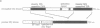Abstract
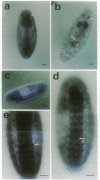
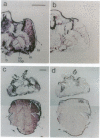
We have isolated a Drosophila melanogaster diacylglycerol kinase (DGK, EC 2.7.1.107) homologue by using a porcine DGK cDNA probe and we have characterized its structure and expression. The DGK cDNA has a single open reading frame that encodes 791 amino acids. The Drosophila and porcine DGKs share a similar carboxyl-terminal region, a putative catalytic domain, which is divided into two separate domains in Drosophila. The DGK gene was mapped to the cytogenetic position 43F1, and its DGK mRNA is abundant both in embryo and in adult fly. By in situ hybridization to sections of adult flies, we demonstrated that the mRNA is present predominantly in the nervous system and muscles, including compound eyes, brain cortex, fibrillar muscle, and tubular muscle. In a 10- to 11-hr embryo, the DGK gene is expressed abundantly in a limited number of cells in the procephalic region and in the ventral nerve cord. The pattern of temporal and spatial expression suggests that the DGK protein has an important function in the adult nervous system and muscle and during the development of the embryonic nervous system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988 Aug 26;54(5):723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E. S., Romero J. M., Doyle S., Elliott R., Murphy G., Carpenter R. floricaula: a homeotic gene required for flower development in antirrhinum majus. Cell. 1990 Dec 21;63(6):1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yoshioka T., Hotta Y. Diacylglycerol kinase defect in a Drosophila retinal degeneration mutant rdgA. J Biol Chem. 1989 Apr 5;264(10):5996–6000. [PubMed] [Google Scholar]
- Jacobs J. R., Goodman C. S. Embryonic development of axon pathways in the Drosophila CNS. II. Behavior of pioneer growth cones. J Neurosci. 1989 Jul;9(7):2412–2422. doi: 10.1523/JNEUROSCI.09-07-02412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Richardson C. N., Glomset J. A. Regulation of diacylglycerol kinase reaction in Swiss 3T3 cells. Increased phosphorylation of endogenous diacylglycerol and decreased phosphorylation of didecanoylglycerol in response to platelet-derived growth factor. J Biol Chem. 1988 Jan 25;263(3):1575–1583. [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Williams B. W., King W. C., Glomset J. A. A membrane-bound diacylglycerol kinase that selectively phosphorylates arachidonoyl-diacylglycerol. Distinction from cytosolic diacylglycerol kinase and comparison with the membrane-bound enzyme from Escherichia coli. J Biol Chem. 1988 Jan 25;263(3):1584–1592. [PubMed] [Google Scholar]
- Masai I., Hotta Y. Genomic organization of a Drosophila phospholipase C, norpA, and molecular lesions in two temperature-sensitive mutants. J Biochem. 1991 Jun;109(6):867–871. doi: 10.1093/oxfordjournals.jbchem.a123472. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane F., Yamada K., Kanoh H. Different effects of sphingosine, R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS Lett. 1989 Sep 25;255(2):409–413. doi: 10.1016/0014-5793(89)81134-2. [DOI] [PubMed] [Google Scholar]
- Sakane F., Yamada K., Kanoh H., Yokoyama C., Tanabe T. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature. 1990 Mar 22;344(6264):345–348. doi: 10.1038/344345a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., de Widt J., van der Wal J., Vandekerckhove J., van Damme J., Gussow D., Ploegh H. L., van Blitterswijk W. J., van der Bend R. L. Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett. 1990 Nov 26;275(1-2):151–158. doi: 10.1016/0014-5793(90)81461-v. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Matsumoto N., Wang P., Inoue H., Yoshioka T., Hotta Y., Osawa T. Purification and partial amino acid sequences of phosphoinositide-specific phospholipase C of Drosophila eye. J Biol Chem. 1990 Sep 5;265(25):14842–14848. [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986 May 15;261(14):6239–6247. [PubMed] [Google Scholar]
- Yoshioka T., Inoue H., Hotta Y. Absence of phosphatidylinositol phosphodiesterase in the head of a Drosophila visual mutant, norpA (no receptor potential A). J Biochem. 1985 Apr;97(4):1251–1254. doi: 10.1093/oxfordjournals.jbchem.a135171. [DOI] [PubMed] [Google Scholar]