Abstract
The herpes virus entry mediator (HVEM) is a member of the tumor necrosis factor receptor superfamily (TNFRSF), and therefore it is also known as TNFRSF14 or CD270 (1,2). In recent years, we have focused on understanding HVEM function in the mucosa of the intestine, particularly on the role of HVEM in colitis pathogenesis, host defense and regulation of the microbiota (2,3,4). HVEM is an unusual TNF receptor because of its high expression levels in the gut epithelium, its capacity to bind ligands that are not members of the TNF super family, including immunoglobulin (Ig) superfamily members BTLA and CD160, and its bi-directional functionality, acting as a signaling receptor or as a ligand for the receptor BTLA. Clinically, Hvem recently was reported as an inflammatory bowel disease (IBD) risk gene as a result of genome wide association studies (5,6). This suggests HVEM could have a regulatory role influencing the regulation of epithelial barrier, host defense and the microbiota. Consistent with this, using mouse models, we have revealed how HVEM is involved in colitis pathogenesis, mucosal host defense and epithelial immunity (3,7). Although further studies are needed, our results provide the fundamental basis for understanding why Hvem is an IBD risk gene, and they confirm that HVEM is a mucosal gatekeeper with multiple regulatory functions in the mucosa.
Keywords: Mucosal immune response, Host defense, TNF, TNF receptor, Inflammation, Epithelial immunity, Innate immunity
HVEM REGULATES COLITIS PATHOGENESIS
HVEM originally was described as a negative regulator in T cells (8), because Hvem-/- T cells were over reactive in vitro exhibiting increased cytokine production. Additionally, Hvem-/- mice were reported to be more susceptible to concanavalin A (ConA)-induced hepatitis and to experimental autoimmune encephalomyelitis (EAE) (8). However, using an animal model of colitis induced by the transfer of CD4+ CD45RBhigh T cells to immune deficient Rag1-/- mice, we and others found that host mice receiving Hvem-/- T cells had reduced colitis induction and pathogenesis (Table I) (7,9). Therefore, in this model, the function of HVEM in T lymphocytes appears to be pro-inflammatory or co-stimulatory for the T-cell response. Although seemingly contradictory, these inconsistent results could be partially explained by the different functions of HVEM in various immune cell types. In ConA-induced hepatitis and EAE, germline Hvem-/- mice were used such that the phenotype could not be solely ascribed to the function of HVEM in T cells. Instead, the overall phenotype was likely the outcome of different HVEM functions (both co-stimulatory and co-inhibitory) in various cell types involved in these two disease models. In contrast, in the T-cell transfer model of colitis, the genotypes of the donor T lymphocytes and the Rag1-/- recipients can be varied to determine the effect of a genetic variation specifically in T cells. Using this model, we and others found Hvem-/- T cells did not expand as efficiently in vivo as wild-type T cells after transfer (7,9), implicating intrinsic HVEM signaling in T lymphocytes as a requirement for in vivo T-cell expansion, and subsequent colitis pathogenesis. There is also a cell intrinsic role for HVEM signals in CD8+ T lymphocytes following exposure to viral or bacterial infections (Table I) (10,11). Although the initial expansion of CD8+ T cells was not affected by the absence of HVEM, long term survival of the activated cells and the generation of systemic and mucosal memory were impaired.
Table I.
Multiple roles of HVEM in the mucosal immune response
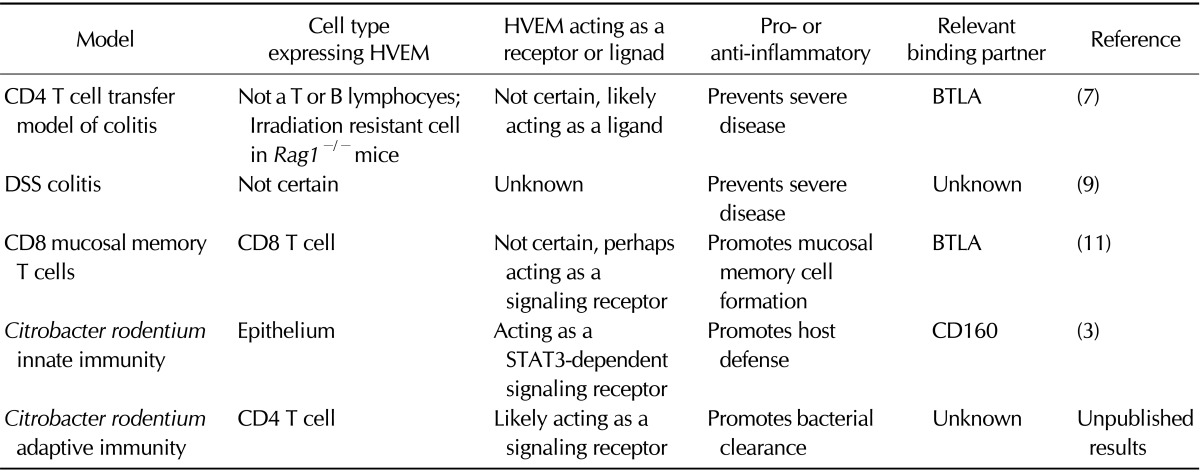
As a reciprocal experiment in the T-cell transfer model of colitis, we made the unexpected observation that the absence of HVEM expression in Rag1-/- hosts led to accelerated and exacerbated disease (7). This striking phenotype revealed for the first time that HVEM signaling in cells other than T or B cells is essential for colitis pathogenesis and likely has an important anti-inflammatory role. To gain further insight into the cell type expressing HVEM that prevents severe colitis, we analyzed recipient mice that were reciprocal Rag-/- bone marrow chimeras. These included mice with Rag-/- bone marrow transferred to irradiated Rag-/- Hvem-/- hosts, as well as the opposite. In mice with Rag-/- bone marrow transferred to irradiated Rag-/- Hvem-/- hosts, disease following T cell transfer was accelerated and more severe, indicating that HVEM expression in an irradiation resistant cell type is responsible for preventing severe colitis. Together, these experiments established that HVEM signaling in T lymphocytes as well as in other cells have opposing effects on colitis pathogenesis.
Consistent with our observation that the effects of HVEM deficiency on intestinal inflammation are cell type-specific, Schaer et al. also showed reduced inflammation when HVEM was not expressed in two colitis models: acute administration of dextran sodium sulfate (DSS) and the T-cell transfer colitis model using Hvem-/- T lymphocytes as donor cells (Table I) (9). They further demonstrated that HVEM expression was required for the expansion and differentiation of CD4+ T cells during intestinal inflammation. The effect of HVEM deficiency on donor T cells was more pronounced than what was observed in our studies, which is likely due to differences in the genetic background of the mice as well as environmental differences between mouse colonies, including the intestinal microbiota. Regardless, their conclusion is consistent with our finding that HVEM is co-stimulatory or pro-inflammatory in T cells and therefore could promote inflammation. This conclusion is consistent with other observations demonstrating that HVEM-mediates NF-κB activation, which likely provides a survival signal for T lymphocyte in vivo such that intrinsic HVEM signaling is required for pathogenic T cells to expand and mediate inflammation.
HVEM MEDIATES MUCOSAL HOST DEFENSE
Our observations in the T-cell transfer colitis model prompted us to further examine the function of HVEM in the intestinal mucosa in fully immune competent mice. Oral infection with Citrobacter rodentium provides a mouse model for acute attaching/effacing enteropathogenic E. coli infection in humans. Infection of wild type mice leads to a well-characterized innate immune response by the epithelium and IL-22 secretion by hematopoietic cells (12,13). The subsequent adaptive immune responses include Th17 cells and the secretion of protective immunoglobulins (Igs). An attractive feature of this model is that lethality and bacterial burdens correlate well with the innate response, while colon pathology and bacterial clearance correlate well with adaptive response. Therefore, the two well-characterized phases during infection facilitate the analysis of both innate and adaptive protective immune responses in a physiological context.
Our earlier finding that the lack of HVEM expression in an irradiation resistant cell type accelerated T-cell transfer-mediated colitis in Hvem-/-Rag1-/- recipients (7), prompted us to explore the role of HVEM in intestinal epithelial cells. Significantly, Hvem mRNA is abundantly expressed by intestinal epithelium, compare to most other TNF receptor family members (preliminary results). Epithelial cells constitute the intestinal barrier whose function is essential for preventing colitis pathogenesis and defending against mucosal infections. We found that Hvem-/- mice showed higher bacterial burdens in colons and feces, increased inflammation, increased bacterial dissemination and reduced survival after C. rodentium infection by oral gavage (Table I) (3). We correlated these phenotypes with a reduction in epithelial innate immunity in the absence of HVEM expression, including decreased production by epithelial cells of anti-microbial peptides, chemokines and pro-inflammatory cytokines. Although the context is different, our results that HVEM expression by intestinal epithelium provides anti-inflammatory and anti-bacterial protection during C. rodentium infection correlates well with the role of HVEM expressed by an irradiation resistant cell type in Hvem-/-Rag1-/- recipients, which is required for preventing accelerated T-cell transfer colitis. Recently, we were able to confirm the importance specifically of epithelial expression of HVEM by infecting mice with an epitheliumspecific HVEM deletion with C. rodentium (preliminary results). We also found Hvem-/- mice had a diminished Th17 cell response in the lamina propria leading to impaired bacteria clearance during C. rodentium infection (preliminary results, Table I). Together, these results provide strong evidence that HVEM expression by epithelium is protective and important for host defense in the mucosa, while HVEM expression by T cells is co-stimulatory or essential for T-cell differentiation and expansion.
HVEM SIGNALING REGULATES EPITHELIUM AND Th17 CELLS IN THE MUCOSA
Because HVEM plays such an essential role in epithelial innate immunity during mucosal infection, we investigated the mechanism of HVEM signaling in epithelial cells. Indeed, either in a mouse colonic epithelial cell line, or in colonic fragment cultures, stimulation with soluble HVEM binding partners, including either BTLA-Ig or recombinant CD160, induced the activation of STAT3 (Table I) (3). Epithelial STAT3 activation promotes the expression of genes important for mucosal immunity and host defense in the intestine (14), such as the gene encoding Reg3γ, an anti-microbial C-type lectin. The anti-microbial response induced by epithelial STAT3 activation provides essential host defense against mucosal bacterial infection including C. rodentium, as evidenced by the ability of Reg3γ-Ig to rescue the lethality in Hvem-/- mice resulting from C. rodentium infection (3). Consistent with this, deletion of STAT3 specifically in epithelium leads to impaired Reg3β/γ expression and increased susceptibility to C. rodentium infection (unpublished results). Together, these results revealed a novel HVEM-STAT3 signaling pathway in the epithelium, which is similar to the epithelial IL-22R-STAT3 pathway. IL-22 interaction with its receptor previously was shown to be essential for epithelial immunity and host protection from C. rodentium (13). We further determined that HVEM-STAT3 signaling pathway, but not IL-22R-STAT3, is dependent on the NF-κB inducing kinase (NIK), suggesting that these two pathways are independent but likely to collaborate to provide optimal STAT3 activation (3). Moreover, the HVEM-STAT3 signaling pathway in epithelium is distinct from the various types of NF-κB signaling mediated by TNF super family receptors, including HVEM.
Signals that activate STAT3, including IL-6, IL-21, and IL-23, are important drivers of Th17 cell differentiation, a component of adaptive immunity which is required for anti-bacterial/fungal immunity. Patients with Hyper-IgE syndrome (HIES), characterized by devastating susceptibility to infections, have dominant-negative mutations of the Stat3 gene and impaired Th17 cell differentiation (15). Importantly, genome-wide association studies (GWAS) have identified not only Hvem, but also Il-23r and Stat3 as genes linked to increased susceptibility to IBD (6). Therefore, an important conclusion from our findings is that STAT3 signaling links HVEM not only to epithelial innate immunity but also to the adaptive Th17 response, both are essential for mucosal host defense and colitis pathogenesis.
During C. rodentium infection, Hvem-/- mice showed a diminished Th17 response in the lamina propria, resulting in impaired bacterial clearance (unpublished results, Table I). This is consistent with our result that HVEM co-stimulation promotes the differentiation of naive T cells cultured under Th17 conditions in vitro (unpublished results). As in the response of Hvem-/- T cells in the models of DSS or T-cell transfer colitis, intrinsic HVEM signaling in T lymphocytes, including Th17 cells, might be critical for differentiation and expansion during the immune response in vivo. We are currently testing the response of T-cell specific HVEM conditional knockout mice in the context of various models of mucosal infection or inflammation to confirm the role of HVEM in T cells during the immune response in vivo.
FUNCTIONAL LIGANDS FOR EPITHELIAL HVEM IN THE MUCOSA
An important question regarding the role of HVEM in epithelial innate immunity is the identity of the binding partner that engages epithelial HVEM and the cell type that provides this molecule. HVEM binding to any one of its three ligands, which include TNF superfamily member 14, also known as Lymphotoxin, shows Inducible expression and competes with herpes simplex virus Glycoprotein D for Herpes virus entry mediator, a receptor expressed by T lymphocytes (LIGHT), BTLA, CD160, leads to different biological outcomes, complicated by the possibility of bi-directional signaling (1). For example, as a ligand, HVEM binding to LIGHT on T cells leads to LIGHT-mediated co-stimulation, while HVEM binding to BTLA or CD160 on T lymphocytes leads to BTLA/CD160-mediated co-inhibition. As a receptor, HVEM can be engaged by either LIGHT, BTLA or CD160, leading to NF-κB activation regardless of the ligand.
During mucosal C. rodentium infection, we found that only Hvem-/- mice were more susceptible to infection, but not Light-/-, Btla-/- or Light-/-Btla-/- mice (3). Interestingly, wild-type mice injected with an anti-CD160 antibody, shown to block the HVEM-CD160 interaction, reproduced the phenotype of Hvem-/- mice in C. rodentium infection in terms of reduced survival, innate immunity and STAT3 phosphorylation. These results provide evidence that CD160 is the non-redundant ligand engaging HVEM in epithelium during host defense against bacterial infection (Table I). We found that CD160 is mostly expressed by CD8αα+ intraepithelial lymphocytes (IEL) while mRNA levels of Light and Btla were low in the IEL compartment (3). Therefore, given their predominant expression of CD160 and the location of IEL in close contact with epithelial cells, it is logical that CD160-mediated signaling of HVEM in epithelial cells is important for host defense. In addition, the population of CD160-expressing CD8αα+ IEL rapidly accumulated at the early stage of C. rodentium infection (3). While one important function of these CD160-expressing IEL is to engage epithelial HVEM for boosting barrier immunity, we still do not fully understand the biological functions of IEL during mucosal infection. Furthermore, it still has not been determined if the IEL-epithelial signaling is bidirectional, with HVEM-mediated CD160 engagement in IEL inducing any biological functions. Whether the HVEM-CD160 interaction is regulated at steady state is currently unknown. It also remains to be determined if there is a continual level of HVEM-STAT3 activation, triggered by CD160 in IEL, for maintaining epithelial innate immunity, which is boosted by IL-22R mediated STAT3 activation after infection, or if the CD160-HVEM interaction is likewise induced. Although the number of CD160 expressing IEL does increase after infection, our unpublished data indicate that the expression level of either CD160 or HVEM does not increase after infection. Furthermore, if such persistent CD160-HVEM signaling occurs, how might this interaction influence the commensal microflora?
SIGNIFICANCE AND RELEVANCE
Our novel findings have revealed new insights about the TNF receptor HVEM. Overall, our laboratory has established an essential role for HVEM in reducing colitis pathogenesis and in enhancing mucosal host defense. HVEM-mediated signals guard at mucosal surfaces against pathogenic bacteria, and HVEM helps to maintain mucosal immune homeostasis, either at steady state or during inflammation and pathogenesis. Furthermore, the mechanism of HVEM-mediated regulation via STAT3 signaling in the mucosa provides a fundamental basis for understanding why Hvem might be a potential risk gene for IBD pathogenesis and also why HVEM could be a promising therapeutic target for boosting host defense and regulating inflammation.
ACKNOWLEDGEMENTS
Supported by grants from NIH RO1 AI061516 (M.K.), PO1 DK46763 (M.K.), NIH F32-DK082249 (J.-W. S.), La Jolla Institute for Allergy and Immunology, Center for Infectious Disease, LIAI-JAN-2011-CID (J.-W. S.) and Career Development Award, Crohn's & Colitis Foundation of America, CCFA-295135 (J.-W. S.).
Abbreviations
- HVEM
herpes virus entry mediator
- TNFSF
tumor necrosis factor super family
- TNFRSF
tumor necrosis factor receptor super family
- Ig
immunoglobulin
- IBD
inflammatory bowel disease
- BTLA
B- and T-lymphocyte attenuator
- LIGHT
lymphotoxin, shows inducible expression and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes
- DSS
dextran sodium sulfate
- EAE
experimental autoimmune encephalomyelitis
- STAT3
signal transducer and activator of transcription 3
- IEL
intraepithelial lymphocyte
- NIK
NF-κB inducing kinase
Footnotes
The authors have no financial conflict of interest.
References
- 1.Murphy L, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 2.Shui JW, Kronenberg M. HVEM: An unusual TNF receptor family member important for mucosal innate immune responses to microbes. Gut Microbes. 2013;4:146–151. doi: 10.4161/gmic.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg MW, Shui JW, Ware CF, Kronenberg M. Regulating the mucosal immune system: the contrasting roles of LIGHT, HVEM, and their various partners. Semin Immunopathol. 2009;31:207–221. doi: 10.1007/s00281-009-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, Murphy KM, Ware CF, Kronenberg M. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205:1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang J, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaer C, Hiltbrunner S, Ernst B, Mueller C, Kurrer M, Kopf M, Harris NL. HVEM signalling promotes colitis. PLoS One. 2011;6:e18495. doi: 10.1371/journal.pone.0018495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn R, Hutchinson T, Murphy KM, Ware CF, Croft M, Salek-Ardakani S. CD8 T cell memory to a viral pathogen requires trans cosignaling between HVEM and BTLA. PLoS One. 2013;8:e77991. doi: 10.1371/journal.pone.0077991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg MW, Huang Y, Wang-Zhu Y, Ware CF, Cheroutre H, Kronenberg M. BTLA interaction with HVEM expressed on CD8(+) T cells promotes survival and memory generation in response to a bacterial infection. PLoS One. 2013;8:e77992. doi: 10.1371/journal.pone.0077992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 14.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]


