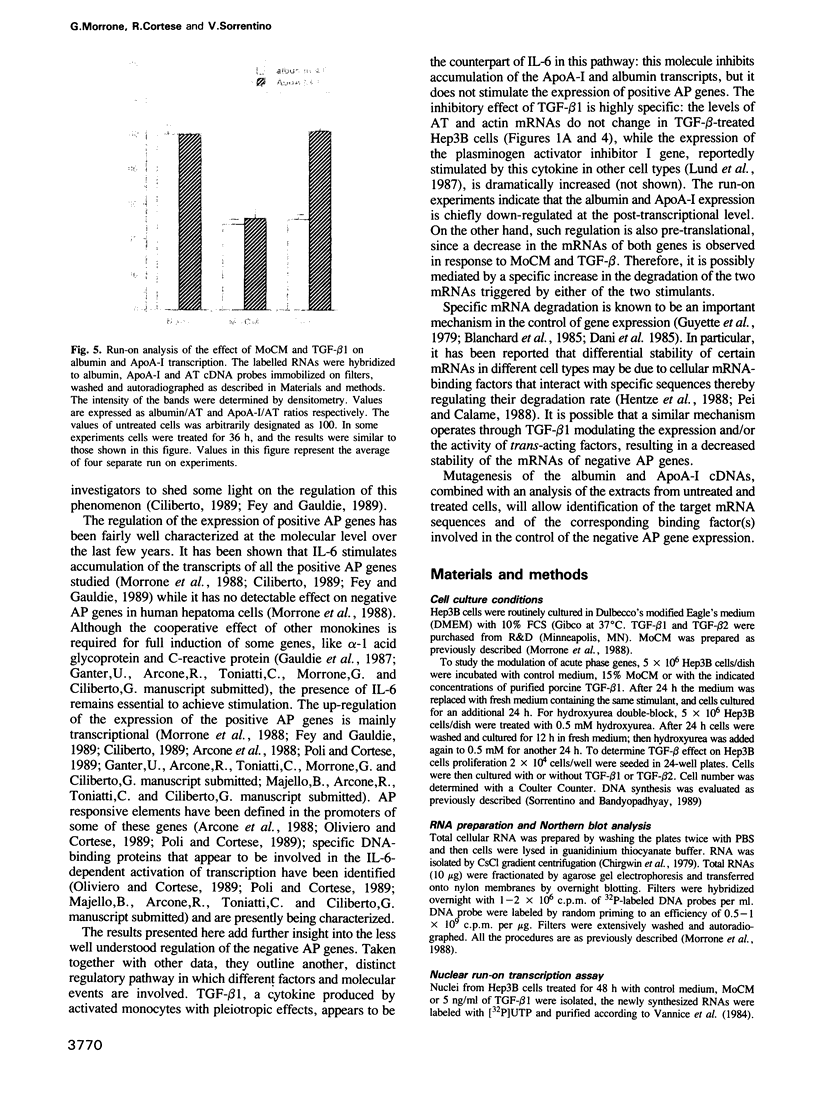
During the acute phase (AP) reaction the expression of a series of liver-specific genes coding for secretory proteins is either stimulated or suppressed by different cytokines released by activated monocytes. Transforming growth factor beta (TGF-beta) is a cytokine that, first identified for its ability to regulate cellular growth, has been gradually recognized to modulate several other functions. We have investigated the effect of TGF-beta on the expression of acute phase genes in liver cells. We found that TGF-beta selectively induces a specific decreases in the amount of mRNAs of genes negatively regulated during AP reaction, like albumin and apolipoprotein A-I (ApoA-I). The inhibitory effect of TGF-beta on the expression of negative AP genes is primarily post-transcriptional and it is very likely to be mediated via an enhancement of the turnover of both albumin and ApoA-I mRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Geiger T., Hirano T., Northoff H., Ganter U., Bauer J., Kishimoto T., Heinrich P. C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. doi: 10.1016/0014-5793(87)80344-7. [DOI] [PubMed] [Google Scholar]
- Arcone R., Gualandi G., Ciliberto G. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucleic Acids Res. 1988 Apr 25;16(8):3195–3207. doi: 10.1093/nar/16.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Muller-Eberhard U. Synthesis of hemopexin and cysteine protease inhibitor is coordinately regulated by HSF-II and interferon-beta 2 in rat hepatoma cells. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1218–1228. doi: 10.1016/0006-291x(87)90778-9. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Piechaczyk M., Dani C., Chambard J. C., Franchi A., Pouyssegur J., Jeanteur P. c-myc gene is transcribed at high rate in G0-arrested fibroblasts and is post-transcriptionally regulated in response to growth factors. Nature. 1985 Oct 3;317(6036):443–445. doi: 10.1038/317443a0. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Riccio A., Andreasen P. A., Nielsen L. S., Kristensen P., Laiho M., Saksela O., Blasi F., Danø K. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987 May;6(5):1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Oliviero S., Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989 Apr;8(4):1145–1151. doi: 10.1002/j.1460-2075.1989.tb03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero S., Morrone G., Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987 Jul;6(7):1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Calame K. Differential stability of c-myc mRNAS in a cell-free system. Mol Cell Biol. 1988 Jul;8(7):2860–2868. doi: 10.1128/mcb.8.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Bandyopadhyay S. TGF beta inhibits Go/S-phase transition in primary fibroblasts. Loss of response to the antigrowth effect of TGF beta is observed after immortalization. Oncogene. 1989 May;4(5):569–574. [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]