Abstract
The rate of biological aging is modulated in part by genes interacting with stressor exposures. Basic research has shown that exposure to short-term stress can strengthen cellular responses to stress (“hormetic stress”). Hormetic stress promotes longevity in part through enhanced activity of molecular chaperones and other defense mechanisms. In contrast, prolonged exposure to stress can overwhelm compensatory responses (“toxic stress”) and shorten lifespan. One key question is whether the stressors that are well understood in basic models of aging can help us understand psychological stressors and human health. The psychological stress response promotes regulatory changes important in aging (e.g., increases in stress hormones, inflammation, oxidative stress, insulin). The negative effects of severe stress are well documented in humans. Potential positive effects of acute stress (stress resistance) are less studied, especially at the cellular level. Can stress resistance slow the rate of aging in humans, as it does in model organisms? If so, how can we promote stress resistance in humans? We urge a new research agenda embracing the continuum from cellular stress to psychological stress, using basic and human research in tandem. This will require interdisciplinary novel approaches that hold much promise for understanding and intervening in human chronic disease.
Key Words: Aging, Stress resistance, Resilience, Stressors, Psychological stress.
The Link Between Stress Resistance and Aging: An Early Geroscience Topic
Geroscience is an interdisciplinary field emerging at the interface of the basic biology of aging and chronic disease. Many laboratories are now adapting their research strategy to take account of aging as a vital factor in the etiology of human disease. This shift has been driven by the many discoveries that suggest that aging mechanisms are tightly linked to disease mechanisms, with macromolecular damage and the ability to respond to damage being a critical link. More recently, commonalities have emerged between aging biology and stress biology. All living organisms experience chronological aging, but the rate of biological aging is fundamentally “elastic,” and modulated by genes, environmental context, and their interaction. For example, in invertebrates, mutations in single genes can dramatically extend lifespan in the laboratory. However, the magnitude of lifespan extension is highly dependent on the environment in which the animals are maintained. Most organisms are sensitive to the type and severity of threats to physical or social survival, naturally embedded in life experiences. Here, we propose that these threats can shape stress responses in long-term ways, sometimes across the lifespan, and consequently the rate of aging. The relationship between rate of aging and an animal’s response to stress is extremely complex (1). However, developing a mechanistic understanding of this relationship could provide new ways to understand and modulate age-related disease.
Stressors and Stress Responses
For cells in culture and simple invertebrates, environmental stressors include any factors that cause cell injury, such as heavy metals, radiation, heat exposure, reactive oxygen species, and osmotic fluctuation. Even in simple invertebrates, there is a continuum of related molecular, hormonal, and behavioral responses to such stressors that are essential to minimize damage, maintain health, and maximize the probability of future reproduction.
In higher level organisms, including humans, stressor exposure has further layers of complexity, including not just some of the physical exposures above, but also common social and psychological stressors. These engage the threat-related neural networks and the interconnected neurohormonal and immune patterns of central to peripheral stress response and related behavioral pathways. In organisms with more highly developed cognitive abilities, the capacity to learn about, imagine, remember, and anticipate stressful situations and their warning signs or loosely related cues can create chronic states of vigilant arousal in the body, even in the absence of observable stressors. On top of this universally conserved survival response, there are multiple individual differences that make some people more vulnerable to stress, where the identical stimuli causes exaggerated stress responses. Understanding this variance across humans is an active area of human stress research with the goal of isolating mechanisms of psychological stress resistance. These individual differences in turn might possibly be associated with hormesis.
The Stress-Aging Paradox: Hormetic Stress Versus Toxic Stress
In simple laboratory animals, stress can be either detrimental or beneficial in an aging context. On one hand, it is fairly clear that stressors that are chronic threats to survival, which have been called “toxic stress,” contribute to disease, and possibly to aging pathology (2). On the other hand, it is also true that limited or manageable stressors may result in physiological benefits, probably due to activation of stress responsive and metabolic alterations. This is commonly called “hormetic stress.” Clearly the duration and to a lesser extent, severity, of the stressor can determine whether it has salutary or damaging effects. Beyond these, there are few factors known to determine whether a stressor will be more hormetic or toxic.
Stress is an inevitable part of life, but a better understanding of how stressors modulate aging biology may allow us to intervene by increasing stress buffers. Stress and aging has been studied in limited contexts to date, almost entirely in laboratory model organisms such as fruit flies and nematode worms. The larger issue is whether the pathways to stress resistance and successful adaptation understood so far are relevant to human aging and if so, to what extent modulation of stress and stress responses could modify the course of human aging.
The Stress-Aging Gap: Model Organisms Versus Human Studies
One important question is whether the systemic responses to stress in humans (either stressful events such as daily hassles, time pressure, and social conflict or chronic stressors such as financial strain, job strain, caregiving, marital strain) have any relationship to the better understood cellular responses to environmental stressors (heat, nutritional, radiation, osmotic stress) commonly applied in laboratory animals that alter rate of aging. There is already a large and consistent human literature showing that chronic stress leads to disease processes, and aging is the biggest factor predicting early disease, so this idea is physiologically plausible. Nevertheless, few studies have focused on processes representing the biology of aging independent of disease. This is in part due to the difficulties of assessing pathways of aging in humans, when we are typically limited to sampling blood rather than other tissues and to observational studies. Thus, to the extent that the study of early disease is independent from the study of basic biology of aging, there is a large gap between basic and clinical research on aging and stress. To bridge this gap necessitates formation of a common knowledge base and taxonomy for describing stressors and stress responses. Such a dialogue can foster collaboration and sharing of methods across disciplines. The fusion of basic and clinical research on stress and aging is an area ripe for inquiry.
Stress Response Pathways and Aging
Basic Research
The deep relationship between stress and aging began to emerge in the early 1990s with studies on simple invertebrate models. It was known that mutations in single genes in the nematode Caenorhabditis elegans resulted in spectacular extensions in lifespan, and experiments were undertaken to understand the molecular basis of this enhanced longevity. Some of these early studies noted that long-lived mutant worms were resistant to oxidative and thermal stress even as young animals. Further studies revealed that these longevity mutants were also resistant to heavy metals, radiation, osmotic stress, and other environmental stressors. This “multiplex stress resistance” (1) seemed to arise from the upregulation of various stress response systems. For example, in C elegans, mutations in genes encoding components of the insulin-signaling pathway enhanced defenses against a broad range of stressors by upregulating the synthesis of molecular chaperones, antioxidant enzymes, and metal-binding proteins.
Molecular chaperones were of particular interest because of their role in promoting protein homeostasis (as further described in this issue (3)). It is now known that during aging, a large variety of cellular proteins accumulate in insoluble forms, indicating a dramatic failure of homeostasis. Mutations leading to stress resistance and longevity slow the accumulation of insoluble proteins. In addition, enhancing chaperone activity via activation of the transcription factor heat shock factor 1 (HSF-1) is sufficient to increase both stress resistance and lifespan in C elegans. Even in isogenic worm populations, there is a correlation between chaperone expression levels and lifespan (4).
Although it appears that enhanced protein homeostasis promotes stress resistance and longevity and provides one of the clearest mechanistic connections between stress and aging, other mechanisms may be equally important. These include oxidative stress and environmental toxins (key factors in some neurological diseases), telomere dysfunction or shortness (a common cause of cellular senescence in human aged tissues, promoting fertile ground for degenerative diseases and cancer), and inflammation (also a key feature of chronic disease).
In addition, there are clear examples of hormesis, where short-term exposure to a stressful event results in longer life (“what doesn’t kill you makes you stronger”). Thermal stressors for short periods of time can extend the lifespan of worms and flies. Metabolic stressors that result in an increase in reactive oxygen species also extend lifespan in some cases (5). It seems likely that the stresses themselves, although damaging (e.g., thermal stress causes widespread sterility in these invertebrates), induce stress response system activation resulting in gene expression changes and metabolic adjustments that promote survival. In the longer term and in the absence of further stresses, this response slows aging. This appears especially true for younger organisms. It’s not clear why this happens, but it may involve the reallocation of energy toward homeostasis, and away from growth or reproduction, thus delaying the aging process.
Mammals and Humans
The relationship between stress responses and lifespan, well demonstrated in simple invertebrates, is less clear in mammals. However, one striking example suggests some conservation of the relationship: fibroblasts cultured from long-lived Snell dwarf mice are very resistant to multiple forms of stress in the culture dish. There is also evidence that cells cultured from long-lived species are stress resistant (6). In addition, calorically restricted mice (and methionine-restricted mice) are resistant to many forms of stress including oxidative stress and long-lived Ames dwarf mice are paraquat resistant. Whether the stress resistance contributes to the longevity of these mice is not yet clear.
In animals including humans, it has been shown that psychological stress changes limbic and prefrontal cortex areas that regulate emotional health and cognitive function. In humans, psychological “stress resistance” in the face of adversity is thought to be promoted by a combination of stress resistant genotypes, temperament and coping behavior, and resources/stress buffers, which might promote greater cell longevity in some cases. However, beyond suggestive correlations, it is not known whether psychological stress resistance, often described as” resilience,” is associated with resistance to multiple stressors at the cellular level or if the terms are merely related conceptually but not mechanistically. This is an empirical question that could be addressed in short-term studies. The larger question of whether psychological stress resilience is linked to health and longevity, in humans, is difficult to study in the current medical paradigm which uses disease risk as outcomes. There are few measures of health, beyond the absence of disease. There have however been studies demonstrating “positive stress”—how certain profiles of acute stress responses are related to better physiological health or better cognitive and behavioral performance (7,8).
In contrast, toxic stress has been well mapped, because of its link with disease pathways. Chronic psychological stress promotes changes in the autonomic, neuroendocrine, metabolic, and immune system, creating a dramatically different biochemical milieu than that of organisms not exposed to stress (2). These changes might affect aging biology and diseases of aging. Although experimental studies on animals have shown the effects of psychological stress on early disease (9,10), there is now converging evidence from multiple population-based studies across different countries that chronic adversity (e.g., job strain, feelings of stress or isolation, childhood adversity) is linked to physiological wear and tear on the stress responsive regulatory systems, promoting multisystem dysregulation, early disease or mortality (2,11–13). A dramatic example of stress effects on later life health is prenatal metabolic or psychological stress effects on offspring, through developmental programming pathways, including calibration of the hypothalamic–pituitary–adrenal axis. The myriad early changes during development predict early cardiovascular and metabolic diseases (14,15). It is unclear how prenatal programming affects basic cellular mechanisms of aging.
Given the pervasiveness and chronicity of stress in modern life, it is highly feasible that differences in emotional and behavioral stress responses may, in part, determine rates of biological aging, not just age-related disease onset. Although it is harder to make inferences in humans without oversimplifying and ignoring complex network systems, it has been illuminating to look at blood-based markers—systemic inflammation, telomere length, and patterns of gene expression. There is some evidence that chronic psychosocial stress predicts indices of biological aging, and this is particularly true when the stressors occur in childhood. For example, early childhood adversity or trauma (abuse, neglect, exposure to violence) is associated with greater levels of inflammation (12), telomere shortness or shortening (16), and a pattern of gene expression regulated by inflammatory signaling (17).
There are some obvious obstacles to examining aging processes in humans—mainly the difficulty in examining the relevant tissues such as stem cells in vivo. The immune cell has been overused within the spectrum of available readouts of human aging status but has been helpful. Telomere length is one of the cellular aging systems that has now been well studied. Telomere length of all leukocytes (or in some studies, specific subsets) may crudely represent stages of replicative senescence, reflect hematopoietic stem cell reserves, or be an index of cumulative exposures to stress mediators (inflammation, stress hormones, insulin resistance). There are now convergent findings, across clinical and large population-based samples, that various aspects of stress (stressor exposure, duration, and suffering – depression, trauma, anxiety) are associated with leukocyte telomere shortness (18). This may be due in part to telomeres’ high sensitivity to multiple types of biochemical stress. There is some early evidence that the initial setting of telomere length at birth may be affected by prenatal stress, although this is a very new area of inquiry (16).
A better ability to assess biomarkers that represent aspects of cellular aging in humans, in multiple tissues, including stem cells and progenitor cells in key areas such as the hippocampus, may help us understand how cellular stress resistance may be fostered by psychological resilience or impaired by toxic stress and if this in turn predicts organismal disease resistance.
Future Questions
As described above, there are both basic model and human examples that show the importance of stress resistance and stress-induced damage in regulating aging and disease. The potential for leveraging this relationship to enhance human health is uncertain, but highly attractive, because stress is modifiable both in the environment and in individuals’ responses. Several key questions need to be addressed to fill in the gaps described above to help clarify the hypothesized stress-longevity nexus, and lead us to the most feasible and promising interventions.
Under What Conditions Is Stress Linked to Disease Processes?
What kinds of stress are linked to aging biology or specific disease processes? What cellular and neuroendocrine changes mediate these links? Can studies of stress biology in small organisms contribute meaningfully to our understanding of the relationship between social-psychological stress and human disease risk? Clearly the type and duration of the stressor is an important determinant of hormesis versus damage. For example, acute psychological stress can enhance cell-mediated immune responses, whereas chronic exposure can dampen it in rodents (19). In animal models, it may be helpful to identify what drives the switch between enhancing versus damaging effects. Further, what are the different effects during the developmental period, early in life, versus in aged organisms? This could move the field toward a finer grained analysis of “stress” so as to better understand good versus bad stress, at which developmental periods stress can have the most salutary versus damaging effects, and the inflection point at which a stressor becomes toxic and overwhelming. Identification of the molecular players involved in this switch is a major gap in the field.
In humans, this question becomes more complex, given the vast range of stressor exposures and types of stress responses. We rely on naturalistic exposures to chronic stressors, and responses range from depression and disease to psychological resiliency. Social factors, such as secure attachment, higher educational attainment, and social support, are already known to have stress-buffering and salutary effects and cannot be ignored in longevity research.
The study of biological predispositions to respond in particular ways under stress is a new frontier. The range of human response to common stressors in part depends on genotype, as shown by recent studies focusing on cell aging mechanisms (20). Although these are relatively small effects, they will contribute to our ability to focus treatments and tailor them, as part of the new movement toward Precision Medicine. Next steps include understanding the range of genotypes, and gene–gene interactions, and how these protect an individual from an adverse environment, as well as Gene × Environment interactions. Subsequently, identifying those most vulnerable and shaping the environment to be most conducive to health and resiliency will need to be major foci for antiaging interventions.
How Do Stress Exposure and the Cellular Response to Stress Regulate Aging? What Experimental Models May Be Useful in Defining the Impact of Stress at a Molecular and Cellular Level?
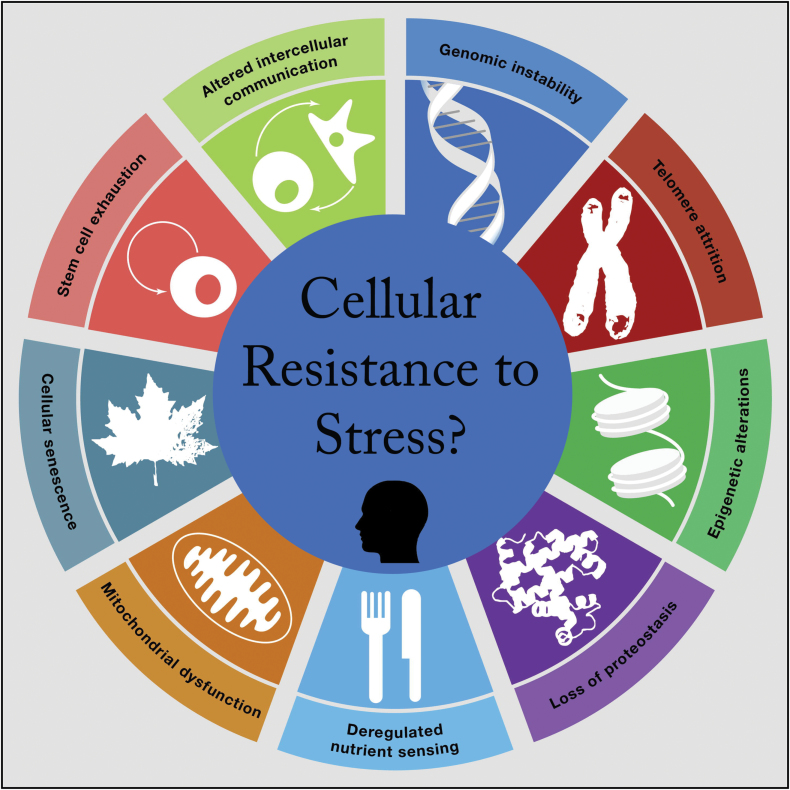
Caloric restriction and GH/IGF-1 deficiency lead to longevity in lower species through certain pathways that change energy metabolism and removal of toxins and can improve response to physiological stressors. None of these studies have examined psychological stressors. There are still fundamental questions in basic research, such as how to understand the stress resistance to longevity relationship mechanistically and what aspects of the relationship might be most open to intervention (21). Of the nine hallmarks of aging recently suggested by López-Otín and coworkers (see Figure 1) (22), some have already been linked to the stress response (3). The pathways in animal studies that have been examined so far include loss of proteostasis and epigenetic changes in specific loci in the brain. In humans, relations have been examined between psychological stress with genomic instability (DNA damage), telomere attrition, and certain patterns of gene expression.
Figure 1.
Stress resistance may be central to the Hallmarks of aging: Does this extend to humans? (adapted with permission from Lopez-Otin et al. (22)).
Other pathways that have not yet been examined in the context of stress include altered intra- and intercellular communication, dysregulated nutrient sensing, mitochondrial health, cell senescence, and stem cell exhaustion. Testing links between these models of aging and stress exposure could provide insights. Examining actual stress response dysregulation or resistance to stress, and how that is causally tied to early cellular events in aging, may be a highly productive line of inquiry. Basic research could examine intracellular signaling, gene expression, and epigenetic changes during “hormetic” and “toxic stress” in basic studies. Human research could examine indices of these aging pathways in disease-free people who have been exposed to high cumulative stress versus those with less exposure.
Can We Harness the Mechanisms of Successful Adaptation to Stress to Slow Aging and Prevent Age-Related Diseases?
If aging is the sum of loss in homeostatic mechanisms in a variety of tissues, then it is not surprising that environmental fluctuations and stressful events should dramatically affect the course of aging
With all this in mind, there have been efforts to find ways to activate endogenous stress response systems without incurring the damaging effects of the stress. It is not yet clear that this can be achieved, because some stress-related damage results from the stress response system itself. However, some small molecules have been shown to promote protein homeostasis partly by inducing aspects of the stress response system. These small molecules also extend lifespan in invertebrates and testing in mammals is underway. Caloric restriction may also promote longevity in part through this stress resistance pathway, although even this relationship is controversial. Caloric restriction does not appear feasible in the general human population, but restriction in rodent systems may provide answers about the intimate connections between nutrition, metabolism, and longevity.
There are some obvious drivers of aging in humans, such as overeating unhealthy food. Rather than the current epidemic of eating excessive empty calories, we need to increase nutrition with reduced calories for healthy longevity or “healthspan.” Although a deeper understanding of aging biology might provide pharmacological interventions, they may be of limited value since people will keep engaging in “pro-aging behavior” like overeating given the toxic food environment. Thus, the basic science of behavior change coupled with the necessary environmental changes, might provide inroads to prevent people from overeating in the first place, and thus might be thought of as another “antiaging” basic science.
How Does Exercise Affect Stress and Stress-Related Diseases?
Exercise is one of the most promising interventions for humans, as it promotes stress resistance in several ways. Animal studies show exercise promotes enhanced telomerase activity, autophagy, and mitochondrial biogenesis, linking metabolic pathways with disease resistance (23). There is also evidence that certain forms of resistance training results in reversal of age-related gene expression trajectories. In humans, there are clear well-known health gains from moderate exercise, systemic benefits across aging related systems, including improved insulin sensitivity and better cognitive function. Many forms of exercise are of course metabolically stressful, resulting in the production of oxygen radicals. Recent reports in the literature suggest that early production of such free radicals might in fact be important for the organism to realize the positive effects of exercise, again suggesting that limited stress might be beneficial. Although the mechanisms of exercise-induced health are only beginning to be elucidated, it is likely that hormesis is at play.
How Do Changes in Gene Expression in Response to Stress Regulate Aging?
Given the increasingly accessible ability to examine whole-genome expression profiles, examining gene expression in states of stress has uncovered several important patterns. Chronic states of adversity tend to be associated with greater expression of genes that are regulated by proinflammatory states, mainly by the transcription factor NF-κb, and in some cases, a downregulation of genes regulated by glucocorticoids (24). Stress suppresses expression of type 1 interferons, which both contribute to vulnerability to infection, and have been linked to longevity (17).
The ability to examine gene expression patterns in large human samples undergoing the same types of adversity may help identify common gene expression pathways as well as specific signaling pathways. This opens up doors to identifying specific signaling pathways that may operate as aging mechanisms. In addition to the traditional line of basic research examining one pathway at a time, through knockout models for example, there is a need to examine a wider range of conditions more relevant to human aging, such as the very common co-occurrence of chronic psychological stress in the context of diet-induced obesity or at least subclinical insulin resistance. Currently, there is no widely agreed upon profile of how the genome responds to “stress.” A more precise taxonomy of stress may be helpful.
Summary
Basic research has revealed pathways for stress resistance, the enhanced adaptation to stress that retards aging. Such stress resistance may be at the heart of many mechanisms of aging, as suggested in Figure 1. Readers are referred elsewhere for detailed reviews of stress resistance and relevance to aging and health (21,25–27). Despite the significance of this finding, there is a large gap between basic and human research in stress and aging. Basic aging research has rarely examined stressors relevant to humans, such as the common co-occurrence of high psychological stress and excess calories. Human research has only just begun to examine the basic pathways of cellular aging. It has rarely examined measures of enhanced adaptation to stress, which might reveal human analogs to “hormetic stress” and “multiplex stress resistance.” It is simply unknown how stress resistance might be applicable to or promotable in humans.
Toxic stress has been better studied. The question of whether physiological stressors such as heavy metals affect human health is not in question. But the problem of ubiquitous chronic psychological stress is a serious problem for human health, as it has been shown to precede and hasten early disease and mortality. How does toxic stress affect the basic biology of aging? If stress pathways are an inherent part of aging, this suggests that some portion of age-related disease and dysfunction is not inevitable but may be modifiable through stress reduction (societal wide and individual). We cannot eliminate all stress. And in fact that is not desirable because with moderate stress comes survival behavior, motivation, and positive striving. It is essential that major societal attempts are focused on making widespread toxic stress more manageable, and this in turn is a promising way to slow the various aspects of aging on a population level.
Translating what we have learned from simple animals to humans, including an examination of pathways along a continuum from cellular to psychological levels of stress, is daunting and will require an interdisciplinary approach. Nonetheless, this is the promise of the emerging field of geroscience and represents a new approach to understanding and intervening in human chronic disease.
Funding
E.S.E. is supported by National Institute of Aging R01 AG0333592-01A1, R01 AG030424-01A2, R01 HL 108821-01, and National Institute of Mental Health R01 MH083784-01A2. G.J.L. is supported by the Larry L. Hillblom Foundation grant , the Glenn Foundation for Medical Research, and National Institutes of Health grant AG042053-02.
Acknowledgments
The authors gratefully acknowledge Richard Miller, Felipe Sierra, and the Geroscience Summit Planning Committee for providing feedback. E.S.E. was a co-founder of Telome Health, Inc.
References
- 1. Lithgow GJ, Miller RA. Determination of aging rate by coordinated resistance to multiple forms of stress. In: Guarente L, Partridge L, Wallace DC, eds. Molecular Biology of Aging. New York, NY: Cold Spring Harbor Laboratory Press; 2008;427–481. 10.1101/087969824.51.427 [Google Scholar]
- 2. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(suppl 2):17180–17185. 10.1073/pnas.1121254109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morimoto RI, Cuervo AM. Proteostasis and aging: Five key questions. J Gerontol. In press.
- 4. Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans . Nat Genet. 2005;37(8):894–898. 10.1038/ng1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92(16):7540–7544. 10.1073/pnas.92.16.7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6(1):1–13. 10.1111/j. 1474-9726.2006.00255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aschbacher K, O’, Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38(9):1698–1708. 10.1016/j.psyneuen.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamieson JP, Nock MK, Mendes WB. Mind over matter: reappraising arousal improves cardiovascular and cognitive responses to stress. J Exp Psychol Gen. 2012;141(3):417–422. 10.1037/a0025719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clin Dermatol. 2013;31(1):18–30. 10.1016/j.clindermatol.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 10. Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73(9):724–730. 10.1097/PSY.0b013e318235be76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens). 2009;8(1):7–22 [DOI] [PubMed] [Google Scholar]
- 12. Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 13. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. 10.1371/journal.pmed.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36(5):445–458. 10.1080/03014460902980295 [DOI] [PubMed] [Google Scholar]
- 15. Entringer S, Wadhwa PD. Developmental programming of obesity and metabolic dysfunction: role of prenatal stress and stress biology. Nestle Nutr Inst Workshop Ser. 2013;74:107–120. 10.1159/000348454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. 10.1016/j.biopsych.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole SW. Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health. 2013;103(suppl 1):S84–S92. 10.2105/AJPH.2012.301183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blackburn EH, Epel ES. Telomeres and adversity: too toxic to ignore. Nature. 2012;490(7419):169–171. 10.1038/490169a [DOI] [PubMed] [Google Scholar]
- 19. Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. 10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- 20. Mitchell C, Hobcraft J, McLanahan S. et al. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci USA. 2014. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 21. Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009;64(2):179–182. 10.1093/gerona/gln072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2011;300(1):E3–10. 10.1152/ajpendo.00512.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slavich GM, Cole SW. The Emerging Field of Human Social Genomics. Clin Psychol Sci. 2013;1(3):331–348. 10.1177/2167702613478594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rattan SI. Targeting the age-related occurrence, removal, and accumulation of molecular damage by hormesis. Ann N Y Acad Sci. 2010;1197:28–32. 10.1111/j.1749-6632.2010.05193.x [DOI] [PubMed] [Google Scholar]
- 26. Demirovic D, Rattan SI. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013;48(1):94–98. 10.1016/j.exger.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 27. LeBourg E. Hormesis, aging, and longevity. Biochim Biophys Acta. 2009;1790(10):1030–1039. 10.1016/j.bbagen.2009.01.004 [DOI] [PubMed] [Google Scholar]