Abstract
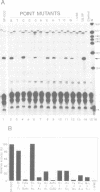
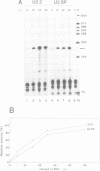
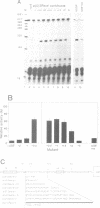
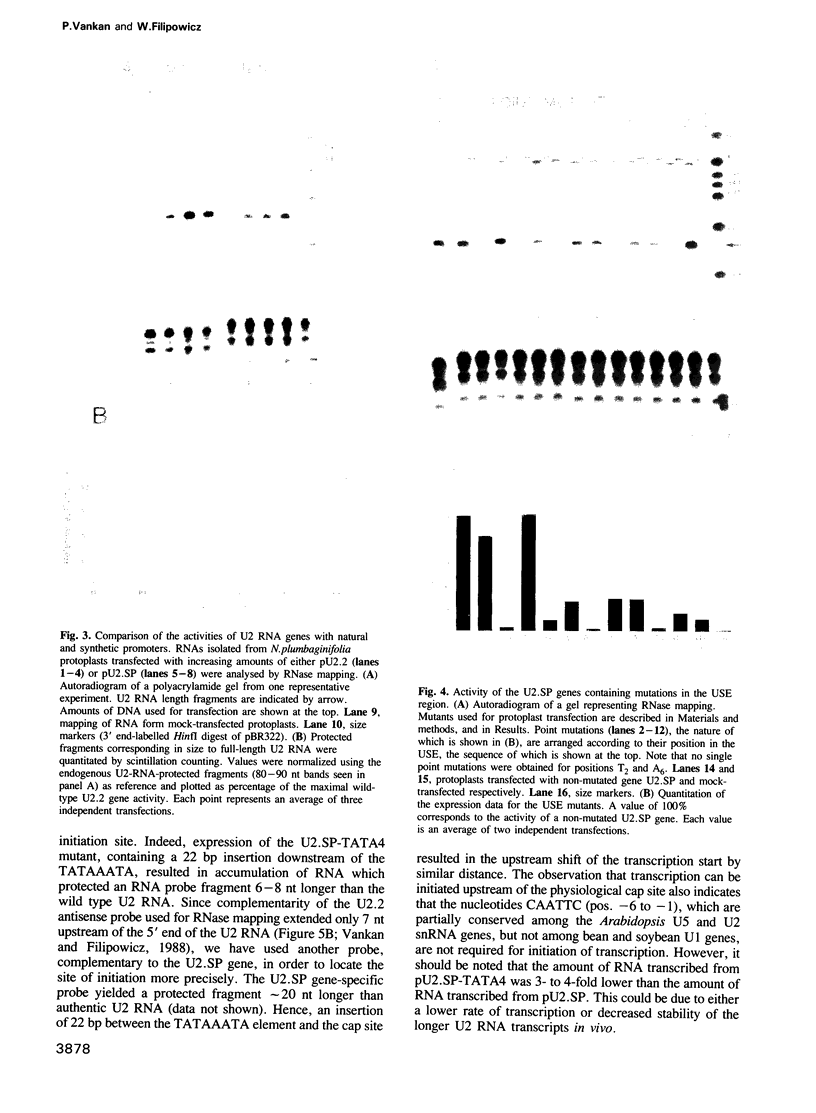
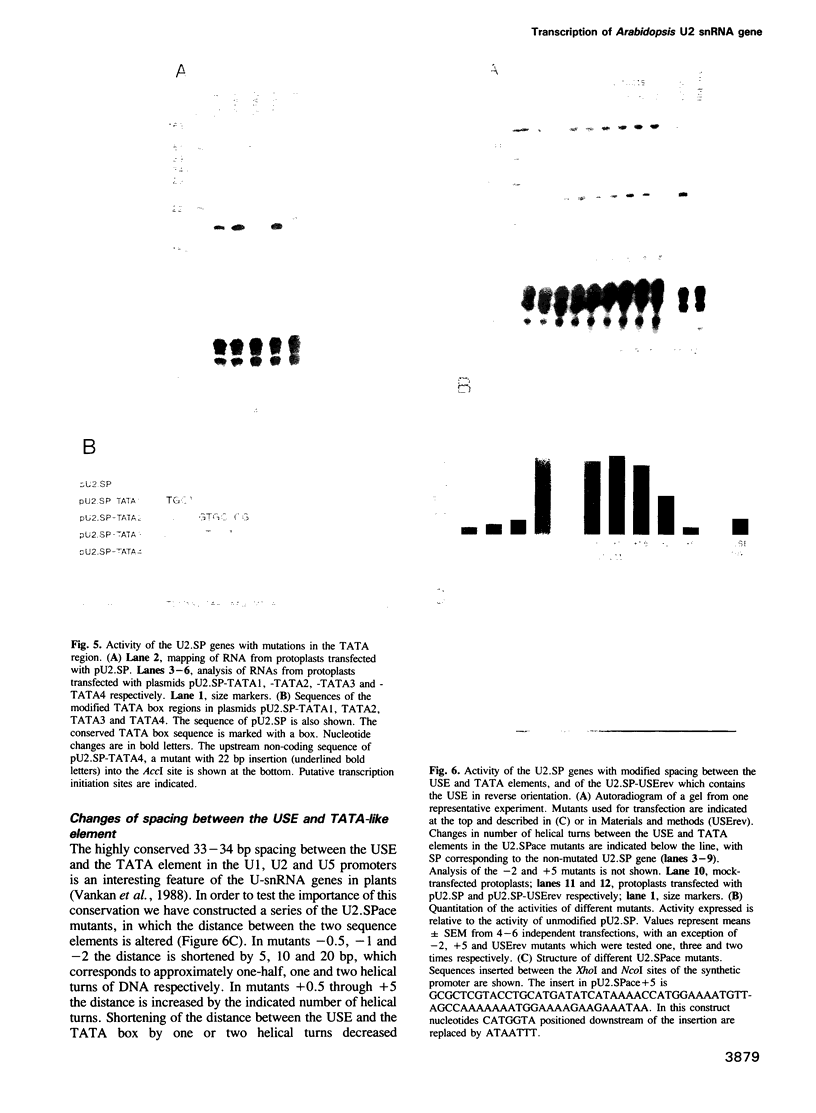
The U2 and U5 snRNA genes of Arabidopsis thaliana contain in their promoter regions two elements with conserved sequence and position. To test the significance of this conservation we have made a construction in which the promoter of the U2 RNA gene is replaced by the synthetic 98 bp long sequence containing the two conserved elements: an upstream sequence element, GTCCCACATCG (USE, pos. -78 to -68), and a TATA-like sequence TATAAATA (-33 to -26), positioned approximately three helical turns apart, as in the wild-type promoter. This synthetic promoter efficiently drove transcription of the U2 gene in transfected protoplasts of Nicotiana plumbaginifolia. The importance of the individual elements and of their position within the promoter was investigated. Deletion of the USE, change of its orientation, and some single point mutations all decreased transcription 10- to 20-fold, and replacement of the TATA-like element by an unrelated sequence inactivated the promoter. Mutants in which the spacing between the USE and TATAAATA was changed were less active but no correlation was observed between promoter activity and insertion of either odd or even numbers of half helical turns. Insertion of a spacer between TATAAATA and the cap site resulted in accumulation of U2 RNA with an extended 5' end, indicating that the TATAAATA element is responsible for selection of the initiation site. The data indicate that the promoters of RNA polymerase II-specific U-snRNA genes in higher plants differ from their animal counter-parts and also from plant mRNA gene promoters. They contain two essential elements, an USE, an element found only in U-snRNA genes, and a TATA element which is indistinguishable from the TATA boxes of mRNA-coding genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Chung J. S., Giglio L., Weiner A. M. Distinct factors with Sp1 and NF-A specificities bind to adjacent functional elements of the human U2 snRNA gene enhancer. Genes Dev. 1987 Oct;1(8):808–817. doi: 10.1101/gad.1.8.808. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Buckland R., Cortese R., Philipson L. Transcription signals in embryonic Xenopus laevis U1 RNA genes. EMBO J. 1985 Jun;4(6):1537–1543. doi: 10.1002/j.1460-2075.1985.tb03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto G., Palla F., Tebb G., Mattaj I. W., Philipson L. Properties of a U1 RNA enhancer-like sequence. Nucleic Acids Res. 1987 Mar 25;15(6):2403–2416. doi: 10.1093/nar/15.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Schenborn E. T. The human U1 snRNA promoter and enhancer do not direct synthesis of messenger RNA. Nucleic Acids Res. 1988 Jul 11;16(13):5827–5840. doi: 10.1093/nar/16.13.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hahn S., Hoar E. T., Guarente L. Each of three "TATA elements" specifies a subset of the transcription initiation sites at the CYC-1 promoter of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8562–8566. doi: 10.1073/pnas.82.24.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Lucito R. Elements required for transcription initiation of the human U2 snRNA gene coincide with elements required for snRNA 3' end formation. EMBO J. 1988 Oct;7(10):3125–3134. doi: 10.1002/j.1460-2075.1988.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Weiner A. M. Formation of the 3' end of U1 snRNA requires compatible snRNA promoter elements. Cell. 1986 Oct 24;47(2):249–258. doi: 10.1016/0092-8674(86)90447-2. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Repeated DNA sequences upstream from HIS1 also occur at several other co-regulated genes in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 25;258(8):5238–5247. [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Nordeen S. K., Vogt K., Edgell M. H. A complete library of point substitution mutations in the glucocorticoid response element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1986 Feb;83(3):710–714. doi: 10.1073/pnas.83.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson L., Bark C., Pettersson U. Identification of proteins interacting with the enhancer of human U2 small nuclear RNA genes. Nucleic Acids Res. 1987 Jul 10;15(13):4997–5016. doi: 10.1093/nar/15.13.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. G., Hellung-Larsen P., Frederiksen S. Synthesis of low molecular weight RNA components A, C and D by polymerase II in alpha-amanitin-resistant hamster cells. Nucleic Acids Res. 1979 Jan;6(1):321–330. doi: 10.1093/nar/6.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmaier M., Tebb G., Mattaj I. W. Functional characterization of X. laevis U5 snRNA genes. EMBO J. 1987 Oct;6(10):3071–3078. doi: 10.1002/j.1460-2075.1987.tb02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Lund E., Dahlberg J. E. The two embryonic U1 RNA genes of Xenopus laevis have both common and gene-specific transcription signals. EMBO J. 1985 Jun;4(6):1529–1535. doi: 10.1002/j.1460-2075.1985.tb03813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin M., Ares M., Jr, Weiner A. M. Human U2 small nuclear RNA genes contain an upstream enhancer. EMBO J. 1986 May;5(5):987–995. doi: 10.1002/j.1460-2075.1986.tb04313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Skuzeski J. T., Lund E., Steinberg T. H., Burgess R. R., Dahlberg J. E. Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J Biol Chem. 1987 Feb 5;262(4):1795–1803. [PubMed] [Google Scholar]
- Mühlbach H. P., Sänger H. L. Viroid replication is inhibited by alpha-amanitin. Nature. 1979 Mar 8;278(5700):185–188. doi: 10.1038/278185a0. [DOI] [PubMed] [Google Scholar]
- Parker R., Simmons T., Shuster E. O., Siliciano P. G., Guthrie C. Genetic analysis of small nuclear RNAs in Saccharomyces cerevisiae: viable sextuple mutant. Mol Cell Biol. 1988 Aug;8(8):3150–3159. doi: 10.1128/mcb.8.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H. D., Tebb G., Mattaj I. W. The Xenopus U2 gene PSE is a single, compact, element required for transcription initiation and 3' end formation. Nucleic Acids Res. 1989 May 25;17(10):3633–3644. doi: 10.1093/nar/17.10.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B., Guthrie C. An essential yeast snRNA with a U5-like domain is required for splicing in vivo. Cell. 1987 Jun 5;49(5):613–624. doi: 10.1016/0092-8674(87)90537-x. [DOI] [PubMed] [Google Scholar]
- Skuzeski J. M., Lund E., Murphy J. T., Steinberg T. H., Burgess R. R., Dahlberg J. E. Synthesis of human U1 RNA. II. Identification of two regions of the promoter essential for transcription initiation at position +1. J Biol Chem. 1984 Jul 10;259(13):8345–8352. [PubMed] [Google Scholar]
- Southgate C., Busslinger M. In vivo and in vitro expression of U7 snRNA genes: cis- and trans-acting elements required for RNA polymerase II-directed transcription. EMBO J. 1989 Feb;8(2):539–549. doi: 10.1002/j.1460-2075.1989.tb03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Vigneron M., Matthes H., Wildeman A., Zenke M., Chambon P. Requirement of stereospecific alignments for initiation from the simian virus 40 early promoter. Nature. 1986 Jan 9;319(6049):121–126. doi: 10.1038/319121a0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Grossniklaus U., Herr W., Hernandez N. Activation of the U2 snRNA promoter by the octamer motif defines a new class of RNA polymerase II enhancer elements. Genes Dev. 1988 Dec;2(12B):1764–1778. doi: 10.1101/gad.2.12b.1764. [DOI] [PubMed] [Google Scholar]
- Vankan P., Edoh D., Filipowicz W. Structure and expression of the U5 snRNA gene of Arabidopsis thaliana. Conserved upstream sequence elements in plant U-RNA genes. Nucleic Acids Res. 1988 Nov 25;16(22):10425–10440. doi: 10.1093/nar/16.22.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. Structure of U2 snRNA genes of Arabidopsis thaliana and their expression in electroporated plant protoplasts. EMBO J. 1988 Mar;7(3):791–799. doi: 10.1002/j.1460-2075.1988.tb02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B. Enhancers and transcription factors in the control of gene expression. Biochim Biophys Acta. 1988 Nov 10;951(1):17–35. doi: 10.1016/0167-4781(88)90021-8. [DOI] [PubMed] [Google Scholar]
- Westin G., Lund E., Murphy J. T., Pettersson U., Dahlberg J. E. Human U2 and U1 RNA genes use similar transcription signals. EMBO J. 1984 Dec 20;3(13):3295–3301. doi: 10.1002/j.1460-2075.1984.tb02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Berk A. Constraints on spacing between transcription factor binding sites in a simple adenovirus promoter. Genes Dev. 1988 Apr;2(4):403–411. doi: 10.1101/gad.2.4.403. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- de Vegvar H. E., Lund E., Dahlberg J. E. 3' end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell. 1986 Oct 24;47(2):259–266. doi: 10.1016/0092-8674(86)90448-4. [DOI] [PubMed] [Google Scholar]
- van Santen V. L., Spritz R. A. Nucleotide sequence of a bean (Phaseolus vulgaris) U1 small nuclear RNA gene: implications for plant pre-mRNA splicing. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9094–9098. doi: 10.1073/pnas.84.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]