Abstract
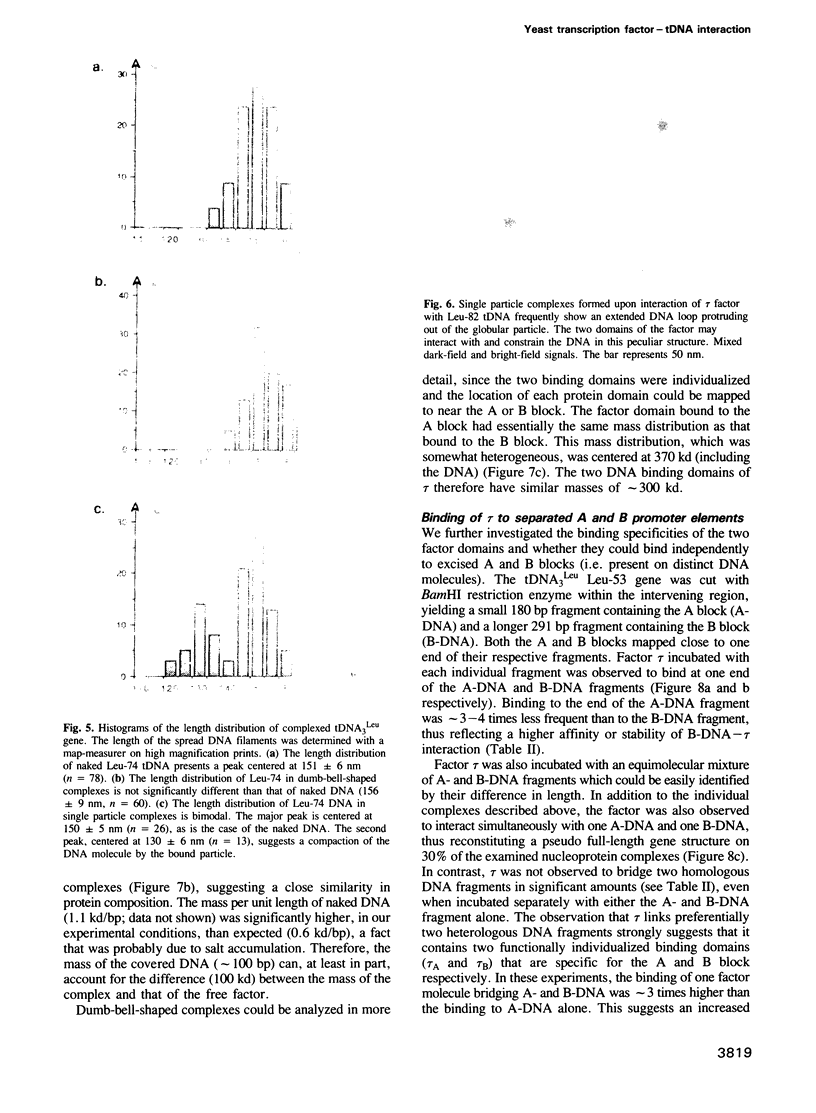
Yeast transcription factor tau interacts with the intragenic promoter of tRNA genes, binding to both the A and B block elements. Affinity-purified tau factor and tau-tDNA complexes were examined by scanning transmission electron microscopy to analyze the structural features of free and DNA bound factor. The free factor appeared as two tightly associated globular domains of roughly similar size (10 nm in diameter) and mass (approximately 300 kd). A combination of these two domains results in a mass for the factor of 510-670 kd. When tau was allowed to interact with recombinant tRNA(3Leu) genes with variable A block-B block spacing, different structures were observed. With short genes, the two globular domains were not resolved and tau appeared as a large particle covering the A and B block region. On the other hand, with genes having a larger A-B distance (53 or 74 bp), mostly dumb-bell-shaped complexes were formed with individualized factor domains bound separately to the A and B blocks. A smaller proportion of the complexes appeared to consist of a large particle bound at only one site, essentially on the B block. Mapping of the binding domains in the DNA showed a good correlation with the respective positions of the A and B promoter elements. Factor binding did not induce a noticeable DNA bending, although with extended genes apparent DNA shortening and cases of DNA looping were observed. Upon cleavage of the tRNA(3Leu) gene between the A and B blocks after or prior to complex formation, the two factor domains remained attached to the same DNA fragment (mostly the B-DNA fragment). In addition, images of protein-linked, reconstituted full-length genes were also observed. These different conformational states of the tau-tDNA complexes probably reflect the dynamic aspect of the interaction of the factor with its DNA target.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. E., Camier S., Sentenac A., Hall B. D. Gene size differentially affects the binding of yeast transcription factor tau to two intragenic regions. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8768–8772. doi: 10.1073/pnas.84.24.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. E., Camier S., Sentenac A., Hall B. D. Gene size differentially affects the binding of yeast transcription factor tau to two intragenic regions. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8768–8772. doi: 10.1073/pnas.84.24.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. E., Gabrielsen O., Hall B. D. Effects of tRNATyr point mutations on the binding of yeast RNA polymerase III transcription factor C. J Biol Chem. 1986 Apr 25;261(12):5275–5282. [PubMed] [Google Scholar]
- Baker R. E., Hall B. D. Structural features of yeast tRNA genes which affect transcription factor binding. EMBO J. 1984 Dec 1;3(12):2793–2800. doi: 10.1002/j.1460-2075.1984.tb02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger P. A., Yoshinaga S. K., Berk A. J. DNA-binding properties and characterization of human transcription factor TFIIIC2. J Biol Chem. 1987 Nov 5;262(31):15098–15105. [PubMed] [Google Scholar]
- Braun B. R., Riggs D. L., Kassavetis G. A., Geiduschek E. P. Multiple states of protein-DNA interaction in the assembly of transcription complexes on Saccharomyces cerevisiae 5S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2530–2534. doi: 10.1073/pnas.86.8.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camier S., Gabrielsen O., Baker R., Sentenac A. A split binding site for transcription factor tau on the tRNA3Glu gene. EMBO J. 1985 Feb;4(2):491–500. doi: 10.1002/j.1460-2075.1985.tb03655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto G., Castagnoli L., Cortese R. Transcription by RNA polymerase III. Curr Top Dev Biol. 1983;18:59–88. doi: 10.1016/s0070-2153(08)60579-7. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Engel A., Baumeister W., Saxton W. O. Mass mapping of a protein complex with the scanning transmission electron microscope. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4050–4054. doi: 10.1073/pnas.79.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P., Coppo A., Fruscoloni P., Benedetti P., Di Segni G., Tocchini-Valentini G. P. Comparative mutational analysis of wild-type and stretched tRNA3(Leu) gene promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8763–8767. doi: 10.1073/pnas.84.24.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R., Leonard K. R. Comparative mass measurement of biological macromolecules by scanning transmission electron microscopy. J Microsc. 1981 Jun;122(Pt 3):275–286. doi: 10.1111/j.1365-2818.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- Gabrielsen O. S., Marzouki N., Ruet A., Sentenac A., Fromageot P. Two polypeptide chains in yeast transcription factor tau interact with DNA. J Biol Chem. 1989 May 5;264(13):7505–7511. [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Jones A. V., Leonard K. R. Scanning transmission electron microscopy of unstained biological sections. Nature. 1978 Feb 16;271(5646):659–660. doi: 10.1038/271659a0. [DOI] [PubMed] [Google Scholar]
- Klekamp M. S., Weil P. A. Yeast class III gene transcription factors and homologous RNA polymerase III form ternary transcription complexes stable to disruption by N-lauroyl-sarcosine (sarcosyl). Arch Biochem Biophys. 1986 May 1;246(2):783–800. doi: 10.1016/0003-9861(86)90335-8. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Stillman D. J., Geiduschek E. P. Specific interactions of Saccharomyces cerevisiae proteins with a promoter region of eukaryotic tRNA genes. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6191–6195. doi: 10.1073/pnas.79.20.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Marzouki N., Camier S., Ruet A., Moenne A., Sentenac A. Selective proteolysis defines two DNA binding domains in yeast transcription factor tau. Nature. 1986 Sep 11;323(6084):176–178. doi: 10.1038/323176a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M., Isaacson M. S., Crewe A. V. Dark field imaging of biological macromolecules with the scanning transmission electron microscope. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1228–1232. doi: 10.1073/pnas.76.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruet A., Camier S., Smagowicz W., Sentenac A., Fromageot P. Isolation of a class C transcription factor which forms a stable complex with tRNA genes. EMBO J. 1984 Feb;3(2):343–350. doi: 10.1002/j.1460-2075.1984.tb01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz P., Weiss E., Colin P., Régnier E., Oudet P. Characterization of SV40 chromatin by mass determination on STEM. Chromosoma. 1986;94(3):189–198. doi: 10.1007/BF00288493. [DOI] [PubMed] [Google Scholar]
- Stillman D. J., Better M., Geiduschek E. P. Electron-microscopic examination of the binding of a large RNA polymerase III transcription factor to a tRNA gene. J Mol Biol. 1985 Sep 20;185(2):451–455. doi: 10.1016/0022-2836(85)90417-6. [DOI] [PubMed] [Google Scholar]
- Stillman D. J., Caspers P., Geiduschek E. P. Effects of temperature and single-stranded DNA on the interaction of an RNA polymerase III transcription factor with a tRNA gene. Cell. 1985 Feb;40(2):311–317. doi: 10.1016/0092-8674(85)90145-x. [DOI] [PubMed] [Google Scholar]
- Waldschmidt R., Jahn D., Seifart K. H. Purification of transcription factor IIIB from HeLa cells. J Biol Chem. 1988 Sep 15;263(26):13350–13356. [PubMed] [Google Scholar]











