Abstract
Those with high baseline stress levels are more likely to develop mild cognitive impairment (MCI) and Alzheimer's Disease (AD). While meditation may reduce stress and alter the hippocampus and default mode network (DMN), little is known about its impact in these populations. Our objective was to conduct a “proof of concept” trial to determine whether Mindfulness Based Stress Reduction (MBSR) would improve DMN connectivity and reduce hippocampal atrophy among adults with MCI. 14 adults with MCI were randomized to MBSR vs. usual care and underwent resting state fMRI at baseline and follow-up. Seed based functional connectivity was applied using posterior cingulate cortex as seed. Brain morphometry analyses were performed using FreeSurfer. The results showed that after the intervention, MBSR participants had increased functional connectivity between the posterior cingulate cortex and bilateral medial prefrontal cortex and left hippocampus compared to controls. In addition, MBSR participants had trends of less bilateral hippocampal volume atrophy than control participants. These preliminary results indicate that in adults with MCI, MBSR may have a positive impact on the regions of the brain most related to MCI and AD. Further research with larger sample sizes and longer-follow-up are needed to further investigate the results from this pilot study.
Keywords: Mild Cognitive Impairment, Alzheimer's Disease, fMRI, Meditation, Default Mode Network, Hippocampus
1. Introduction
Over 50% of those with Mild Cognitive Impairment (MCI), a transitional state between normal aging and dementia, will develop dementia within five years [9]. Despite the clinical and public health significance of MCI, there are no known therapies preventing progression to dementia. In older adults, high levels of perceived stress are associated with a higher risk of developing MCI and AD. [33, 34] Meditation has been shown to decrease perceived stress, cortisol levels and improves well-being. [11, 22] Mindfulness Based Stress Reduction (MBSR) is a standardized, widely tested mind/body intervention (>100 published trials) that teaches mindfulness meditation and yoga[18]. Cross-sectional studies show meditators have more hippocampal gray matter concentration [15] and volumes [24] than controls and the hippocampus is selectively activated during meditation [23]. A recent longitudinal study showed that after MBSR, participants had increased hippocampal gray matter density compared to before [14]. The hippocampus, a critical site of episodic memory, progressively atrophies from normal aging to MCI to AD [12]. The default mode network (DMN), a set of brain regions that are more active at rest in the “default” state, (e.g. medial prefrontal cortex [MPFC], posterior cingulate cortex [PCC], hippocampus, and lateral/inferior parietal cortex), may also be especially impacted by meditation.[3, 8, 16, 19, 27]. The DMN involves the same areas affected by cerebral atrophy, reduced metabolism, and amyloid in early AD/MCI [4]. DMN connectivity may be a noninvasive biomarker useful for assessing an intervention's impact in adults with MCI [10].
Could meditation improve the functional connectivity of the DMN and reduce the rate of hippocampal atrophy in adults with MCI? In this context, we conducted a pilot randomized controlled trial in adults with MCI to test our hypotheses that MBSR would 1) increase regional functional connectivity in the areas of the DMN, specifically the MPFC, hippocampus, and PCC; 2) slow the rate of hippocampal atrophy.
2. Methods
2.1 Study population
We recruited 14 participants from 2010-2011 from Beth Israel Deaconess Medical Center's (BIDMC) Cognitive Neurology Unit. The study was approved by BIDMC's human subjects review board and registered with the NIH clinical trials database (Clinicaltrials.gov), NCT01605448. Inclusion criteria (based on the Alzheimer's Disease Neuroimaging Initiative criteria[1] and the research operational definition of MCI[12]): adults 55-90 years with MCI determined by a neurologist through history, physical, and neuropsychological testing (including Wechsler Memory scale IV, Mini Mental Status Exam, Clinical Dementia Rating). Exclusion criteria: actively practicing meditation/yoga; any history of brain lesions or major head trauma.
2.2 Study design
This study was a prospective, randomized controlled pilot clinical trial with participants randomized 2:1 to either MBSR or usual care (and then offered MBSR at study conclusion). We randomized our participants 2:1 into the intervention and control, respectively, as we wanted to increase our experience with participants undergoing the active intervention. We used permuted block randomization with randomly varying block size to generate treatment assignment.
2.3 MBSR intervention
The class met weekly × 8 for 2 hours, plus one “mindfulness retreat day.” Mindfulness, defined as non-judgmental moment to moment awareness, was cultivated through sitting and walking meditation, body scan, and mindful movement (yoga). Home practice (30 min/day) was encouraged with standard guided audio recordings.
2.4 fMRI acquisition and analyses
Participants underwent an fMRI at baseline and 8 weeks using a 3T Siemens whole body scanner with echo-planar imaging capability using a 32-channel radio-frequency head coil at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital. During the resting state fMRI scan, subjects were asked to keep their eyes open and look at a darkened screen for 6 minutes. The scan acquisition included 47 slices with thickness of 3 mm, TR 3000ms, TE 30ms, a 3×3mm in–plane spatial resolution, and FoV 216 mm. T1 weighted MPRAGE type structural images were acquired using the following parameters: voxel size 1.2×1.2×1.2mm, TR 2.2s, TE 1.54ms, flip angle 7 degrees, slices 144, field of view: 230.
2.5 Seed-based functional connectivity analyses
Seed based functional connectivity analysis was performed using methods employed in previous functional connectivity studies [17, 21, 28] using the fcfast script developed by Randy Buckner's group at Athinoula A. Martinos Center for Biomedical Imaging.
(http://cnlwiki.pbworks.com/w/page/13165363/One%20Step%20Funcitonal%20Connectivity%20Analysis%20Script). The PCC (Peak at 8 -56 30 with 3mm radius) was used as seed because of its importance in the DMN. Seed coordinates were used in a previous study on exercise training in older adults[29] . In brief, functional data were preprocessed to decrease image artifacts, between-slice timing differences, and to eliminate differences in odd/even slice intensity. Data were then spatially smoothed using a Gaussian kernel of 6mm full-width at half-maximum and temporally filtered (0.009Hz<f<0.08Hz). Several spurious or nonspecific sources of variance were removed by regression of the following variables: (1) six movement parameters computed by rigid body translation and rotation during preprocessing, (2) mean whole brain signal, (3) mean brain signal within the lateral ventricles, and (4) the mean signal within a deep white matter region of interest (ROI). Temporally shifted versions of these waveforms were also removed by inclusion of the first temporal derivatives in the linear model.
A functional connectivity analysis produced coefficients for each previously defined seed-voxel correlation using Pearson correlation analysis. Fisher's r-to-z transformation was used to convert correlation maps into z maps. Random effect models were applied for second level analysis. A two sample t-test on the pre- vs. post differences between the MBSR and control groups (MBSR [pre-post] – control [pre-post]) was calculated to explore the difference between the two groups. Based on our hypothesis, we defined our a priori regions of interest (ROI) as the MPFC and hippocampus.. . An initial threshold of p < 0.005 was applied in data analysis. To correct for multiple comparisons, Monte Carlo Simulations with the program AlphaSim program in AFNI were applied for the priori ROI using a template based on Anatomical Automatic Labeling tool box. The results showed that a voxel-wise threshold p < 0.005 with 29 voxels has a corrected threshold of p < 0.05 at the cluster level for priori ROIs. A threshold of p<0.005 uncorrected and p<0.05 corrected (family-wise error, FWE) at the cluster level was used for non-ROI.
2.6 MRI volumetric analyses
To explore the potential treatment effect on changes in brain structures, we compared the bilateral hippocampi volume change (in mm3) between the two groups after the intervention compared to baseline. Data analysis were applied using freesurfer (http://surfer.nmr.mgh.harvard.edu/). We used the automated procedure for labeling different brain structures, and getting their volumetric measures, as previously described in detail [7]. This automated MRI process of measuring hippocampal volume has been validated in adults with MCI and AD [5]. The automatic segmentations were visually inspected for accuracy. The bilateral hippocampal volumes were assessed by analyzing the median change in the volume from baseline to 8 weeks using a Wilcoxon rank sum test. Whole brain volume analyses of the cortical and subcortical gray matter, white matter, and total intracranial volume were similarly assessed.
2.7 Statistical analyses for clinical measures
Participants also completed the Alzheimer's Disease Assessment Scale, cognitive subscale (ADAS-cog) as a global cognitive measure, although the study was not powered to detect anticipated clinical differences. Additional neuropsychological tests and measures of “well-being” were considered exploratory and are reported elsewhere [32]. Weekly phone calls assessed for adverse events. All analyses were blinded and performed on an intention-to-treat basis. SAS software, version 9.2 (Research Triangle Park, NC) was used for descriptive statistics of adherence, baseline characteristics, and for analyzing the median change in the ADAS-cog scores from baseline to 8 weeks with the Wilcoxon rank sum test.
3. Results
Of the 14 subjects randomized, 13 subjects completed the two fMRI scan sessions (one subject did not participate in the second scan due to a schedule conflict) (see supplemental figure, the Consort Flow Diagram, for recruitment breakdown). The baseline characteristics of participants in the MBSR vs. control groups did not differ by age or MMSE (see supplemental table). Briefly, the mean age of the participants in the MBSR group was 73 years (±8) and in the control 75 years (±7) and the MMSE was 27 (±2) in both groups. There were no adverse events reported related to the study protocol, mean class attendance was 7.9 out of 9, and mean (± SD) daily home practice was 26 minutes (± 20).
3.1 fMRI resting state
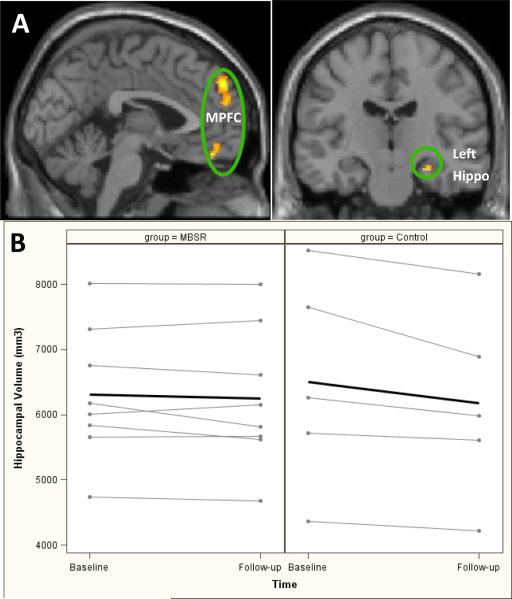
Seed-based resting state functional connectivity changes between the MBSR group and control group [intervention (post-pre) –control (post-pre)] are presented in Figure 1A and Table 1. Results showed that after treatment, those in the MBSR group had significantly increased functional connectivity between the PCC and bilateral MPFC, and between the PCC and left hippocampus as compared with the control group; no regions showed significant differences in the opposite contrast [control (post-pre)-intervention (post-pre)]).
Figure 1. Resting state functional connectivity, and hippocampal volume results 1A. Resting state functional connectivity results.
These images are representations of statistical results of a two sample t-test on the pre- vs. post differences between the MBSR and control groups (MBSR [pre-post] - control [pre-post]). The results showed increased functional connectivity between the Posterior Cingulate Cortex (PCC) and Medial Prefrontal Cortex (MPFC) and between the PCC and left hippocampus (hippo) after MBSR as compared with control condition.
1B. Hippocampal volume results: individual and average (bolded) hippocampal volumes from baseline to follow-up in the intervention (MBSR, Mindfulness Based Stress Reduction) and control groups
Table 1.
fMRI results showing difference between groups [intervention (post-pre) - control (post - pre)]
| Contrasts | Brain Region | Zmax | Cluster size | Peak Coordinates X Y X (MNI) |
|---|---|---|---|---|
| Intervention > Control | Bilateral superior MPFCa | 3.93 | 191 | 0 50 40 |
| Bilateral orbital MPFCa | 3.44 | 66 | 4 50 −10 | |
| Left hippocampus | 3.10 | 28 −22 16 | ||
| Control > Intervention | no region above the threshold |
MPFC=Medial Prefrontal Cortex
3.2 MRI volumetric analyses
Total brain, cortical and sub-cortical gray matter and white matter volumes showed no significant differences on pre- and post-treatment changes between the two groups. As expected in adults with MCI, the bilateral hippocampi of both groups atrophied from baseline to follow-up, however those in the MBSR group had a trend towards less atrophy compared to controls (median [Q1, Q3] bilateral hippocampal volume difference from baseline to follow-up in MBSR vs. control, -32 mm3 [-179, 69] vs. -274 mm3 [-368, -137], p=0.07) and as seen in Figure 1B, which shows the individual and average (bolded line) hippocampal volume data between the 2 groups.
3.3 Measure of cognition
No significant changes were detected for median [Q1, Q3] change from baseline for MBSR vs. control for ADAS-cog (-0.5, [-4, 0.5] vs. 0 [-1, 2], p=0.46).
4. Discussion
In the first study to our knowledge reporting the impact of MBSR on fMRI among patients with MCI, we found that adults with MCI in the MBSR group had increased functional connectivity between the PCC and MPFC and left hippocampus and trends of less hippocampal atrophy in follow-up compared to controls. Given that the areas of the brain important for the DMN are specifically affected in AD and the hippocampus preferentially atrophies in AD, MBSR may affect the regions of the brain most sensitive to MCI and AD.
The DMN results from our study are consistent with recent research showing that meditation may increase certain connections within the DMN [8, 16, 20, 27], although research in this area is conflicting [3] and the exact role of the DMN in meditation may be more complex[13]. While it may be surprising to see hippocampal volume changes after an 8 week meditation intervention, Holzel and colleagues also showed structural changes in the hippocampus after a similar 8 week MBSR intervention [14]. In addition, our findings correspond to a similar 8 week pilot study of a meditation intervention in cognitively impaired adults that showed meditation increased cerebral blood flow in the prefrontal cortex [25]. Other studies have also shown meaningful neural changes after brief meditation interventions: one demonstrated that a mind-body training program improved white matter efficiency with diffusion tensor imaging after only 4 weeks [26], and another that showed measurable neuroplastic changes associated with pain reduction after only 4 days of meditation training [35].
Other research has also demonstrated that stress reducing non-pharmacological interventions, such as aerobic exercise, may be protective against the development of dementia, and the mechanisms may be through attenuation or reversal of hippocampal atrophy and improved DMN connectivity.[2, 6] Tai chi, another mind/body practice that similarly improves psychological well-being [30], may also create structural brain changes with specific increases in regional cortical thickness. [31] If some component of cognitive decline is a function of stress-induced hippocampal changes, then meditation may impact the hippocampus as a stress-reducing technique thereby improving cognitive reserve. As previously reported, this intervention showed non-significant trends of reduced stress and improved well-being in the adults with MCI who participated in MBSR, with improvements seen in the Resilience Scale, Perceived Stress Scale, Quality of Life-Alzheimer's Disease, Herth Hope Index, and Life-Orientation Test-Revised.[32] Through its impact on the DMN, meditation may improve self-related processing and emotional well-being in adults with MCI. Meditation may possibly change the resting state into a meditative experience [3], and thus the changes seen post-intervention reflect this new meditative resting state.
This study's main limitation is its small sample size and thus our results should be interpreted as preliminary. Our study was not powered to detect differences on measures of cognition, but we report elsewhere [32] that most data suggest trend towards improvement for measures of cognition and well-being. We do not have long-term follow-up to determine dementia progression. The intervention of MBSR involves factors beyond mindfulness meditation (e.g. social and intellectual engagement, a weekly time commitment and instructor attention) and since our usual care control group did not adequately control for these factors, the brain changes may be reflective of more than just mindfulness meditation. Nevertheless, the aim of this pilot study was to test the feasibility, effect size and variability of the treatment, and provide valuable preliminary data for the design of a larger sample size study. A separate study with a larger sample size will improve the reliability of the current study. Despite these limitations, the improved DMN functional connectivity and the trend of less atrophy in the MBSR group vs. control group are interesting given that these were our a priori hypotheses and we used non-parametric statistical analyses to account for our small sample size and adjust for potential outliers.
5. Conclusions
This small randomized neuroimaging trial reports on an innovative and novel “proof of concept” study that demonstrates the neural effects of a non-pharmacological intervention on a priori targeted brain networks in adults with MCI. This pilot study demonstrated that in adults most susceptible to the development of dementia, MBSR may reduce hippocampal atrophy and improve functional connectivity in the same areas of the brain most affected by the disease process. For a condition without a standard treatment and with potential progression to AD, this study provides preliminary evidence that an intervention with limited side effects may be of potential benefit to patients with few other options for improvement. Given the absence of therapies for this population, further studies with longer follow-up and larger sample sizes powered to detect clinical improvements are urgently needed.
Supplementary Material
Supplemental Figure: CONSORT flow diagram of eligibility assessment, exclusion, inclusion, and analysis.
Highlights.
*We conducted a randomized trial of meditation for Mild Cognitive Impairment (MCI)
*Meditation may increase functional connectivity in the default mode network in MCI
*Mediation may reduce hippocampal volume atrophy in MCI
*Meditation may have a positive impact on brain regions most related to dementia
*Further research with larger sample sizes and longer-follow-up are needed
Acknowledgments
This study was supported by the Harvard Medical School Osher Research Center, the Division of General Medicine and Primary Care at BIDMC and NIH National Center for Complementary and Alternative Medicine (NCCAM) K24 AT004095. In addition, this work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers).
We acknowledge the research support of: National Research Service Award Number T32AT000051 (REW); NIH NCCAM funding of: K24 AT004095 (TK), K24AT000589 (RSP), K01 AT003459 (CK), KO1AT003883 (JK), R21AT004497 (JK), R01AT006364 (JK); NIH NIDA funding of R03AT218317 (JK); NIH UL1 RR 02758 (RD). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
We gratefully acknowledge the assistance of Alexandra Cheetham, Neha Trivedi, and Drs. Peter Wayne, Maulik Purohit, Reisa Sperling, and David Eisenberg for their support and help in completing this project.
Abbreviations used, in order of appearance beginning with abstract
- MCI
Mild Cognitive Impairment
- AD
Alzheimer's Disease
- DMN
Default mode network
- MBSR
Mindfulness Based Stress Reduction
- MPFC
Medial prefrontal cortex
- PCC
Posterior cingulate
- BIDMC
Beth Israel Deaconess Medical Center
- ADAS-cog
Alzheimer's Disease Assessment Scale, cognitive subscale
- MMSE
Mini-Mental Status Exam
- CDR
Clinical Dementia Rating
- ROI
Region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- 1.Alzheimer's Disease Neuroimaging Initiative Protocol.
- 2.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A. 2011;108:20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Schmansky NJ, Greve DN, Salat DH, Buckner RL, Fischl B. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 8.Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen NK, McClernon FJ, Greeson JM, Sobin P. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond, Evidence-based complementary and alternative medicine : eCAM. 2012;2012:680407. doi: 10.1155/2012/680407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 10.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 12.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr., Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci. 2012;6:38. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang JH, Jung WH, Kang DH, Byun MS, Kwon SJ, Choi CH, Kwon JS. Increased default mode network connectivity associated with meditation. Neurosci Lett. 2011;487:358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients With Fibromyalgia Display Less Functional Connectivity In The Brain's Pain Inhibitory Network. Mol Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Random House; New York: 1990. [Google Scholar]
- 19.Kang DH, Jo HJ, Jung WH, Kim SH, Jung YH, Choi CH, Lee US, An SC, Jang JH, Kwon JS. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. Neuroimage. 2011;56:290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krygier JR, Heathers JA, Shahrestani S, Abbott M, Gross JJ, Kemp AH. Mindfulness meditation, well-being, and heart rate variability: A preliminary investigation into the impact of intensive Vipassana meditation. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013 doi: 10.1016/j.ijpsycho.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- 24.Luders E, Thompson PM, Kurth F, Hong JY, Phillips OR, Wang Y, Gutman BA, Chou YY, Narr KL, Toga AW. Global and regional alterations of hippocampal anatomy in long-term meditation practitioners. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: a preliminary study. J Alzheimers Dis. 2010;20:517–526. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- 26.Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci U S A. 2012;109:10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, Courtemanche J, Lavarenne AS, Marrelec G, Benali H, Beauregard M. Impact of meditation training on the default mode network during a restful state. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 29.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Bannuru R, Ramel J, Kupelnick B, Scott T, Schmid CH. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC complementary and alternative medicine. 2010;10:23. doi: 10.1186/1472-6882-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei GX, Xu T, Fan FM, Dong HM, Jiang LL, Li HJ, Yang Z, Luo J, Zuo XN. Can taichi reshape the brain? A brain morphometry study. PLoS One. 2013;8:e61038. doi: 10.1371/journal.pone.0061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells RE, Kerr CE, Wolkin J, Dossett M, Davis RB, Walsh J, Wall RB, Kong J, Kaptchuk T, Press D, Phillips RS, Yeh G. Meditation for adults with mild cognitive impairment: a pilot randomized trial. J Am Geriatr Soc. 2013;61:642–645. doi: 10.1111/jgs.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 35.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: CONSORT flow diagram of eligibility assessment, exclusion, inclusion, and analysis.