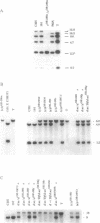
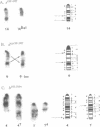
Abstract
Specific-locus experiments have previously shown melphalan to be mutagenic in all male germ-cell stages tested and particularly so in early spermatids. All but 2 of 24 specific-locus mutations recovered were tested genetically, cytogenetically, and/or molecularly. At least 12 of 15 tested mutations recovered from postspermatogonial stages but only 1 of 7 mutations recovered from stem-cell or differentiating spermatogonia gave evidence of being deletions or other rearrangements. Melphalan-induced mutations, thus, confirm the pattern of dependence of mutation structure on germ-cell stage that had been shown earlier for other chemicals. Results of the present investigation illustrate the capabilities of combined genetic, cytogenetic, and molecular analyses for characterizing the nature of specific-locus mutations. Fine-structure molecular mapping of long regions surrounding specific loci has been greatly facilitated by the availability of genetic reagents (particularly, deletion complexes) generated in specific-locus experiments over the course of decades. Reciprocally, this mapping permits increasingly detailed characterization of the nature of lesions induced by mutagenic exposures of germ cells, adding great powers for qualitative analysis of mutations to the specific-locus test. Cytogenetic and genetic investigations also provide evidence on lesion type, especially for loci at which mutations cannot yet be analyzed molecularly. Melphalan, like chlorambucil, can generate many mutations, a high proportion of which are deletions and other rearrangements, making this chemical valuable for generating mutations (at any locus) amenable to molecular access.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cacheiro N. L., Russell L. B. Evidence that Linkage Group IV as well as linkage Group X of the mouse are in chromosome 10. Genet Res. 1975 Apr;25(2):193–195. doi: 10.1017/s0016672300015597. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- EVANS E. P., BRECKON G., FORD C. E. AN AIR-DRYING METHOD FOR MEIOTIC PREPARATIONS FROM MAMMALIAN TESTES. Cytogenetics. 1964;3:289–294. doi: 10.1159/000129818. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Wakulchik M., Haq A. K., Halaban R., Kestler D. Sequence analysis of mouse tyrosinase cDNA and the effect of melanotropin on its gene expression. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- RUSSELL L. B., RUSSELL W. L. Genetic analysis of induced deletions and of spontaneous nondisjunction involving chromosome 2 of the mouse. J Cell Comp Physiol. 1960 Nov;56(Suppl 1):169–188. doi: 10.1002/jcp.1030560415. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. L. X-ray-induced mutations in mice. Cold Spring Harb Symp Quant Biol. 1951;16:327–336. doi: 10.1101/sqb.1951.016.01.024. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Bangham J. W., Hunsicker P. R., Cacheiro N. L., Kwon B. S., Jackson I. J., Russell L. B. Genetic and molecular analysis of chlorambucil-induced germ-line mutations in the mouse. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1416–1420. doi: 10.1073/pnas.87.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M. Chemical mutagenesis and fine-structure functional analysis of the mouse genome. Trends Genet. 1991 Jan;7(1):15–21. doi: 10.1016/0168-9525(91)90016-j. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Russell L. B., Copeland N. G., Jenkins N. A. Molecular genetic analysis of the dilute-short ear (d-se) region of the mouse. Genetics. 1986 Feb;112(2):321–342. doi: 10.1093/genetics/112.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B. Definition of functional units in a small chromosomal segment of the mouse and its use in interpreting the nature of radiation-induced mutations. Mutat Res. 1971 Jan;11(1):107–123. doi: 10.1016/0027-5107(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Russell L. B. Functional and structural analyses of mouse genomic regions screened by the morphological specific-locus test. Mutat Res. 1989 May;212(1):23–32. doi: 10.1016/0027-5107(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Hunsicker P. R., Cacheiro N. L., Bangham J. W., Russell W. L., Shelby M. D. Chlorambucil effectively induces deletion mutations in mouse germ cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3704–3708. doi: 10.1073/pnas.86.10.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S., Raymer G. D. Analysis of the albino-locus region of the mouse: IV. Characterization of 34 deficiencies. Genetics. 1982 Mar;100(3):427–453. doi: 10.1093/genetics/100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Selby P. B., von Halle E., Sheridan W., Valcovic L. The mouse specific-locus test with agents other than radiations: interpretation of data and recommendations for future work. Mutat Res. 1981 May;86(3):329–354. doi: 10.1016/0165-1110(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarsky E., Favor J., Jackson I. J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics. 1990 Oct;126(2):443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]