Abstract
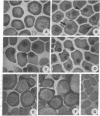
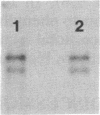
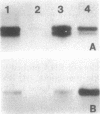
The major cytoskeletal proteins alpha-spectrin, beta-spectrin, and ankyrin are synthesized and assembled into a supportive membrane skeleton during erythroid differentiation. Information on the temporal appearance of mRNA and protein species is essential for understanding both the cytoskeletal assembly process and the function of various isoforms. We have isolated highly enriched populations of fetal erythroid cells at various stages of maturation. mRNAs for erythroid ankyrin, alpha-spectrin, and beta-spectrin were expressed at all stages but there were differences in transcript types and levels. The ratio of 9-kilobase (kb) to 7.5-kb erythroid ankyrin transcripts decreased markedly during differentiation, but there was no change in the ratio of the 10.1-kb and 9.3-kb erythroid beta-spectrin transcripts. The relative amounts of ankyrin, alpha-spectrin, and beta-spectrin mRNA increased during yolk sac cell differentiation, whereas only alpha-spectrin mRNA increased during differentiation of the fetal liver cells. The amounts of beta-spectrin mRNA exceeded the amounts of alpha-spectrin mRNA in the early precursors from both yolk sac and fetal liver; protein synthetic levels showed the same pattern. The 16-day fetal peripheral reticulocytes, on the other hand, had the adult mRNA and protein synthetic ratios with alpha/beta greater than 1. The data indicate that at least two mechanisms exist to meet changing erythroid membrane cytoskeletal requirements during development in utero: (i) stage-specific processing of the mRNA for the major cytoskeletal linker protein ankyrin and (ii) developmentally regulated alpha/beta-spectrin protein synthetic rates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Birkenmeier C. S., McFarland-Starr E. C., Barker J. E. Chromosomal location of three spectrin genes: relationship to the inherited hemolytic anemias of mouse and man. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8121–8125. doi: 10.1073/pnas.85.21.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Chang H., Langer P. J., Lodish H. F. Asynchronous synthesis of erythrocyte membrane proteins. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3206–3210. doi: 10.1073/pnas.73.9.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Prenant M., Leung A., Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989 Aug 15;74(3):1112–1120. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chui D. H., Patterson M., Bayley S. T. Unequal alpha and beta globin mRNA in reticulocytes of normal and mutant f/f fetal mice. Br J Haematol. 1980 Mar;44(3):431–439. doi: 10.1111/j.1365-2141.1980.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Cioe L., Curtis P. Detection and characterization of a mouse alpha-spectrin cDNA clone by its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1367–1371. doi: 10.1073/pnas.82.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioè L., Laurila P., Meo P., Krebs K., Goodman S., Curtis P. J. Cloning and nucleotide sequence of a mouse erythrocyte beta-spectrin cDNA. Blood. 1987 Oct;70(4):915–920. [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991 May 5;266(13):8273–8280. [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J., Mohandas N., Tchernia G., Kan Y. W. Molecular basis of hereditary elliptocytosis due to protein 4.1 deficiency. N Engl J Med. 1986 Sep 11;315(11):680–685. doi: 10.1056/NEJM198609113151105. [DOI] [PubMed] [Google Scholar]
- Davies K. A., Lux S. E. Hereditary disorders of the red cell membrane skeleton. Trends Genet. 1989 Jul;5(7):222–227. doi: 10.1016/0168-9525(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Eisen H., Bach R., Emery R. Induction of spectrin in erythroleukemic cells transformed by Friend virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3898–3902. doi: 10.1073/pnas.74.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geiduschek J. B., Singer S. J. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979 Jan;16(1):149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Marchesi V. T. The binding of vimentin to human erythrocyte membranes: a model system for the study of intermediate filament-membrane interactions. J Cell Biol. 1985 Jun;100(6):1955–1961. doi: 10.1083/jcb.100.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J., Glenney P. Co-expression of spectrin and fodrin in Friend erythroleukemic cells treated with DMSO. Exp Cell Res. 1984 May;152(1):15–21. doi: 10.1016/0014-4827(84)90225-8. [DOI] [PubMed] [Google Scholar]
- Hall T. G., Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987 Aug 5;262(22):10537–10545. [PubMed] [Google Scholar]
- Hanspal M., Kalraiya R., Hanspal J., Sahr K. E., Palek J. Erythropoietin enhances the assembly of alpha,beta spectrin heterodimers on the murine erythroblast membranes by increasing beta spectrin synthesis. J Biol Chem. 1991 Aug 25;266(24):15626–15630. [PubMed] [Google Scholar]
- Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987 Sep;105(3):1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Yoon S. H., Yu H., Hanspal J. S., Lambert S., Palek J., Prchal J. T. Molecular basis of spectrin and ankyrin deficiencies in severe hereditary spherocytosis: evidence implicating a primary defect of ankyrin. Blood. 1991 Jan 1;77(1):165–173. [PubMed] [Google Scholar]
- Inaba M., Maede Y. A new major transmembrane glycoprotein, gp155, in goat erythrocytes. Isolation and characterization of its association to cytoskeleton through binding with band 3-ankyrin complex. J Biol Chem. 1988 Nov 25;263(33):17763–17771. [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem. 1988 Jul 25;263(21):10212–10218. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J Cell Physiol. 1988 Oct;137(1):65–74. doi: 10.1002/jcp.1041370108. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Mueller T. J. The role of erythropoietin in the production of principal erythrocyte proteins other than hemoglobin during terminal erythroid differentiation. J Cell Physiol. 1986 Feb;126(2):259–265. doi: 10.1002/jcp.1041260216. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Rana S. S. Changes in erythroid membrane proteins during erythropoietin-mediated terminal differentiation. J Cell Physiol. 1987 Dec;133(3):438–448. doi: 10.1002/jcp.1041330304. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert S., Yu H., Prchal J. T., Lawler J., Ruff P., Speicher D., Cheung M. C., Kan Y. W., Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. From genes to structural morphogenesis: the genesis and epigenesis of a red blood cell. Cell. 1987 Nov 6;51(3):345–356. doi: 10.1016/0092-8674(87)90631-3. [DOI] [PubMed] [Google Scholar]
- Lehnert M. E., Lodish H. F. Unequal synthesis and differential degradation of alpha and beta spectrin during murine erythroid differentiation. J Cell Biol. 1988 Aug;107(2):413–426. doi: 10.1083/jcb.107.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Tse W. T., Menninger J. C., John K. M., Harris P., Shalev O., Chilcote R. R., Marchesi S. L., Watkins P. C., Bennett V. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990 Jun 21;345(6277):736–739. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- Peters L. L., Birkenmeier C. S., Bronson R. T., White R. A., Lux S. E., Otto E., Bennett V., Higgins A., Barker J. E. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nb mice. J Cell Biol. 1991 Sep;114(6):1233–1241. doi: 10.1083/jcb.114.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Huima T., Redman C. M. Biosynthesis of spectrin and its assembly into the cytoskeletal system of Friend erythroleukemia cells. J Cell Biol. 1986 Jul;103(1):103–113. doi: 10.1083/jcb.103.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. E., Chasis J. A., Mohandas N. Identification of a functional role for human erythrocyte sialoglycoproteins beta and gamma. Blood. 1987 Apr;69(4):1068–1072. [PubMed] [Google Scholar]
- Rossi G. B., Aducci P., Gambari R., Minetti M., Vernole P. Presence of spectrin in untreated Friend erythroleukemic cells. Its accumulation upon treatment of the cells with dimethyl sulfoxide. J Cell Physiol. 1978 Dec;97(3 Pt 1):293–304. doi: 10.1002/jcp.1040970304. [DOI] [PubMed] [Google Scholar]
- Rybicki A. C., Heath R., Wolf J. L., Lubin B., Schwartz R. S. Deficiency of protein 4.2 in erythrocytes from a patient with a Coombs negative hemolytic anemia. Evidence for a role of protein 4.2 in stabilizing ankyrin on the membrane. J Clin Invest. 1988 Mar;81(3):893–901. doi: 10.1172/JCI113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Birkenmeier C. S., Lux S. E., Barker J. E. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nb allele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3117–3121. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]