Abstract
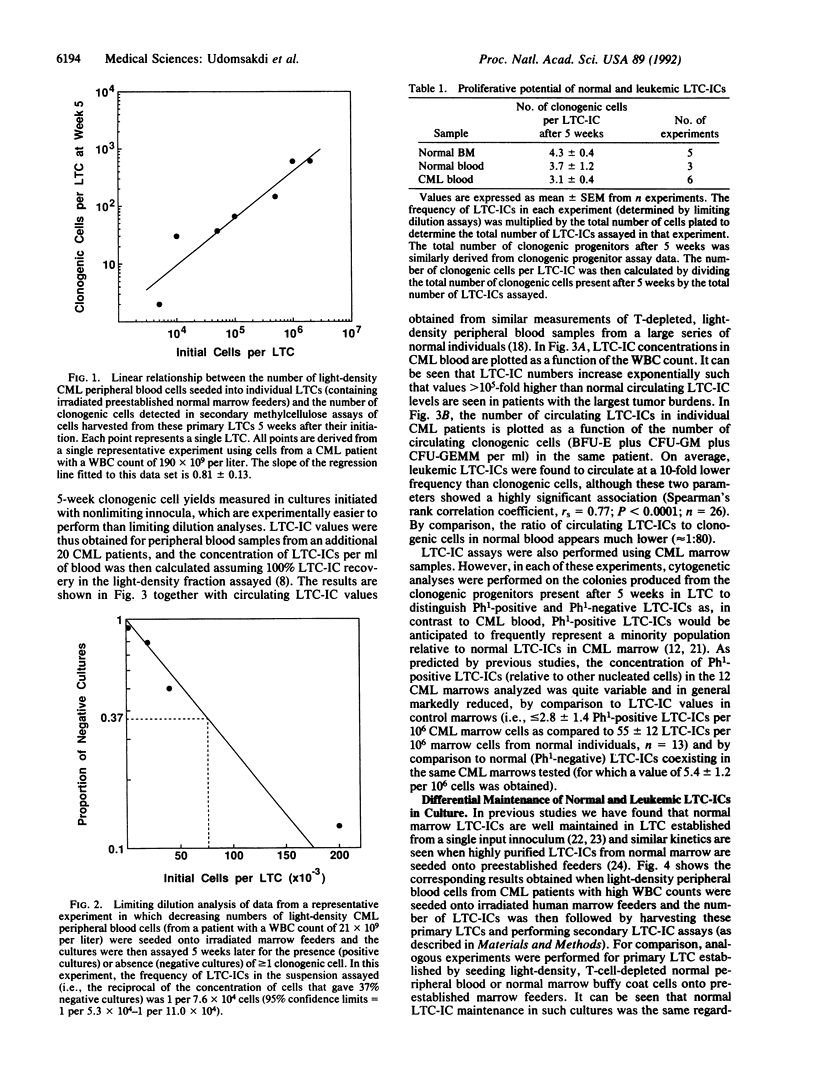
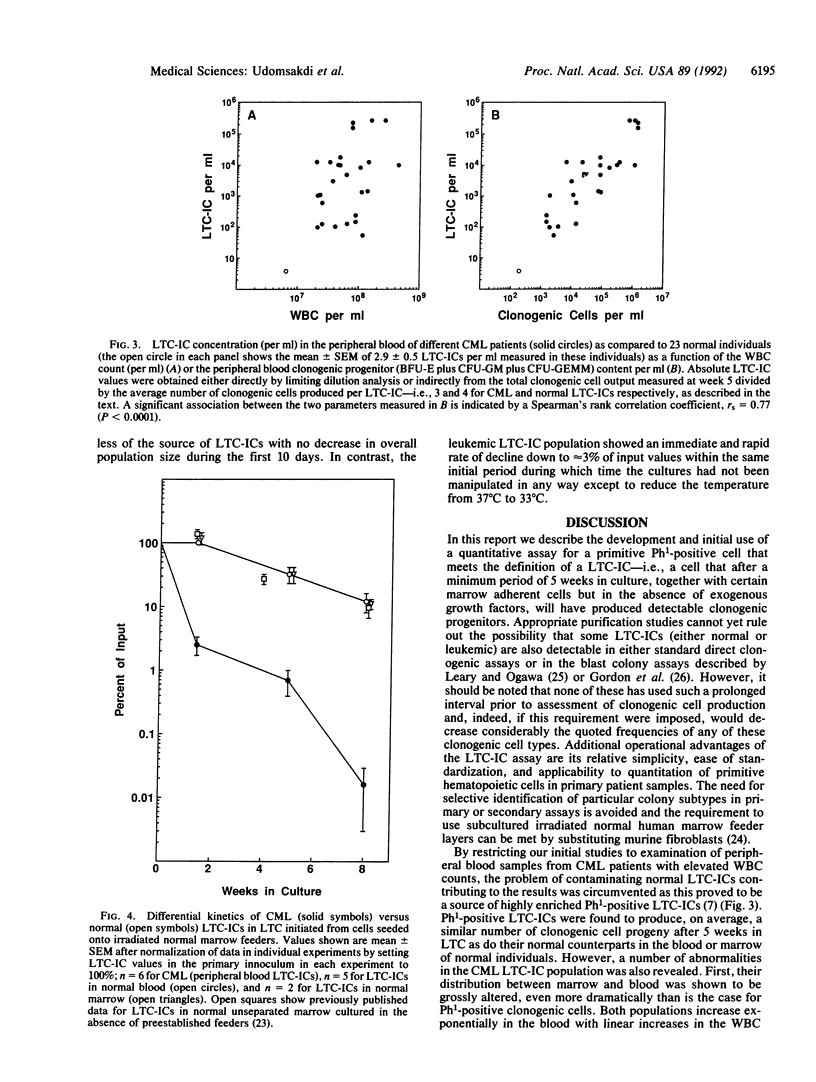
In this report we describe a quantitative in vitro assay for the most primitive type of leukemic precursors yet defined in patients with chronic myeloid leukemia (CML). This assay is based on the recently described "long-term culture-initiating cell" (LTC-IC) assay for primitive normal human hematopoietic cells. Such cells, when cocultured with competent fibroblast feeder layers, give rise after a minimum of 5 weeks to multiple single and multilineage clonogenic progenitors detectable in secondary semisolid assay cultures. Similar cultures initiated by seeding a highly enriched source of leukemic cells from patients onto normal feeders showed the clonogenic cell output after 5 weeks to be linearly related to the input innoculum over a wide range down to limiting numbers of input cells, thus allowing absolute frequencies of leukemic LTC-ICs to be determined using standard limiting dilution analysis techniques. Leukemic LTC-IC concentrations in CML marrow were found to be decreased, on average to less than 10% of the normal LTC-IC concentration in normal marrow, but were greatly increased (up to greater than 10(5) times) in CML blood. Assessment of the number of clonogenic cells produced per leukemic LTC-IC by comparison to normal blood or marrow LTC-IC values showed this function to be unchanged in leukemic LTC-ICs [i.e., 3.1 +/- 0.4 clonogenic cells per CML LTC-IC (mean +/- SEM, n = 6) versus 3.7 +/- 1.2 (n = 3) and 4.3 +/- 0.4 (n = 5), respectively, for normal blood and marrow LTC-ICs]. In contrast, leukemic LTC-IC maintenance in LTC proved to be highly defective by comparison to normal LTC-IC of either blood or marrow origin. Thus, when cells from primary LTC were subcultured into secondary LTC-IC assays, leukemic LTC-IC rapidly declined (greater than 30-fold) within the first 10 days of culture, whereas normal LTC-IC numbers remained unchanged during this period. These findings illustrate how self-maintenance and differentiation events in primitive human hematopoietic cells can be differentially modulated by an oncogenic process and provide a framework for further studies of their manipulation, analysis, and therapeutic exploitation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett M. J., Eaves C. J., Phillips G. L., Kalousek D. K., Klingemann H. G., Lansdorp P. M., Reece D. E., Shepherd J. D., Shaw G. J., Eaves A. C. Successful autografting in chronic myeloid leukaemia after maintenance of marrow in culture. Bone Marrow Transplant. 1989 Jul;4(4):345–351. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Coller H. A., Coller B. S. Poisson statistical analysis of repetitive subcloning by the limiting dilution technique as a way of assessing hybridoma monoclonality. Methods Enzymol. 1986;121:412–417. doi: 10.1016/0076-6879(86)21039-3. [DOI] [PubMed] [Google Scholar]
- Coulombel L., Kalousek D. K., Eaves C. J., Gupta C. M., Eaves A. C. Long-term marrow culture reveals chromosomally normal hematopoietic progenitor cells in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. N Engl J Med. 1983 Jun 23;308(25):1493–1498. doi: 10.1056/NEJM198306233082502. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dowding C. R., Gordon M. Y., Goldman J. M. Primitive progenitor cells in the blood of patients with chronic granulocytic leukemia. Int J Cell Cloning. 1986 Sep;4(5):331–340. doi: 10.1002/stem.5530040505. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Kalousek D. K., Eaves C. J. Unregulated proliferation of primitive chronic myeloid leukemia progenitors in the presence of normal marrow adherent cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5306–5310. doi: 10.1073/pnas.83.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves A. C., Henkelman D. H., Eaves C. J. Abnormal erythropoiesis in the myeloproliferative disorders: an analysis of underlying cellular and humoral mechanisms. Exp Hematol. 1980;8 (Suppl 8):235–247. [PubMed] [Google Scholar]
- Eaves C. J., Cashman J. D., Sutherland H. J., Otsuka T., Humphries R. K., Hogge D. E., Lansdorp P. L., Eaves A. C. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann N Y Acad Sci. 1991;628:298–306. doi: 10.1111/j.1749-6632.1991.tb17260.x. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gartler S. M., Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. C., Szilvassy S. J., Eaves C. J., Humphries R. K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C., Eaves C. J., Kalousek D. K. Fluorodeoxyuridine synchronization of hemopoietic colonies. Cancer Genet Cytogenet. 1987 Jan;24(1):1–6. doi: 10.1016/0165-4608(87)90077-x. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Hibbin J. A., Kearney L. U., Gordon-Smith E. C., Goldman J. M. Colony formation by primitive haemopoietic progenitors in cocultures of bone marrow cells and stromal cells. Br J Haematol. 1985 May;60(1):129–136. doi: 10.1111/j.1365-2141.1985.tb07393.x. [DOI] [PubMed] [Google Scholar]
- Goto T., Nishikori M., Arlin Z., Gee T., Kempin S., Burchenal J., Strife A., Wisniewski D., Lambek C., Little C. Growth characteristics of leukemic and normal hematopoietic cells in Ph' + chronic myelogenous leukemia and effects of intensive treatment. Blood. 1982 Apr;59(4):793–808. [PubMed] [Google Scholar]
- Kantarjian H. M., Vellekoop L., McCredie K. B., Keating M. J., Hester J., Smith T., Barlogie B., Trujillo J., Freireich E. J. Intensive combination chemotherapy (ROAP 10) and splenectomy in the management of chronic myelogenous leukemia. J Clin Oncol. 1985 Feb;3(2):192–200. doi: 10.1200/JCO.1985.3.2.192. [DOI] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Blast cell colony assay for umbilical cord blood and adult bone marrow progenitors. Blood. 1987 Mar;69(3):953–956. [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E., Hansen P. W., Christiansen S. E. Isolation of human T and B lymphocytes by E-rosette gradient centrifugation. Characterization of the isolated subpopulations. J Immunol Methods. 1980;33(4):323–336. doi: 10.1016/0022-1759(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Thacker J. D., Hogge D. E. The effects of interleukin 6 and interleukin 3 on early hematopoietic events in long-term cultures of human marrow. Exp Hematol. 1991 Nov;19(10):1042–1048. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Lansdorp P. M., Thacker J. D., Hogge D. E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991 Aug 1;78(3):666–672. [PubMed] [Google Scholar]
- Sutherland H. J., Lansdorp P. M., Henkelman D. H., Eaves A. C., Eaves C. J. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci U S A. 1990 May;87(9):3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]
- Udomsakdi C., Eaves C. J., Sutherland H. J., Lansdorp P. M. Separation of functionally distinct subpopulations of primitive human hematopoietic cells using rhodamine-123. Exp Hematol. 1991 Jun;19(5):338–342. [PubMed] [Google Scholar]


