Abstract
Antimicrobial peptides have attracted much interest as a novel class of antibiotics against a variety of microbes including antibiotics resistant strains. In this study, a new cationic antimicrobial peptide Hp1404 was identified from the scorpion Heterometrus petersii, which is an amphipathic α-helical peptide and has a specific inhibitory activity against gram-positive bacteria including methicillin-resistant Staphylococcus aureus. Hp1404 can penetrate the membrane of S. aureus at low concentration, and disrupts the cellular membrane directly at super high concentration. S. aureus does not develop drug resistance after multiple treatments with Hp1404 at sub MIC concentration, which is possibly associated with the antibacterial mechanism of the peptide. In addition, Hp1404 has low toxicity to both mammalian cells (HC50 = 226.6 µg/mL and CC50 > 100 µg/mL) and balb-c mice (Non-toxicity at 80 mg/Kg by intraperitoneal injection and LD50 = 89.8 mg/Kg by intravenous injection). Interestingly, Hp1404 can improve the survival rate of the MRSA infected balb-c mice in the peritonitis model. Taken together, Hp1404 may have potential applications as an antibacterial agent.
Introduction
The increasing frequency of antibiotic resistance among microorganisms is becoming a more and more serious problem, which has outpaced the development of new antibiotics [1], [2], [3]. It is urgently needed to discover new and more effective antimicrobial agents. As a potential source of these agents, antimicrobial peptide (AMPs) are ubiquitous in nature, which can be found in microorganisms [4], insects [5], amphibians [6], mammals [7], and plants [8]. They are produced as a part of the innate immune system defense, and show potent antimicrobial activity against a broad spectrum of microorganisms including resistant strains [9]. Interestingly, the mechanisms of AMPs's action are different from conventional antibiotics, most of which kill microorganisms rapidly by disrupting the integrity of the cytoplasmic membrane [10], [11]. Some of them can also interfere with the intracellular processes, such as affecting cell-wall biosynthesis pathway, inhibiting protein biosynthesis, or interacting with nucleic acids [12]. These properties make them the attractive candidates for the development of new antimicrobial agents in overcoming microbial resistance. At least 2300 different AMPs have been studied (http://aps.unmc.edu/AP/main.php) during the last three decades, and several AMPs have been investigated as therapeutic agents in the past decade [9].
As a living fossil, scorpion has survived over 400 million years on earth, and developed diverse venom peptides for successful survival during its long-term evolution [13]. So far, more and more AMPs have been identified from scorpion venoms, which can be divided into disulphide-bridged and non-disulphide-bridged peptides. Scorpine, a triple disulphide-bridge AMP from the scorpion Pandinus imperator has anti-bacterial and anti-malaria activities [14]. Non-disulphide-bridged AMPs Pandinins (from the scorpion Pandinus imperator) and IsCTs (from the scorpion Opisthacanthus madagascariensis) are α-helical polycationic peptides and have antimicrobial activity against both gram-positive bacteria and gram-negative bacteria [15], [16], [17]. The non-disulphide-bridged AMP Vejovine from the scorpion Vaejovis mexicanus can inhibit the growth of multidrug resistant clinical isolates of gram-negative bacteria [18]. These findings make scorpion venom as a potential source for discovering AMPs. We focus our interest on the scorpion species Heterometrus petersii, which usually inhabits in tropical to subtropical rainforests. Various kinds of bacteria can grow and proliferate in this kind of living environment, which is conducive to the evolution of the scorpion venom to contain more AMPs.
In this study, a new AMP named Hp1404 was characterized from the venomous gland cDNA library of the scorpion Heterometrus petersii. Hp1404 is an amphipathic α-helical peptide. The in vitro antibacterial activities of Hp1404 peptide were then investigated using both standard and resistant strains. The mechanism of Hp1404 against bacteria was further explored in our work. Finally, we tested the toxicities of Hp1404 against mammalian cells and mice and the protective effect of Hp1404 against infection to evaluate its potential application as an antibacterial agent.
Materials and Methods
Ethics statement
The scorpion Heterometrus petersii used in this work was obtained from a scorpion breeding base in Hubei, province of China. The female balb-c (18–21 g) mice were obtained from the Animal Facility at Wuhan University Zhong Nan Hospital. The mice were maintained under standard conditions of humidity (50 ± 5%), temperature (25 ± 2°C) and dark and light cycles (12 h each) with free access to food and water. When met certain clinical criteria or at the end of the experiments, the mice were humanely euthanized (anesthetized by intra-peritoneal injection of pentobarbital, and sacrifice by cervical dislocation). All animal experiments were approved by the Institutional Animal Care and Use Committee of Wuhan University. The fresh human red blood cells (hBRCs) were from healthy donor Zhongjie Li, who is the author of this manuscript. The hBRCs-related experiment was approved by the Ethics Committee of the College of Life Sciences of Wuhan University.
cDNA library construction and sequencing
Twenty specimens of H. petersii were collected and milked for 2 days by electrical stimulation. Total RNA was prepared from the glands by using TRIzol reagent (Invitrogen). Poly (A) mRNA was purified by using a Poly (A) Tract mRNA isolation system (Promega). The cDNA library was constructed with the Superscript plasmid system cDNA library construction kit (Gibco/BRL). cDNAs were cloned into the pSPORT1 plasmid (Gibco/BRL) and transformed into Escherichia coli DH5α cells. cDNA clones were randomly chosen and sequenced to obtain a reliable representation of the toxin content in the venom gland. Positive clones were identified by using an ABI Prism 377XL DNA sequencer with a universal T7 promoter primer.
Sequence and secondary structure analysis
Sequence analysis was carried out by using BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi), DNAMAN, and GENRUNR. The secondary structure was analyzed by using the online program Heliquest (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py) and measured by circular dichroism (CD) spectroscopy. The CD assay was performed at room temperature at the UV range of 190–250 nm at a concentration of 0.1 mg/mL in: (i) water, (ii) 30% TFE/H2O, or (iii) 70% TFE/H2O. Spectra were collected from three separate recordings.
Peptide synthesis and bacterial strains
The peptides used in this study were synthesized by GL Biochem (Shanghai, China) with amidated C-terminus, and with a purity of >95%. Staphylococcus aureus AB94004, S. aureus ATCC25923, S. aureus ATCC6538, S. aureus AB208193, S. epidermidis AB208187, S. epidermidis AB208188, Micrococcus luteus AB93113, Bacillus subtilis AB91021, E. coli AB94012, E. coli ATCC25922, Pseudomonas aeruginosa AB93066, Pseudomonas aeruginosa ATCC9027 and Pseudomonas aeruginosa ATCC27853 were purchased from the China Center of Type Culture Collection (CCTCC). MRSA P1381 and MRSA P1374 were obtained from the 302nd military hospital of Beijing, China. Enterococcus faecium, Streptococcus agalactiae were clinical strains from a hospital.
Antimicrobial assays
The antimicrobial activity was determined in the broth microdilution assay from the procedure recommended by the Clinical and Laboratory Standards Institute with some modifications. Briefly, bacteria were cultured in Luria-Bertani (LB) medium to OD600 = 0.6 at 37 °C, then diluted to 105–106 cfu/mL in LB medium, and peptide was serially diluted in 0.9% saline. 160 µL of the bacterial suspension and 40 µL of peptide dilution at varying concentrations were added into 96-well plates, and then incubated for 16 h at 37 °C with continuous shaking at 250 rpm. The minimum inhibitory concentration (MIC) was determined as the lowest peptide concentration at which no bacterial growth was observed.
Competition assays
A total of 1 mg/mL of Hp1404 peptide solution was mixed with an equal volume of 5 mg/mL of the lipoteichoic acid (LTA, sigma-aldrich: L2515) or lipopolysaccharide (LPS, sigma-aldrich: L3012) solution. Then, the MICs of Hp1404 treated with LTA or LPS against S. aureus AB94004 were measured. The experiment was verified by three independent trials.
Time-killing kinetics
S. aureus AB94004 was cultured in LB medium to exponential phase (OD600 = 0.5), then diluted to ca. 107 cfu/mL in LB medium, and treated with different concentrations of peptide solutions. Aliquots were taken at defined intervals and washed with 0.9% saline, and then diluted appropriately in the saline and plated on LB agar. The plates were incubated at 37 °C for 24 h, and the colony forming units (CFU) were counted.
Confocal laser-scanning microscopy
The assay was measured according to the method [19] with some modifications. S. aureus AB94004 was cultured to mid logarithmic phase (ca. 107 cfu/mL), harvested by centrifugation, and washed and resuspended with 15 mM sodium phosphates buffer (pH 7.4). After incubated with 12.5 µg/mL fluorescein isothiocyanate (FITC)-labeled peptide at 37 °C for 20 min, the cells were washed. The glass slide was immersed in the suspension for 10 min to let the cells immobilized, and then examined by an Olympus IX70 confocal laser-scanning microscope.
Transmission electron microscopy (TEM)
The exponential phase bacteria S. aureus AB94004 (ca. 107 cfu/mL) was incubated with Hp1404 at the final concentration of 2 × MIC or 5 × MIC or without Hp1404 for 20 min at 37 °C. Samples were harvested by centrifugation, and washed with 0.9% salt solution. The bacteria were fixed with 2% glutaraldehyde in 0.1 M PBS for 1 h, and with 1% osmium tetroxide at 4 °C for another 1 h. After fixation, the bacteria were stained with 1% uranyl acetate and then dehydrated by using a series of graded ethyl alcohols. After this, the samples were embedded in epoxy resins and stained with 1% uranyl acetate and lead citrate. The samples were then semi-thin sectioned and examined by using a HITACHIH-8100 transmission electron microscope.
Fluorescence measurements
S. aureus AB94004 was cultured to exponential phase (ca. 107 cfu/mL), then the fluorescence dye SYTOX green (Invitrogen) was added at a final concentration of 5 µM. After incubated for 10 min, the peptides were added at a final concentration of 1 × MIC, 2 × MIC. Fluorescence was measured at the excitation and emission wavelengths of 488 and 525 nm, respectively.
Drug resistant assays
The initial MIC of Hp1404 and control antibiotics kanamycin against S.aureus AB94004 was obtained as described above. Bacteria from duplicate wells at the concentration of 1/2-MIC were then diluted to 105∼106 cfu/mL in LB medium for the new MIC assay. The experiment was repeated each day for 15 passages.
Checkerboard assay
The checkerboard assay is widely used for evaluation of properties of different antibacterial combinations [20]. Kanamycin was serially diluted in 0.9% saline in the presence of a constant amount of peptide, equal to one-quarter of the peptide MIC. Then the combination MIC was measured. The fractional inhibitory concentration index (FICI) was used to determine the synergy between antimicrobial agents. Synergy was defined as an FICI ≤ 0.5. Indifference or absence of interaction was defined as 0.5 < FICI < 4. Antagonism was defined as FICI > 4.The FICI was calculated as follows:  , cMIC: MIC in combination.
, cMIC: MIC in combination.
Hemolytic activity
Fresh hRBCs from healthy donors were washed 3 times in 0.9% saline, and resuspended in the same salt solution to a concentration of 2% (v/v). 100 µL of peptide solution and 100 µL of RBC suspension were added to the wells of 96-well plate, which made the final concentration of hBRCs was 1% (v/v). After incubated for 1 h at 37 °C with gently shaking, samples were centrifuged at 1000 g for 10 min. The supernatant (100 µL) from each well was transferred to a new 96-well plate and the absorbance was measured at 490 nm. 0.9% saline and 1% Triton X-100 was served as negative and positive controls, respectively. 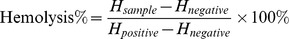 . H: absorbance at 490 nm.
. H: absorbance at 490 nm.
Cell culture and MTT assay
The HFF, HEK293T and A375 cell lines were cultured in Dulbecco's modified Eagle medium supplemented with 100 µg/mL streptomycin, 100 U/mL penicillin and 10% (v/v) fetal bovine serum, and maintained in a humidified chamber with 5% CO2 at 37 °C. Cytotoxicity of Hp1404 against the mammalian cells was evaluated by MTT assays. Cells (6000 cells/well) were pre-seeded on sterilized 96-well plates in 100 µL medium and incubated for 24 h. Dilutions of peptides in the same medium (100 µL) were added and incubated for another 24 h. Then 20 µL of 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well and the plates were incubated for an additional 4 h. The supernatant was removed, and 100 µL dimethyl sulfoxide was added to the wells to dissolve any remaining precipitate. Absorbance at 570 nm was measured using an ELISA reader. No peptide and 0.1% Triton X-100 was served as negative and positive controls, respectively. The viability percentage was calculated according to the equation: 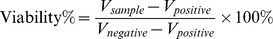 . V: absorbance at 570 nm.
. V: absorbance at 570 nm.
Acute toxicity
The acute systemic toxicity of Hp1404 to mice was evaluated by the determination of the LD50 (50% lethal dose). After being dissolved in 0.9% saline, Hp1404 was given to female balb-c mice (18–21 g) by intraperitoneal (i.p.) or intravenous (i.v.) injection at the designated doses in 0.4 mL, six mice per group. The number of mice surviving at each group was monitored for up to a period of 7 days after treatment, and the values of LD50 were calculated by the Karber method [21].
Mouse peritonitis model
The mouse peritonitis model was based on a previously described protocol [22]. Female balb-c mice (18–21 g) were infected by i.p. injection of 0.5 mL of the MRSA P1381 inoculum (108 CFU) in 0.9% saline containing 5% (wt/vol) mucin. One hour after bacterial administration, mice (6 mice per group) were treated with a single dose of peptide by (i) i.p. or (ii) i.v. injection. Vancomycin and 0.9% saline treatment were used as positive and negative controls, respectively. The mice were observed 2–4 times daily for 7 days. The mice were euthanized if they became moribund (unresponsive to external stimuli, can not swing the neck, and can not eat and drink), and counted as non-survivor.
Results
Characterization and analysis of Hp1404
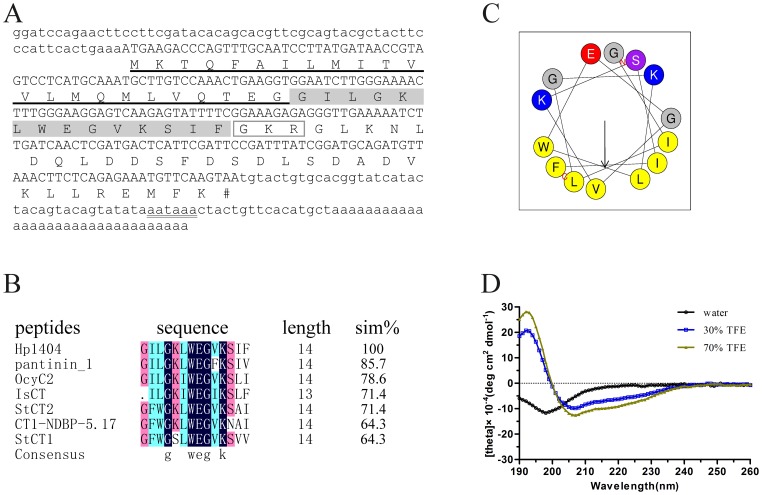
After systemic screening the clones from the venom gland cDNA library of the scorpion H. petersii, we obtained a new peptide precursor named Hp1404. As shown in Figure 1A, the cDNA sequence of Hp1404 consists of a 5′ UTR of 62 nt, an ORF of 210 nt, and a 3′ UTR of 92 nt. A single polyadenylation signal (AATAAA) was found 16 nt upstream of the poly (A) tail. The ORF of 210 nt encodes a precursor that consists of 69 amino acid residues, which contains a putative 23-residue signal peptide (http://www.cbs.dtu.dk/services/SignalP/) and a 46-residue propeptide. According to previous studies [23], [24], further bioinformatic analysis indicated that the propeptide starts with a conserved posttranslational processing signal Gly-Lys-Arg at positions 38 to 40, and would result in a 14-residue mature peptide (GILGKLWEGVKSIF) with C-terminal amidation.
Figure 1. Sequence analysis, multiple alignment, and secondary structure analysis of Hp1404 peptide.
(A) cDNA and deduced amino acid sequences of Hp1404.The deduced amino acid residues are shown below the corresponding nucleotide sequences. The signal peptide residues are underlined. The mature peptide residues are shaded in gray.The potential cleavage site for prohormone convertase is marked in rectangle. The potential polyadenylation signal aataaa is double underlined. (B) Multiple alignment of Hp1404 with other antimicrobial peptides. Sim%, the percentage of sequence identity relative to Hp1404. The different color represents the different homology level. StCT1 and StCT2 from the scorpion Scorpiops tibetanus, CT1-NDBP-5.17 from the scorpion Urodacus yaschenkoi, IsCT from the scorpion Opisthacanthus madagascariensis, OcyC2 from the scorpion Opisthacanthus cayaporum, pantinin 1 from the scorpion Pandinus imperator. (C) Helical wheel diagram of Hp1404 determined by the Heliquest method. The representation of Hp1404 as a helical wheel shows the hydrophilic face and hydrophobic face. (D) CD spectra of Hp1404 peptide (100 µg/mL) in water alone or with 30 or 70% aqueous TFE.
Multiple sequence alignment revealed that Hp1404 shared a high identity (Figure 1B) with the AMPs StCT1 [25], CT1-NDBP-5.17 [26], IsCT [27], StCT2 [28], OcyC2 [29], and pantinin 1 [30], which suggests that Hp1404 peptide may have antibacterial activity.
Secondary structure predicted by using the online program Heliquest (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py) indicated that Hp1404 was a typical amphipathic α-helix, the helical wheel is divided into two parts: one part is the hydrophobic face, and the other is the hydrophilic face (Figure 1C). Such structure was further confirmed by the CD spectral analysis. As shown in Figure 1D, Hp1404 exhibited only a large negative peak at 198 nm in water, indicating a random coil structure, while Hp1404 exhibited a large positive peak at around 195 nm and large negative bands at 208 and 220 nm in TFE, indicating predominance of α-helices [31], [32]. These results suggested that Hp1404 could form an amphipathic helical structure in the appropriate membrane environment.
Antimicrobial activity in vitro
As shown in Table 1, Hp1404 has anti-bacterial activity with MICs of 6.25–25 µg/mL against gram-positive bacteria including MRSA and other clinical strains, but it can not inhibit the growth of gram-negative bacteria at the concentration of 100 µg/mL.
Table 1. MICs of Hp1404 against bacteria.
| Strains | MIC | |
| μg/mL | μM | |
| Gram-positive | ||
| Staphylococcus aureus AB94004 | 12.5 | 8.08 |
| Staphylococcus aureus ATCC25923 | 6.25 | 4.04 |
| Staphylococcus aureus ATCC6538 | 12.5 | 8.08 |
| Staphylococcus aureus AB208193 | 12.5 | 8.08 |
| Staphylococcus epidermidis AB208187 | 25 | 16.16 |
| Staphylococcus epidermidis AB208188 | 25 | 16.16 |
| Micrococcus luteus AB93113 | 12.5 | 8.08 |
| Bacillus subtilis AB91021 | 12.5 | 8.08 |
| Staphylococcus aureus MRSA P1381 | 12.5 | 8.08 |
| Staphylococcus aureus MRSA P1374 | 25 | 16.16 |
| Enterococcus faecium | 12.5 | 8.08 |
| Streptococcus agalactiae | 25 | 16.16 |
| Gram-negative | ||
| Escherichia coli AB94012 | > 100 | > 64.6 |
| Escherichia coli ATCC25922 | > 100 | > 64.6 |
| Pseudomonas aeruginosa AB93066 | > 100 | > 64.6 |
| Pseudomonas aeruginosa ATCC9027 | > 100 | > 64.6 |
| Pseudomonas aeruginosa ATCC27853 | > 100 | > 64.6 |
LTA and LPS competition assays
To determine whether LTA or LPS interfere with the antimicrobial activity of Hp1404, MICs of Hp1404 mixed with LTA or LPS against S. aureus were measured. Our results (data not shown) showed that the MICs of Hp1404 treated with LTA or LPS were 12.5 µg/mL, which were the same as the untreated Hp1404.
Antibacterial mechanism
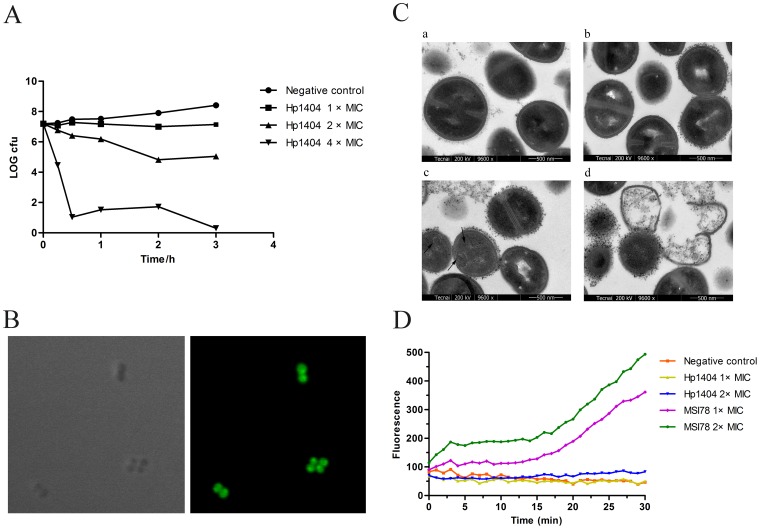
To gain insights into the antibacterial mechanism of Hp1404, S. aureus AB94004 was selected as the model bacteria. Firstly, the time-killing kinetics was studied. As shown in Figure 2A, the killing rate increased along with the increasing of the peptide concentration. The viable colony number only decreased 15% in 1 h at the concentration of 2 × MIC, but it decreased approximately 85% in 30 min at the concentration of 4 × MIC.
Figure 2. Antibacterial mechanism of the peptide Hp1404.
(A) Time-kill kinetics of Hp1404 against S. aureus AB94004.The assay was performed by determining the counts of surviving bacteria, 0 h represents bacteria before treated. (B) Confocal fluorescence microscopic images of S. aureus treated with FITC-Hp1404. Left: normal image; right: fluorescence image. (C) Transmission electron microscopy of S. aureus treated with Hp1404. a: negative control. b: treated with Hp1404 at the concentration of 1×MIC for 20 min. c-d: treated with Hp1404 at the concentration of 5×MIC for 20 min. (D) Fluorescence measurements. Negative control, 0.9% saline. MSI78 is an amphipathic α-helical peptide with an antibacterial mechanism of disrupting the membrane. The MIC of MSI78 against S. aureus AB94004 is 6.25 µg/mL.
To explore the target site of Hp1404 in S. aureus AB94004, the distribution of Hp1404 in bacteria was investigated by confocal laser-scanning microscopy. As shown in Figure 2B, FITC-labeled Hp1404 penetrated the bacterial cell membrane and accumulated in the cytoplasm.
To determine the direct influence of Hp1404 on the bacteria, TEM was used to examine the ultrastructure changes of the bacteria. As shown in Figure 2C-a, untreated cells of S. aureus were round, proliferating cell with intact cell wall and well-defined membrane, and the intracellular DNA region displayed a heterogeneous electron density. At the concentration of 2 × MIC, the cell wall and membrane had no changes (Figure 2C-b). But, special structures could be observed in the cytoplasm (Figure 2C-c: marked by arrow) and some cells were completely lysed (Figure 2C-d) at the concentration of 5 × MIC.
To further assess whether Hp1404 could influence the integrity of the membrane, the ability of permeabilizing the cell membrane of Hp1404 was also tested with the fluorescent nucleic acid stain SYTOX. As shown in Figure 2D, no fluorescence increase was apparent over 30 min when S. aureus cells were exposed to SYTOX and Hp1404, compared with a rapid fluorescence increase upon exposure to MSI78, which is an amphipathic α-helical peptide with a antibacterial mechanism of disrupting the membrane [33].
Drug resistant assay
The emergence of drug resistance in bacteria for conventional antibiotic treatments is a major public-health concern. The development of resistance to Hp1404 was evaluated in S. aureus AB94004. As shown in Figure 3, the multiple treatments with kanamycin (MIC: 6.25 µg/mL) gave rise to drug resistance as early as passage 2, and the MIC increased about 32-fold after the course of 9 passages, while there was no change in the MIC for Hp1404 during the course of 15 passages. These results indicated that it is difficult for S. aureus to develop resistance to Hp1404.
Figure 3. Resistance development of S. aureus AB94004 treated by Hp1404 or kanamycin.
Checkerboard assay
To evaluate whether Hp1404 exhibits synergistic effects with kanamycin, the checkerboard assay was performed. Our results showed that the fractional inhibitory concentration index was 0.5, which demonstrated that Hp1404 exhibited synergistic effects with kanamycin.
In vitro toxicity
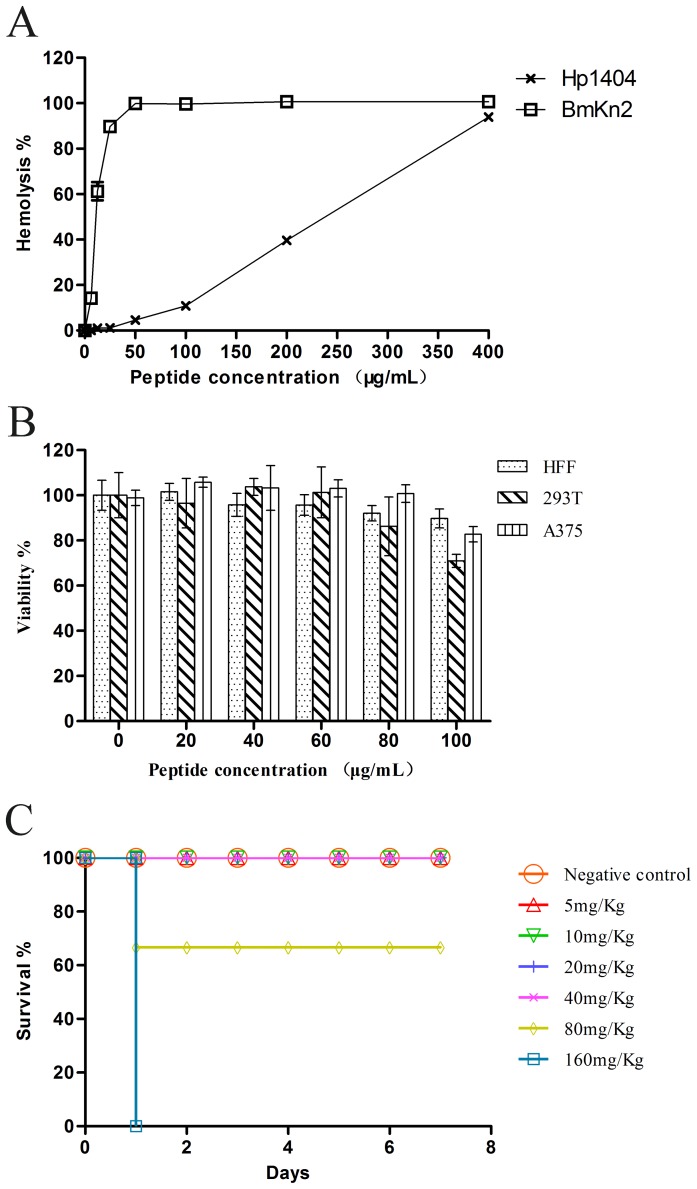
In order to assess the toxicity of the peptide, the hemolytic activity to hRBCs and cytotoxicity to mammalian cell lines were tested. In comparison with BmKn2 [34], a highly hemolytic peptide, Hp1404 has a low hemolytic activity (about 10%) at the concentration 100 µg/mL (Figure 4A), and the HC50 (50% hemolytic concentration) of the peptide Hp1404 is 226.6 µg/mL (146.5 µM). The MTT tests showed that the peptide only has about 10–30% cytotoxicity to the cells be tested at the concentration of 100 µg/mL (Figure 4B), and the CC50 (50% cytotoxic concentration) is > 100 µg/mL (64.6 µM). Taken together, the toxicity of Hp1404 to the mammalian cells is lower than that to the sensitive bacteria.
Figure 4. Toxicity of Hp1404 in vitro and in vivo.
(A) Hemolytic activity of Hp1404 against human red blood cells. BmKn2 is a highly hemolytic antimicrobial peptide from the scorpion Mesobuthus martensii Karsch. (B) Cytotoxicities of Hp1404 against HFF, HEK293T and A375 cell lines. Cytotoxicity was measured by MTT assay. (C) Acute toxicity of Hp1404 to mice by intravenous injection.
In vivo toxicity
Acute toxicity was examined to evaluate the in vivo toxicity of Hp1404. With the dose up to 80 mg/Kg, the mice injected with Hp1404 by i.p showed no immediate adverse events, and all the treated mice survived in the 7-day study period (data not shown). In the i.v. injection treatment, the mice injected with Hp1404 also showed no immediate adverse events at the dose of 5 or 10 mg/Kg, but had a 33.3% (2/6 mice) mortality at the dose of 80 mg/Kg and 100% (6/6 mice) mortality at the dose of 160 mg/Kg (Figure 4C). Thus, the LD50 of Hp1404 to mice is 89.8 mg/Kg by i.v. injection.
In vivo antibacterial activity
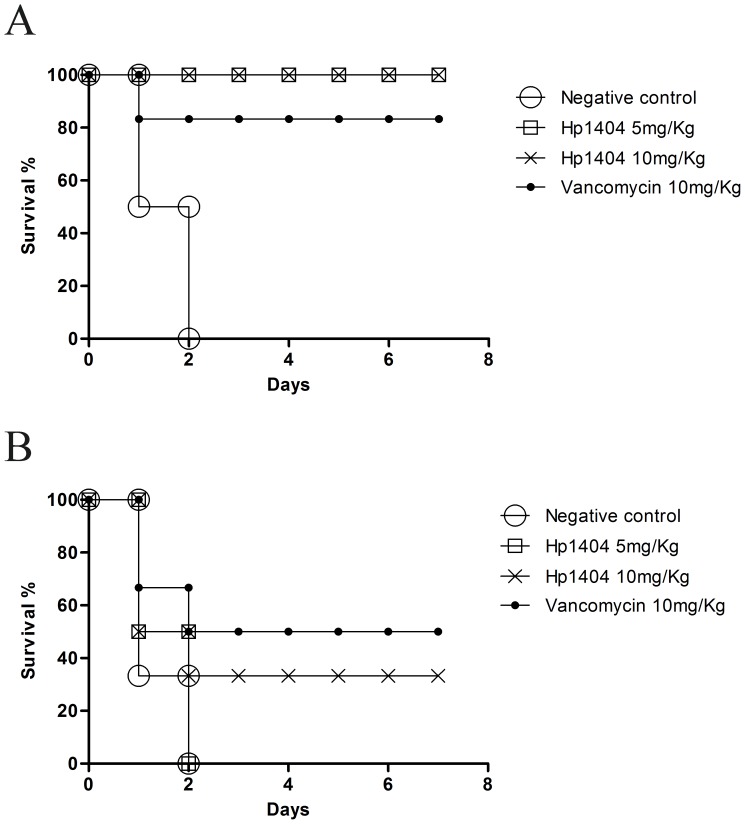
To further investigate the antibacterial activity of Hp1404 peptide in vivo, the potential therapeutic efficacy of the peptide Hp1404 was evaluated by a mouse peritonitis model. As shown in Figure 5A, all six mice in the negative control group died after inoculated with MRSA P1381 for 48 h, but the mice treated with the dose of 5 or 10 mg/Kg Hp1404 by i.p. injection showed a survival rate of 100%. On the other hand, the mice treated with the dose of 10 mg/Kg Hp1404 by i.v. injection also showed a survival rate of 33.3% (2/6 mice) in the 7-day study period (Figure 5B), but it was not as effective as the dose of 5 mg/Kg by i.p. All these data indicate that the peptide Hp1404 still has anti-bacterial activity in vivo.
Figure 5. Antibacterial activity of Hp1404 in vivo.
(A) Therapeutic efficacy of Hp1404 on MRSA infected mice by intraperitoneal injection. (B) Therapeutic efficacy of Hp1404 on MRSA infected mice by intravenous injection.
Discussion
In the present study, we indentified a new cationic AMP Hp1404 with a net charge of +1 from the scorpion H. petersii. In contrast to conventional AMPs with wide spectra of activity, Hp1404 just has a specific potent antimicrobial activity against gram-positive bacteria (Table 1). Previous studies showed that the interaction between AMPs and LPS or LTA is important to the activity of AMPs [12]. Our results showed that the antibacterial activity of Hp1404 was not interfered with LTA or LPS, which was different from the peptide Kn2–7, a scorpion AMP derivative studied in our previous work [35]. It implied that LPS or LTA might not be the primary antimicrobial action site of Hp1404. Our further data by biolayer interferometry experiments showed that Hp1404 did not interact with LPS or LTA indeed (data not shown). Thus, the mode of action of Hp1404 may be different from that of most classical AMPs [35]. Though Hp1404 do not interact with LTA, two factors may let it successful reach the action sites and perform its activity against gram-positive bacteria: (i) The structure of the gram-positive bacteria cell wall. Such as S. aureus cell, which has a loosely arrayed network on the cell surface, consisting of fibrils and pores ranging in size from 50 to 500 Å [36], [37], while the Hp1404 molecule (14aa) is about 20–50 Å. These pores are large enough to let the peptide molecules to pass through them. (ii) Hp1404 is a cationic peptide, while there are a lot of negatively charged molecules outside the bacterial cell, the electrostatic bonding between Hp1404 and bacteria will encourage the cationic peptide to pass through the cell wall [11].
Structure analysis suggested that Hp1404 was an amphipathic and α-helical peptide (Figure 1C, 1D). Usually, natural AMPs with such structure are considered to be membrane-lytic peptides [38], [39], which kill bacteria in 2 to 3 min after initial exposure [40], [41]. The killing of S. aureus by Hp1404 peptide was a rather slow process (Figure 2A), which suggested that Hp1404 might not be a classical membrane-lytic peptide. Such assumption was confirmed by TEM, which showed that the membrane was not lysed by Hp1404 at low MIC concentration (Figure 2C-b). Our Fluorescence measurements also showed that Hp1404 did not disrupt the membrane at low MIC concentration (Figure 2D). But, it is interestingly that the FITC-Hp1404 can penetrate the cell membrane of S. aureus and accumulate in the cytoplasm at low concentration (Figure 2B), which is similar to the linear α-helical peptide Buforin II [19]. As a short peptide with the length of 14 amino acid residues, Hp1404 may be via a floodgate mechanism to translocate to the cytoplasm [42]. As we know, after penetrating the membrane, AMPs can inhibit the macromolecular biosynthesis or interact with specific vital components inside the microorganisms (43, 46, 47). Thus, Hp1404 may have an unknown intracellular target, which needs further study to be confirmed in the future. On the other hand, Hp1404 may interact with the membrane and lead to the lateral expansion of the membrane area at high MIC concentration, which result in forming mesosome-like structures (Figure 2C-c), and lead to cell lysis (Figure 2C-d). It is the same as PGLa [40]. Due to the complex mechanism of action, it is difficult for S. aureus to develop resistance to Hp1404 (Figure 3), compared to conventional antibiotics.
Although AMPs have advantages over conventional antibiotics, there remain obstacles that prevent their therapeutic use: (i) Most AMPs, especially the α-helical AMPs, are toxic to mammalian cells due to less selectivity for target cells. For example, the MICs of bee venom peptide melittin against bacteria are 0.2-2 µM, while it has a CC50 of 0.7 µM, and has a HC50 of 0.51 µM [43], [44]. (ii) The loss of antimicrobial activity under physiological conditions or in serum also hampers the clinical applications of AMPs [45], [46]. There is a significant loss of in vitro activity of hBD-1 as the salt concentration increased from 50 mM to 125 mM [47], and the activity of LL-37 was completely suppressed in human serum [48]. In this study, it was found that Hp1404 has a low toxicity toward hRBC (HC50: 146.5µM) and human cell lines (10–30% toxicity at 64.6 µM). Moreover, the MICs of Hp1404 were tested under the salt concentration of about 170 mM, and Hp1404 still had in vivo effect under the normal physiological condition (Figure 5). However, our further experiments showed that serum decreased the activity of Hp1404 (data not shown), which may be the reason why the one dose of Hp1404 at 10 mg/Kg injected by i.v. did not cure all the MRSA P1381 infected mice (Figure 5B). Although Hp1404 does not have a good stability in serum, its low toxicity toward both mammalian cells and mice and non-inducing drug resistance suggest that it has the potential for applications.
In summary, Hp1404 is a cationic AMP from the scorpion H. petersii, and has a specific activity against gram-positive bacteria including MRSA. Hp1404 can penetrate the membrane of S. aureus at low MIC concentration, and can also interact with the membrane and result in forming mesosome-like structures at high MIC concentration. Moreover, S. aureus does not develop drug resistance after multiple treatments with Hp1404. Furthermore, Hp1404 has low toxicities to both mammalian cells and mice at its effective antibacterial concentrations, and can effectively protect mice from the infection of MRSA. Thus, Hp1404 may have potential applications as an antibacterial agent.
Funding Statement
This work is supported by the grants from National Key Basic Research Program in China (http://www.973.gov.cn/English/AreaList.aspx): Nos. 2010CB529800, and 2010CB530100. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10: S122–129. [DOI] [PubMed] [Google Scholar]
- 2. Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36: 697–705. [DOI] [PubMed] [Google Scholar]
- 3. Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE Jr (2004) Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 38: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 4. Fitton JE, Dell A, Shaw WV (1980) The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett 115: 209–212. [DOI] [PubMed] [Google Scholar]
- 5. Boman HG, Hultmark D (1987) Cell-free immunity in insects. Annu Rev Microbiol 41: 103–126. [DOI] [PubMed] [Google Scholar]
- 6. Park S, Park SH, Ahn HC, Kim S, Kim SS, et al. (2001) Structural study of novel antimicrobial peptides, nigrocins, isolated from Rana nigromaculata. FEBS Lett 507: 95–100. [DOI] [PubMed] [Google Scholar]
- 7. Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1: 440–464. [DOI] [PubMed] [Google Scholar]
- 8. Morassutti C, De Amicis F, Skerlavaj B, Zanetti M, Marchetti S (2002) Production of a recombinant antimicrobial peptide in transgenic plants using a modified VMA intein expression system. FEBS Lett 519: 141–146. [DOI] [PubMed] [Google Scholar]
- 9. Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19: 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462: 55–70. [DOI] [PubMed] [Google Scholar]
- 11. Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3: 238–250. [DOI] [PubMed] [Google Scholar]
- 12. Fjell CD, Hiss JA, Hancock RE, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11: 37–51. [DOI] [PubMed] [Google Scholar]
- 13. Zeh DW (1990) (1990) The Biology of Scorpions. Science Gary Polis A, Ed. Stanford University Press, xxvi, 587 pp., illus. $85 249: 1176–1177. [Google Scholar]
- 14. Conde R, Zamudio FZ, Rodriguez MH, Possani LD (2000) Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett 471: 165–168. [DOI] [PubMed] [Google Scholar]
- 15. Corzo G, Escoubas P, Villegas E, Barnham KJ, He W, et al. (2001) Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem J 359: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai L, Corzo G, Naoki H, Andriantsiferana M, Nakajima T (2002) Purification, structure-function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem Biophys Res Commun 293: 1514–1522. [DOI] [PubMed] [Google Scholar]
- 17. Lee K, Shin SY, Kim K, Lim SS, Hahm KS, et al. (2004) Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem Biophys Res Commun 323: 712–719. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez-Aponte CA, Silva-Sanchez J, Quintero-Hernandez V, Rodriguez-Romero A, Balderas C, et al. (2011) Vejovine, a new antibiotic from the scorpion venom of Vaejovis mexicanus. Toxicon 57: 84–92. [DOI] [PubMed] [Google Scholar]
- 19. Park CB, Kim HS, Kim SC (1998) Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun 244: 253–257. [DOI] [PubMed] [Google Scholar]
- 20. Cassone M, Otvos L Jr (2010) Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev Anti Infect Ther 8: 703–716. [DOI] [PubMed] [Google Scholar]
- 21. Banerjee TK (1993) Estimation of acute toxicity of ammonium sulphate to the fresh water catfish, Heteropneustes fossilis I. Analysis of LC50 values determined by various methods. Biomed Environ Sci 6: 31–36. [PubMed] [Google Scholar]
- 22. Domenech A, Ribes S, Cabellos C, Dominguez MA, Montero A, et al. (2004) A mouse peritonitis model for the study of glycopeptide efficacy in GISA infections. Microb Drug Resist 10: 346–353. [DOI] [PubMed] [Google Scholar]
- 23. Isaac R, Schoofs L, Williams TA, Veelaert D, Sajid M, et al. (1998) A novel peptide-processing activity of insect peptidyl-dipeptidase A (angiotensin I-converting enzyme): the hydrolysis of lysyl-arginine and arginyl-arginine from the C-terminus of an insect prohormone peptide. Biochem J 330 (Pt 1): 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradbury AF, Smyth DG (1991) Peptide amidation. Trends Biochem Sci 16: 112–115. [DOI] [PubMed] [Google Scholar]
- 25. Yuan W, Cao L, Ma Y, Mao P, Wang W, et al. (2010) Cloning and functional characterization of a new antimicrobial peptide gene StCT1 from the venom of the scorpion Scorpiops tibetanus. Peptides 31: 22–26. [DOI] [PubMed] [Google Scholar]
- 26. Luna-Ramirez K, Quintero-Hernandez V, Vargas-Jaimes L, Batista CV, Winkel KD, et al. (2013) Characterization of the venom from the Australian scorpion Urodacus yaschenkoi: Molecular mass analysis of components, cDNA sequences and peptides with antimicrobial activity. Toxicon 63: 44–54. [DOI] [PubMed] [Google Scholar]
- 27. Dai L, Yasuda A, Naoki H, Corzo G, Andriantsiferana M, et al. (2001) IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis. Biochem Biophys Res Commun 286: 820–825. [DOI] [PubMed] [Google Scholar]
- 28. Cao L, Li Z, Zhang R, Wu Y, Li W, et al. (2012) StCT2, a new antibacterial peptide characterized from the venom of the scorpion Scorpiops tibetanus. Peptides 36: 213–220. [DOI] [PubMed] [Google Scholar]
- 29. Silva EC, Camargos TS, Maranhao AQ, Silva-Pereira I, Silva LP, et al. (2009) Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum. Toxicon 54: 252–261. [DOI] [PubMed] [Google Scholar]
- 30. Zeng XC, Zhou L, Shi W, Luo X, Zhang L, et al. (2013) Three new antimicrobial peptides from the scorpion Pandinus imperator. Peptides 45C: 28–34. [DOI] [PubMed] [Google Scholar]
- 31. Ranjbar B, Gill P (2009) Circular dichroism techniques: biomolecular and nanostructural analyses- a review. Chem Biol Drug Des 74: 101–120. [DOI] [PubMed] [Google Scholar]
- 32. Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1: 2876–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallock KJ, Lee DK, Ramamoorthy A (2003) MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J 84: 3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng XC, Wang SX, Zhu Y, Zhu SY, Li WX (2004) Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii Karsch. Peptides 25: 143–150. [DOI] [PubMed] [Google Scholar]
- 35. Cao L, Dai C, Li Z, Fan Z, Song Y, et al. (2012) Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS One 7: e40135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Touhami A, Jericho MH, Beveridge TJ (2004) Atomic force microscopy of cell growth and division in Staphylococcus aureus. J Bacteriol 186: 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meroueh SO, Bencze KZ, Hesek D, Lee M, Fisher JF, et al. (2006) Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc Natl Acad Sci U S A 103: 4404–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang L, Harroun TA, Weiss TM, Ding L, Huang HW (2001) Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 81: 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaguchi S, Huster D, Waring A, Lehrer RI, Kearney W, et al. (2001) Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys J 81: 2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, et al. (2010) Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother 54: 3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y, Huang J, Chen Y (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Azad MA, Huttunen-Hennelly HE, Ross Friedman C (2011) Bioactivity and the first transmission electron microscopy immunogold studies of short de novo-designed antimicrobial peptides. Antimicrob Agents Chemother 55: 2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, et al. (2009) Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest 119: 2830–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, et al. (2006) Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother 50: 2608–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacob L, Zasloff M (1994) Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found Symp 186: 197–216 discussion 216–123. [DOI] [PubMed] [Google Scholar]
- 46. Phadke SM, Islam K, Deslouches B, Kapoor SA, Beer Stolz D, et al. (2003) Selective toxicity of engineered lentivirus lytic peptides in a CF airway cell model. Peptides 24: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 47. Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, et al. (1997) Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88: 553–560. [DOI] [PubMed] [Google Scholar]
- 48. Deslouches B, Islam K, Craigo JK, Paranjape SM, Montelaro RC, et al. (2005) Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother 49: 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]