Abstract
TMC genes encode a broadly-conserved family of multipass integral membrane proteins in animals1,2. Human TMC1 and TMC2 are deafness genes required for hair cell mechantransduction; however, the molecular functions of these and other TMC proteins have not been determined3-6. We show here that the C. elegans TMC-1 gene encodes a sodium sensor that functions specifically in salt taste chemosensation. TMC-1 is expressed in the ASH polymodal avoidance neurons, where it is required for salt-evoked neuronal activity and behavioural avoidance of high concentrations of NaCl. However, tmc-1 has no effect on responses to other stimuli sensed by the ASH neurons including high osmolarity and chemical repellents, indicating a specific role in salt sensation. When expressed in mammalian cell culture, TMC-1 generates a predominantly cationic conductance activated by high extracellular sodium but not by other cations or uncharged small molecules. Thus, TMC-1 is both necessary for salt sensation in vivo and sufficient to generate a sodium-sensitive channel in vitro, identifying it as a likely candidate ionotropic sensory receptor.
TMC1 is an important deafness gene in humans1,3 , and mutant mice carrying semidominant (Beethoven) or recessive (deafness) TMC1 alleles are hearing-deficient3,4. TMC1 is expressed in cochlear hair cells, and is required for hair cell function5. Recently, knockout mice containing deletions of both TMC1 and a closely related gene, TMC2, were shown to lack hair cell mechanosensory potentials6. TMC1 and TMC2 are members of a larger family of putative multipass transmembrane proteins that includes eight proteins in mammals, one in Drosophila, and two in C. elegans2,3, one of which (tmc-2) is enriched mechanoreceptors7. However, it is not known whether TMC genes encode channel proteins, nor whether TMC1 and TMC2 are components of the hair cell mechanotransducer or are required indirectly for its activity.
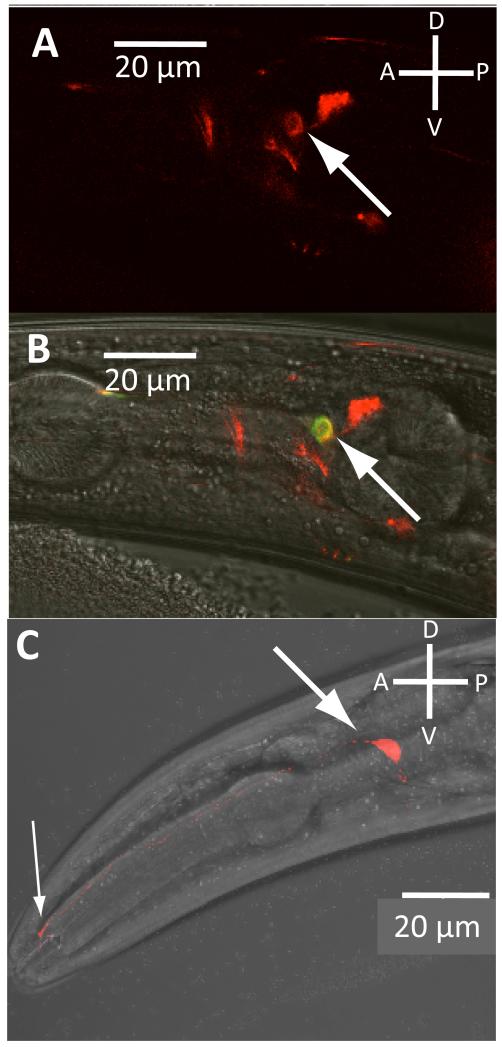
To learn more about the function of the C. elegans tmc-1 gene, we first investigated its expression pattern. In transgenic lines expressing a fluorescent reporter under the tmc-1 promoter, expression was observed in a small number of neurons, including the ASH neurons, which are important for sensing chemical repellents8, high osmolarity and nose touch9 (Figure 1A, B). Expression was also seen in other sensory neurons, including the ADFs, ASEs, ADLs and PHAs (Supplemental Figures 1-2). To investigate the subcellular distribution of TMC-1, we expressed a translational fusion between the tmc-1 cDNA and mCherry under the ASH promoter gpa-11 (Figure 1C). This TMC-1::mCherry fusion was localised to the cell body as well as the sensory cilia, the site of sensory transduction. These results suggested a possible role for TMC-1 in ASH sensory function.
Figure 1. Expression of TMC-1 in chemosensory neurons. A-B.
Expression of the tmc-1::mCherry promoter fusion in the ASH neurons. Animals expressing the extrachromosomal array ljEx470[tmc-1::mCherry] as well as ljEx239[sra-6::YC3.60] which specifically labels the ASH neurons. Panel A shows a red fluorescence image showing the expression pattern of the tmc-1::mCherry reporter; the ASH neuron is indicated by the arrow. Panel B is a DIC/fluorescence image of the same animal showing colabelling of tmc-1::mCherry with sra-6::YC3.60 in ASH Expression was also seen in the following neurons: ADF, ASE, PHA, ADL, CEP, NSM, and at lower frequency, AWC, AFD and BAG (see also Supplemental Figures 1 and 2). C. Localization of a tmc-1::mCherry protein fusion in the ASH neurons. Shown is a DIC/fluorescence image of a strain carrying a transgenic array (ljEx483[gpa-11::tmc-1::mCherry]) in which the tmc-1 cDNA is fused in frame to mCherry and expressed in ASH under the gpa-11 promoter. The ASH sensory cilia (thin arrows) and cell body (thick arrow) are indicated.
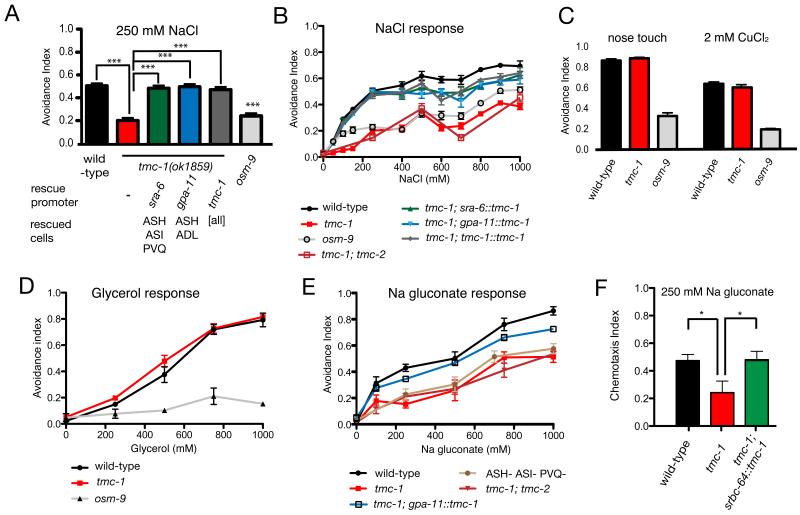
To investigate the possible role of TMC-1 in sensory transduction, we assayed the effect of a tmc-1 deletion allele on on ASH-mediated behaviours. The ASH neurons are important for avoidance of diverse noxious stimuli, including nose touch, hyperosmolarity, heavy metals, acids, and high concentrations of salt10-13. tmc-1 mutant animals showed no apparent defect in nose touch avoidance (Figure 2C), suggesting mechanosensation in ASH was unaffected by TMC-1. To assess ASH chemosensory function, we presented animals with various repulsive chemical stimuli, including CuCl2, glycerol, SDS, and high salt, and assayed escape behaviour8. We observed that tmc-1 mutants were strongly defective in the avoidance of NaCl concentrations above 100 mM (Figure 2A, B). In contrast, responses to other soluble repellents as well as to hyperosmolarity were indistinguishable from wild-type (Figure 2C, D), indicating that tmc-1 is not required generally for ASH function. In contrast, a loss-of-function mutation in the TRPV channel osm-9 affected avoidance to all these stimuli (Figure 2). Expression of a tmc-1 cDNA under the control of either the sra-6 or gpa-11 promoters, whose expression overlaps only in ASH, rescued the tmc-1 salt avoidance defect (Figure 2A, B), indicating that tmc-1 functions in ASH. A loss-of-function mutation in the paralogous tmc-2 gene did not enhance the tmc-1 mutant phenotype (Figure 2B). These results are consistent with TMC-1 acting as a salt sensor in ASH.
Figure 2. tmc-1 is specifically required for high salt avoidance behaviour. A.
Effect of tmc-1 on 250mM NaCl avoidance. For each genotype at least 370 animals were tested in population drop assays8. Avoidance index (A.I.) indicates the fraction of animals reversing following stimulus application; error bars for these and other panels indicates SEM. One-way ANOVA with Bonferroni correction was used to test significance. tmc-1(ok1859) animals were significantly different from wild-type (p<0.0005), while all tmc-1 rescue lines were significantly different from tmc-1(ok1859) (p<0.0005), but not from wild-type. B. Dose response for NaCl avoidance. For each data point, at least 170 animals were tested. At all concentrations tmc-1(ok1859) were significantly different from wild-type (p<0.005) and all tmc-1 rescue lines were significantly different from tmc-1(ok1859) (p<0.05). C. Effect of tmc-1 on other ASH-dependent escape behaviours. For each genotype at least 260 animals were tested for nose touch and at least 320 for CuCl2 avoidance. No significant difference was observed between wild type and tmc-1(ok1859). D. Dose response for glycerol avoidance. For each data point, at least 70 animals were tested. No significant difference was detected between wild-type and tmc-1(ok1859) across all concentrations. E. Dose response for Na gluconate avoidance. For each data point, at least 70 animals were tested (except ablated animals, for which a minimum of 25 animals were tested). tmc-1(ok1859) was significantly different from wild-type across all concentrations (p<0.05). The tmc-1; gpa-11::tmc-1 rescue line was significantly different from tmc-1(ok1859) (p<0.05). F. Chemotaxis behaviour of wild-type, tmc-1 mutant and animals with heterologous tmc-1 expression in ASK to Na gluconate. At least 790 animals were assayed for each genotype. Wild type was significantly different from tmc-1(ok1859) (p<0.05) and tmc-1; srbc-64::tmc-1 was significantly different (p<0.05) from tmc-1(ok1859).
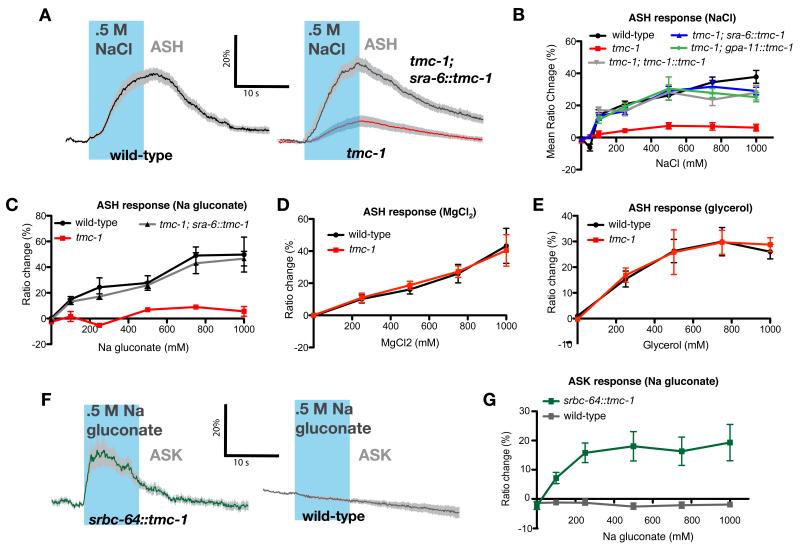
The whole-cell neuronal responses of ASH neurons can be measured directly in intact animals by calcium imaging14. We used a transgenic line expressing a genetically-encoded calcium indicator in ASH to assay the effects of tmc-1 on calcium transients in the ASH cell body evoked by increases in external salt concentration15. In wild-type animals, we observed large calcium transients in ASH in response to high concentrations of NaCl (Figure 3A), consistent with behavioural studies13. In contrast, the ASH neurons of the tmc-1 deletion mutant showed little response to high salt (Figure 3A, B). This salt response defect could be rescued by expression of wild-type tmc-1 under the control of either the sra-6 or gpa-11 promoters (Figure 3A, B). tmc-1 mutant animals showed normal calcium responses to other stimuli that activate ASH, including glycerol, copper, SDS and nose touch, consistent with the behavioural data indicating that only salt sensation is defective in these animals (Figure 3E; Supplemental Figure 4). Thus, tmc-1 is specifically required for salt sensation in the ASH neurons.
Figure 3. tmc-1 is specifically required for ASH chemosensory responses to salt.
A. ASH calcium responses to external salt stimulation. Shown are averaged traces of ASH calcium transients in wild-type, tmc-1 mutant and ASH-rescued animals in response to a 10s NaCl concentration upstep of 0-500 mM. Traces indicate average percent ΔR/R0 where R is the fluorescence emission ratio and R0 the baseline ratio; the gray band represents SEM. Statistical significance for all experiments was evaluated by one-way ANOVA with Bonferroni correction. The time of the NaCl upstep is shown in blue; n≥13. B. Quantification of ASH calcium responses in mutant and rescued animals. 17<n<51 animals were tested for each data point; error bars for this and other panels indicate SEM. For all concentrations ≥100 mM, the response of tmc-1 was statistically different from wild-type and all rescue lines (p<.05). C. ASH calcium responses to sodium gluconate. 5<n<19 animals were tested for each data point. For all concentrations ≥100 mM, the response of tmc-1 was statistically different from wild-type and the rescue line (p<.05). D. ASH calcium responses to MgCl2. 4<n<12 animals were tested for each data point. For all concentrations the response of tmc-1 was not statistically different from wild-type. E. ASH calcium responses to glycerol. 5<n<38 animals were tested for each data point. For all concentrations the response of tmc-1 was not statistically different from wild-type. F. ASK calcium responses to sodium gluconate. Shown are averaged R/R0 traces in 12 wild-type, and 15 srbc-64::tmc-1 animals, which express TMC-1 heterologously in ASK, in response to a 10 second stimulation with 0.5 M sodium gluconate. G. Quantification of ASK calcium responses to sodium gluconate. 7<n<24 animals were tested for each data point. For all concentrations ≥100mM, the response of srbc-64::tmc-1 was statistically different from wild-type (p<.05).
To examine the specificity of the TMC-1-dependent salt response, we used calcium imaging and behavioural assays to test the responses of ASH to salts other than NaCl. We observed that several chloride salts, including KCl, CaCl2 and MgCl2, evoked escape responses and ASH calcium transients when presented in high concentrations These responses were unaffected (for MgCl2 and CaCl2) or only slightly affected (for KCl) by the tmc-1 mutation, suggesting that TMC-1 is not required to sense these salts (Figure 3D, Supplemental Figures 3B, 4C and 5A-B). We also observed that escape responses and ASH calcium transients were evoked by sodium acetate and sodium gluconate. We found that these responses were strongly reduced in the tmc-1 deletion mutant and rescued cell-autonomously in ASH (Figure 3C, Supplemental Figures 3A and 6). The sodium gluconate avoidance phenotype of tmc-1 mutants was only slightly enhanced by ASH ablation and was similar to that of ASH-ablated wild-type animals (Figure 2E; Supplemental Figure 3A), indicating that TMC-1 mediates most of the sodium response in ASH, and most of the TMC-1-independent response is sensed by other neurons. Mutation of tmc-2 did not enhance the tmc-1 mutant phenotype (Figure 2E). Together, these data suggest that the ASHneurons contain a TMC-1-dependent salt sensor that responds specifically to sodium ions.
The finding that TMC-1 is specifically required for sodium responses in ASH raised the possibility that TMC-1 might itself be the salt-sensing receptor. To address this possibility, we tested whether heterologous expression of TMC-1 in worm neurons could confer the ability to sense sodium. We used the srbc-64 promoter to express TMC-1 cell-specifically in the ASKs, amphid neurons that do not respond to salt (Figure 3F-G) and use a cGMP-dependent mechanism to generate a calcium off-response to lysine and pheromones16,17 . In these srbc-64::tmc-1 animals, we observed strong calcium responses to sodium gluconate in the ASK neurons (Figure 3F, G), indicating that expression of tmc-1 was sufficient to confer salt sensitivity. Moreover, srbc-64::tmc-1 animals exhibited behavioural responses to sodium in a tmc-1 mutant background (though surprisingly, attraction rather than repulsion), indicating the functionality of the ectopic TMC-1-mediated salt responses in ASK (Figure 2F). These results suggest that TMC-1 can be sufficient to generate a salt receptor in vivo.
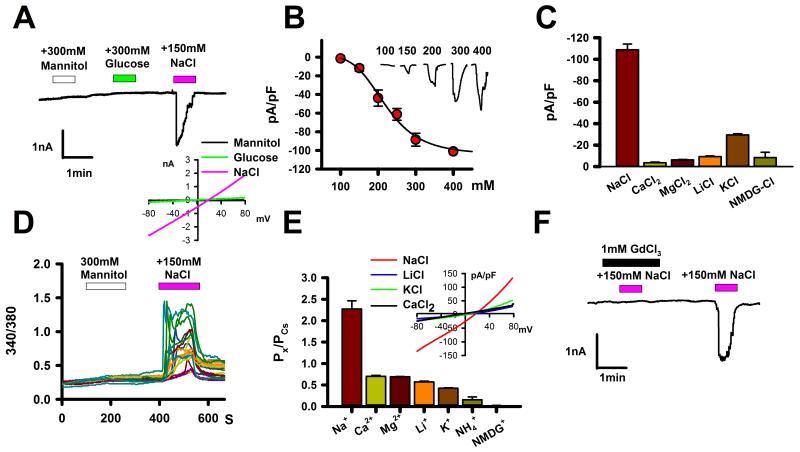
To further investigate this possibility, we expressed a tmc-1 cDNA in CHO-K1 cells recorded electrophysiological responses of the transfected cells to external salt stimulation. The expressed TMC-1 protein was localized to the plasma membrane, as verified by confocal fluorescence imaging (Supplemental Figure 8A-C). A 150 mM upstep in NaCl (from a baseline of 140 mM) evoked inward currents in TMC-1-expressing cells (Figure 4A); in contrast, hypertonic stimulation using glucose, mannitol or n-methyl-d-glucamine (NMDG) chloride did not activate currents in these cells (Figure 4A and Supplemental Figures 8E, 10D). Assaying over a range of NaCl concentrations, we estimated an EC50 for the TMC-1-dependent currents of 220 mM, with a threshold of approximately 140 mM and a plateau at approximately >400 mM (Figure 4B). The Hill plot of the NaCl dose-response curve rose steeply (Hill coefficient: 4.5) suggesting cooperative gating by external salt. Together, these results indicate that heterologously-expressed TMC-1 induces the formation of ion channels that are specifically sensitive to extracellular NaCl.
Figure 4. Sodium-sensitive cation currents in TMC1-expressing cells. A.
Inward currents were elicited in TMC-1-expressing CHO-K1 cells by external addition of 150 mM NaCl, but not by 300mM mannitol or glucose, in whole-cell voltage clamp recordings at -60 mV (n=7) using native chloride conditions (see methods). Unless otherwise indicated (i.e. panels B and E) the external buffer contained 140 mM NaCl. When GFP was co-transfected, 76% of GFP-positive cells showed the inward current reponses; mock-transfected or untransfected cells lacked such responses (Supplemental Figure 8D). Inset: Representative current-voltage relationship of NaCl-induced inward currents in TMC-1-expressing CHO-K1 cells. B. NaCl dose-response for inward currents of TMC-1-expressing cells. Filled circles represent mean responses to extracellular NaCl (n=9-17 for each point); error bars for this and other panels indicates SEM. Fitting to the Hill equation gives a Hill coefficient of 4.5 and EC50 of 220 mM. C. Activation of currents in TMC-1 CHO-K1 cells by other salts. Shown are mean current responses at -60 mV upon addition of 300 mM of the indicated salt to the recording buffer (n=6-11). D. Intracellular Ca2+ levels were elevated in TMC-1 CHO-K1 cells in response to addition of external 150 mM NaCl but not to 300 mM mannitol (n=34). Mock-transfected or untransfected cells lacked such responses, as did transfected cells in Ca2+-free external buffer (Supplemental Figure 9). E. Relative cation permeabilities of TMC-1 calculated from the reversal potentials of basal currents with CsCl in the internal solution and the indicated chloride salt in the external solution. Error bars indicate SEM. Inset: representative current-voltage relationships for different external salts in TMC-1-expressing CHO-K1 cells. F. 1 mM GdCl3 blocked NaCl-induced inward currents in TMC-1-expressing CHO-K1 cells (n=11).
We performed additional experiments to learn more about the properties of the TMC-1-dependent channels. Chloride salts other than NaCl did not evoke robust current increases in TMC-1-expressing CHO-K1 cells (Figure 4C, Supplemental Figure 10A-C). In contrast, sodium salts with other anions (including Na-gluconate) all elicited currents (Supplemental Figure 11, data not shown), suggesting that Na+ is critical for TMC-1 activation. In the presence of external calcium, extracellular NaCl evoked Ca2+ transients, suggesting that the expressed channels are permeable to Ca2+ ions (Figure 4D), although indirect effects might contribute to this calcium signal. By measuring the reversal potentials of basal currents18, we quantified ion permeabilities of TMC-1 conductances: Na+ ions were the most permeable (2.3 fold greater than Cs+), whereas Ca2+, K+ and the large cation NMDG+ had lower indices (70%, 42% and 2% of Cs+ permeability, respectively; Figure 4E). Cl− and other anions were about 7-12 times less permeable than Na+ or K+ (Supplemental Figure 11F-H). Comparable permeability ratios of Cl− to Na+ and K+ (8.7% and 6%), despite the two cations’ different permeabilities, may indicate that Cl− must be chaperoned by countercations to permeate TMC-1 (Supplemental Figure 11F). Although TMC-1-dependent currents were insensitive to piezo-driven membrane displacement (Supplemental Figure 8F), responses to external salt were inhibited by GdCl3, a non-specific blocker of stretch-activated channels (Figure 4F and Supplemental Figure 8G), which also blocks the tmc-1-dependent response of ASH to sodium gluconate (Supplemental Figure 7). Overall, TMC-1 expression generates sodium-sensitive, gadolinium-blockable currents with a high Na+ permeability, properties consistent with a salt sensor.
The production of salt-sensitive channels in TMC-1-expressing cells raises the possibility that TMC-1 itself might form a bona fide ion channel. Though the evidence is not definitive, several lines of evidence support this possibility. When we expressed TMC-1 heterologously in other mammalian cell lines (including HEK293T and HeLa cells), we observed sodium-sensitive inward currents similar to those in TMC-1 CHO-K1 cells (Supplemental Figure 12A, B). This, together with the expression of an ectopic salt sensor in ASK (Figure 3F-G) suggest that additional cell-type specific ionotropic effectors are not required to express TMC-1-dependent ion channels. Moreover, TMC-1-dependent currents were neither voltage-sensitive nor significantly blocked by micromolar amiloride, suggesting that a DEG/ENaC or voltage-gated sodium channel is not involved (Supplemental Figure 12C-E). These results are all consistent with TMC-1 encoding an ionotropic sensory receptor.
In summary, TMC-1 is a putative ion channel required for salt sensation in C. elegans, and meets several critical criteria expected of an authentic salt sensor. First, heterologously-expressed TMC-1 forms salt-activated channels that respond specifically to extracellular sodium. Thus, external salt alters the properties of TMC-1 in vitro in a manner consistent with the requirements for in vivo chemosensation. Second, the ASH neurons of tmc-1 mutants are strongly defective in responses to sodium, but respond normally to other stimuli including hyperosmolarity and chemical repellents. Thus, TMC-1 is specifically required in vivo for neuronal responses to salt. Finally, tmc-1 mutants show strong, modality-specific defects in chemosensory behaviour with a focus in the ASH neurons. Thus, the neuronal responses that require tmc-1 are clearly linked to sensory behaviour. Taken together, these results argue strongly that TMC-1 is a salt sensor in C. elegans chemosensory neurons and may comprise a salt-activated channel.
Although much is known about the molecular mechanisms of sweet, sour, umami and bitter taste, the molecular basis of salt taste is less well-understood. In C. elegans, detection of salt concentration changes by neurons mediating chemosensory attraction requires a cGMP-gated channel15,19,20; however, the receptors that might link this channel’s activity to extracellular salt concentration are unknown. ASH responses to salt, as well as chemical, osmotic and mechanical stimuli, also require the TRPV channel OSM-9 21, which may function as a nonspecific amplifier rather than a receptor22. In mammals and insects, low-concentration salt taste requires the sodium-selective ENaC channel23,24, whereas mammalian high-concentration salt taste may involve TRPV125. However, on their own these channels, though sodium-permeable, are inhibited by external sodium26,27. In contrast, the Hill coefficient of TMC-1-dependent channels indicates they are not just sodium permeable, but sodium-activated28, properties optimally-suited for a salt chemosensor.
TMC proteins have been previously implicated in processes of sensory transduction, in particular hearing, but their mechanistic role in these processes has been unclear5,6. Our results indicate that C. elegans TMC-1 may comprise an ion channel gated by a sensory stimulus, raising the possibility that other TMC proteins may constitute channels as well. We speculate that TMC proteins, including but not limited to mammalian TMC1, may function generally as ionotropic sensory receptors, though potentially gated by stimuli other than sodium. Since mammals contain several TMC proteins of unknown function, it is possible one or more of these may play a role in sensory transduction processes such as touch or salt taste. With the recent finding that mammalian TMC1 and TMC2 are required for cochlear hair cell mechanotransduction6, these molecules must be considered strong candidates for the mammalian hair cell mechanotransducer.
Methods
Drop test assays
The repellent drop test was performed on unseeded NGM plates essentially as described8. 10 to 30 animals were picked from a culture plate and placed on a plate without food for a few seconds to avoid transferring food to the assay plates. The animals were then placed on the assay plate, allowed to settle for 10 minutes, then assayed using a capillary to delivery the stimulus. A drop of the stimulus solution was delivered near the tail of a moving animal; the response of a single animal to each drop delivered was recorded as either positive (when the animal stops moving forwards and reverses) or negative (when the animal continues moving forward) for escape behaviour. To consider the response positive the avoidance reaction must be observed within a 4 second interval following the stimulation. Each animal was tested only once. All salts were dissolved in 1 mM MgSO4, 1 mM CaCl2 and 5 mM KPO4. CuCl2 was dissolved in M13 buffer. The animals lacking the ASH, ASI and PVQ neurons expressed the egl-1 cell death gene under the control of the sra-6 promoter (see strain list in Supplemental Methods). Statistical comparison between two strains was done using the t-test. Where more than one comparison was made, ANOVA with Bonferroni correction for multiple comparisons was used instead.
Calcium imaging of ASH and ASK neurons
Filter-dichroic pairs were excitation, 400–440; excitation dichroic 455; CFP emission, 465–495; emission dichroic 505; YFP emission, 520–550. Individual adult worms (~24hours past L4) were glued with Dermabond 2-Octyl Cyanoacrylate glue to pads composed of 2% agarose in extracellular saline (5mM KCl, 1mM CaCl2, 5mM MgCl2, 20mM d-glucose and 10mM HEPES buffer, pH 7.2). Worms used for calcium imaging had similar levels of cameleon expression in sensory neurons as inferred from initial fluorescence intensity. The animals were placed under the microscope in a perfusion chamber (RC-26GLP,Warner Instruments) under constant flow rate (0.4 ml/min) of neuronal buffer using a perfusion pencil (AutoMate). Outflow was regulated using a peristaltic pump (Econo Pump, Biorad). Repellents were delivered using the perfusion pencil and manually controlled valves. Solutions contained the indicated amount of salt and in addition 1 mM MgSO4, 1 mM CaCl2 and 5 mM KPO4. For salt experiments, the osmolarity of the pre and post stimulus solution was adjusted to match that of the stimulus using sucrose as described15; similar results were obtained when sucrose was not included in the buffer solution (data not shown). The pH was adjusted to pH 7. Glycerol was dissolved in M13 buffer to a final concentration of 1M or 0.5M. Additional details of these experiments are in Supplemental Materials.
Electrophysiology
Unless otherwise specified, the internal solution consisted of (in mM) 140 CsCl, 5 EGTA, 10 HEPES, 2.0 MgATP, 0.2 NaGTP titrated to pH 7.2 with CsOH. For the native Cl-condition, 20 KCl plus 120 K-gluconate was used instead of 140 CsCl. The external bath solution except for Figure 4E cation permeability experiments consisted of (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, titrated to pH 7.4 with NaOH. Responses were sampled at 20 kHz and calculated using Clampfit 10.3 software (Molecular Devices). To minimize junction potentials, we used a 3 M KCl agar salt bridge as the reference electrode. To generate the curve in Figure 4B, the sodium concentration in the external buffer was changed to the indicated concentration; concentrations of other solutes were unchanged. The curve was fitted by the Hill equation (I=Imax/(1+(EC50/[NaCl])n); I, current response; Imax, maximum current response from a given cell).
Cation permeabilities of TMC-1 were determined by current-voltage relationship experiments by using the following cationic solutions. The internal solution consisted of (in mM) 150 CsCl and 10 HEPES (pH 7.3 with CsOH). The monovalent external solutions consisted of (in mM) 300 NaCl (or 150 KCl or 150 LiCl for a measurement of K+ or Li+ permeability respectively) and 10 HEPES. The divalent external solutions consisted of (in mM) 100 CaCl2 (or 100 MgCl2 for a measurement of Mg2+ permeability) and 10 HEPES. Reversal potentials were obtained from the current-voltage curves under an 800-millisecond voltage ramp protocol ranging from −80 mV to +80 mV at a holding potential of −60 mV. When the basal currents of a given cell were small, averaged current-voltage curves from multiple ramp trials on a same cell were used to obtain the reversal potential. To completely wash external NaCl before other cation applications, 150 CsCl plus 10 HEPES were used. The ratio of cation permeability (PX/PCs) was determined for each test cation X from the reversal potential of the basal current (see Supplemental Material for more detail). Permeability ratios: 2.27±0.19 for Na+ (n=13), 0.42±0.09 for K+ (n=14), 0.57±0.01 for Li+ (n=11), 0.70±0.02 for Ca2+ (n=11), 0.69±0.06 for Mg2+ (n=17), 0.15±0.06 for NH4 (n=5) and 0.02±0.01 for NMDG+ (n=17) (mean±SEM).
Supplementary Material
Acknowledgements
We thank the Caenorhabditis Genetics Center and M. de Bono for strains, and A. Patapoutian, J.R. Holt, J. Hao, B. Zhao, D. Miller and R. Branicky for helpful suggestions and comments on the manuscript. This research was supported by the Medical Research Council (WRS) and grants to SWH from the National Research Foundation of Korea (2012000540) and Korea Health technology R&D Project of Ministry of Health & Welfare (A111373).
References
- 1.Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics. 2003;82:300–308. doi: 10.1016/s0888-7543(03)00154-x. [DOI] [PubMed] [Google Scholar]
- 2.Keresztes G, Mutai H, Heller S. TMC and EVER genes belong to a larger novel family, the TMC gene family encoding transmembrane proteins. BMC genomics. 2003;4:24. doi: 10.1186/1471-2164-4-24. doi:10.1186/1471-2164-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurima K, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nature genetics. 2002;30:277–284. doi: 10.1038/ng842. doi:10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 4.Vreugde S, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nature genetics. 2002;30:257–258. doi: 10.1038/ng848. doi:10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- 5.Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. The Journal of physiology. 2006;574:677–698. doi: 10.1113/jphysiol.2005.095661. doi:10.1113/jphysiol.2005.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima Y, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. The Journal of clinical investigation. 2011;121:4796–4809. doi: 10.1172/JCI60405. doi:10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CJ, et al. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multidendritic nociceptor in C. elegans. Developmental biology. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. doi:10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilliard MA, Bargmann CI, Bazzicalupo PC. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr. Biol. 2002;12:730–734. doi: 10.1016/s0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harbor Symp. Quant. Biol. 1990;55:529–538. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Sambongi Y, et al. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999;10:753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- 12.Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. The EMBO journal. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. doi:10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hukema RK, Rademakers S, Dekkers MP, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. The EMBO journal. 2006;25:312–322. doi: 10.1038/sj.emboj.7600940. doi:10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilliard MA, et al. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. The EMBO journal. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. doi:10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, et al. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. doi:10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi T, et al. In vivo calcium imaging of OFF-responding ASK chemosensory neurons in C. elegans. Biochimica et biophysica acta. 2009;1790:765–769. doi: 10.1016/j.bbagen.2009.03.032. doi:10.1016/j.bbagen.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Jang H, et al. Neuromodulatory State and Sex Specify Alternative Behaviors through Antagonistic Synaptic Pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. doi:10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. The Journal of biological chemistry. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. doi:10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 19.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 21.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in C. elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geffeney SL, et al. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. doi:10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, et al. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. doi:10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyall V, et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. The Journal of physiology. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. doi:10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta T, Imagawa T, Ito S. Novel gating and sensitizing mechanism of capsaicin receptor (TRPV1): tonic inhibitory regulation of extracellular sodium through the external protonation sites on TRPV1. The Journal of biological chemistry. 2008;283:9377–9387. doi: 10.1074/jbc.M709377200. doi:10.1074/jbc.M709377200. [DOI] [PubMed] [Google Scholar]
- 27.Chraibi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. The Journal of general physiology. 2002;120:133–145. doi: 10.1085/jgp.20028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, MacKinnon R. Ion binding affinity in the cavity of the KcsA potassium channel. Biochemistry. 2004;43:4978–4982. doi: 10.1021/bi049876z. doi:10.1021/bi049876z. [DOI] [PubMed] [Google Scholar]
- 29.Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nature neuroscience. 2010;13:610–614. doi: 10.1038/nn.2537. doi:10.1038/nn.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. The EMBO journal. 2011;30:1110–1122. doi: 10.1038/emboj.2011.22. doi:10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.