Abstract
Polyclonal antibodies were prepared against synthetic peptides corresponding to four different extramembrane segments of the rat glucagon receptor. The antibodies bound specifically to native glucagon receptor as judged by immunofluorescence microscopy of cultured cells expressing a synthetic gene for the receptor. Antibodies to peptides designated PR-15 and DK-12 were directed against amino acid residues 103-117 and 126-137, respectively, of the extracellular N-terminal tail. Antibody to peptide KD-14 was directed against residues 206-219 of the first extracellular loop, and antibody to peptide ST-18, against the intracellular C-terminal tail, residues 468-485. The DK-12 and KD-14 antibodies, but not the PR-15 and ST-18 antibodies, could effectively block binding of 125I-labeled glucagon to its receptor in liver membranes. Incubation of these antibodies with rat liver membranes resulted in both a decrease in the maximal hormonal binding capacity and an apparent decrease in glucagon affinity for its receptor. These effects were abolished in the presence of excess specific peptide antigen. In addition, DK-12 and KD-14 antibodies, but not PR-15 and ST-18 antibodies, interfered with glucagon-induced adenylyl cyclase activation in rat liver membranes and behaved as functional glucagon antagonists. These results demonstrate that DK-12 and KD-14 antibodies are pharmacologically active glucagon antagonists and strongly suggest that residues 126-137 of the N-terminal tail and residues 206-219 of the first extracellular loop contain determinants of ligand binding and may comprise the primary ligand-binding site on the glucagon receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
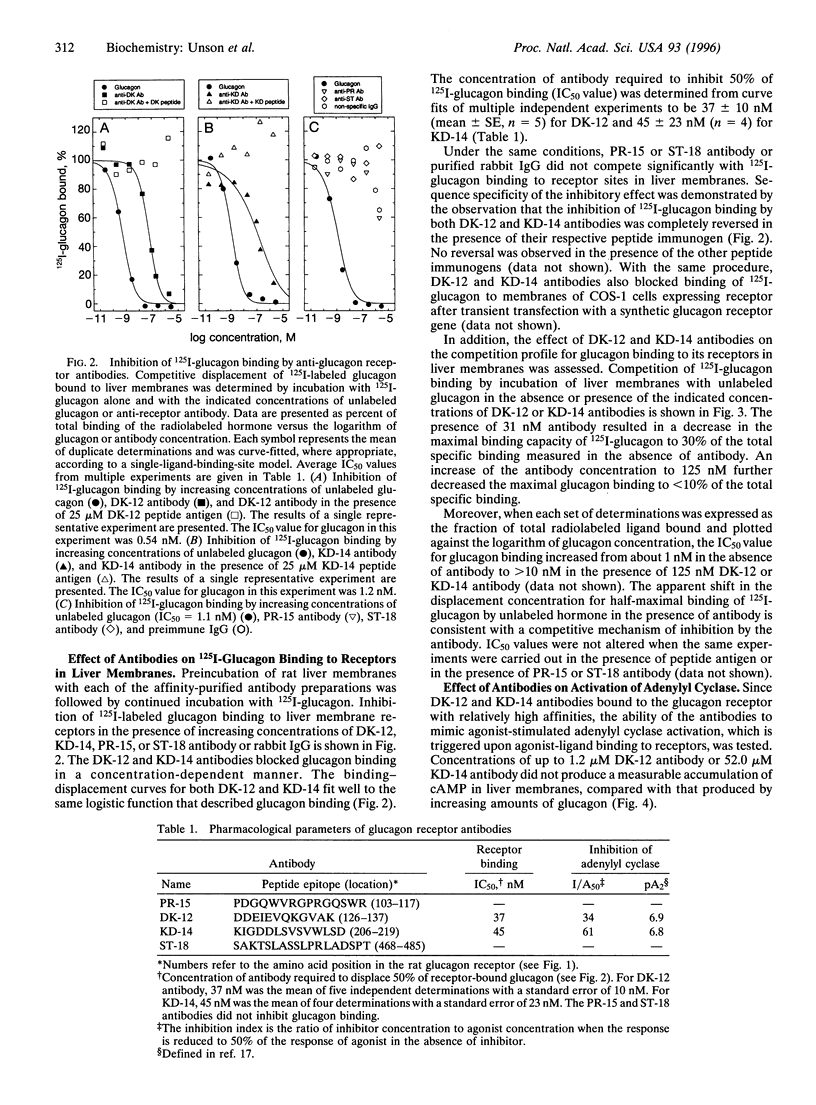
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
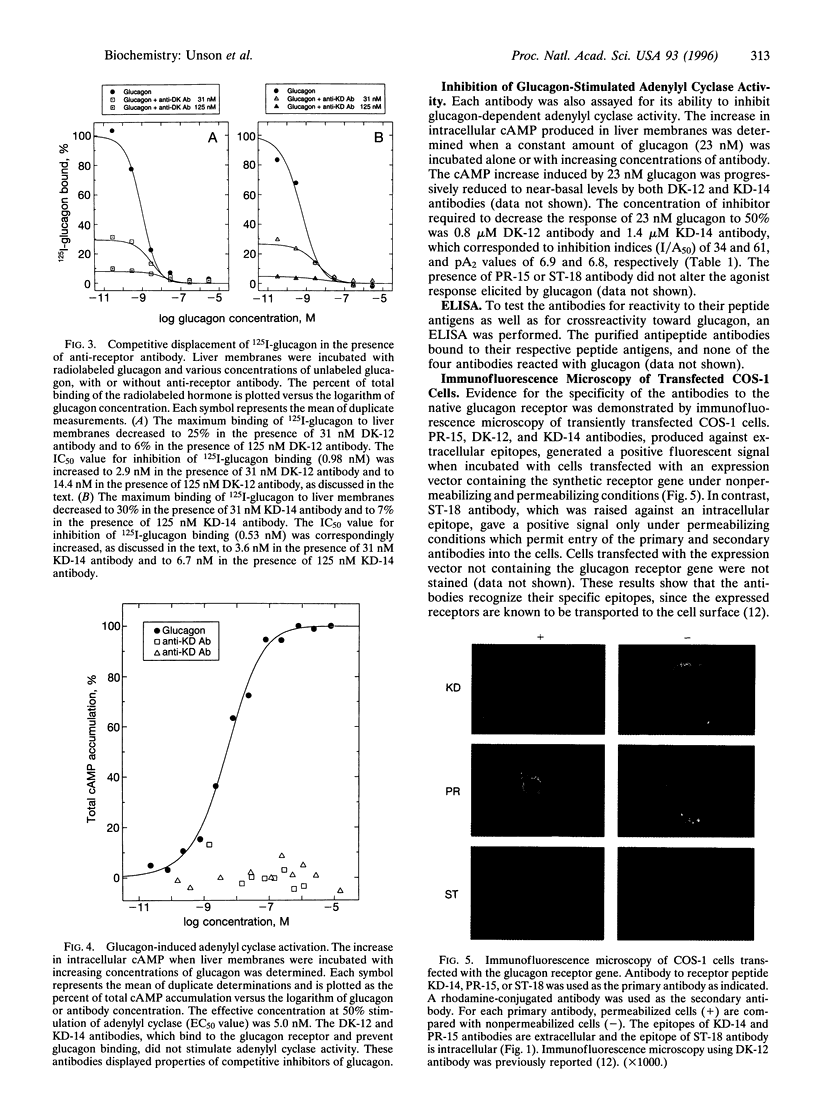
- Buggy J. J., Livingston J. N., Rabin D. U., Yoo-Warren H. Glucagon.glucagon-like peptide I receptor chimeras reveal domains that determine specificity of glucagon binding. J Biol Chem. 1995 Mar 31;270(13):7474–7478. doi: 10.1074/jbc.270.13.7474. [DOI] [PubMed] [Google Scholar]
- Burcelin R., Li J., Charron M. J. Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene. 1995 Oct 27;164(2):305–310. doi: 10.1016/0378-1119(95)00472-i. [DOI] [PubMed] [Google Scholar]
- Carruthers C. J., Unson C. G., Kim H. N., Sakmar T. P. Synthesis and expression of a gene for the rat glucagon receptor. Replacement of an aspartic acid in the extracellular domain prevents glucagon binding. J Biol Chem. 1994 Nov 18;269(46):29321–29328. [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Hjorth S. A., Schambye H. T., Greenlee W. J., Schwartz T. W. Identification of peptide binding residues in the extracellular domains of the AT1 receptor. J Biol Chem. 1994 Dec 9;269(49):30953–30959. [PubMed] [Google Scholar]
- Holtmann M. H., Hadac E. M., Miller L. J. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J Biol Chem. 1995 Jun 16;270(24):14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Jelinek L. J., Lok S., Rosenberg G. B., Smith R. A., Grant F. J., Biggs S., Bensch P. A., Kuijper J. L., Sheppard P. O., Sprecher C. A. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993 Mar 12;259(5101):1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- Jüppner H., Schipani E., Bringhurst F. R., McClure I., Keutmann H. T., Potts J. T., Jr, Kronenberg H. M., Abou-Samra A. B., Segre G. V., Gardella T. J. The extracellular amino-terminal region of the parathyroid hormone (PTH)/PTH-related peptide receptor determines the binding affinity for carboxyl-terminal fragments of PTH-(1-34). Endocrinology. 1994 Feb;134(2):879–884. doi: 10.1210/endo.134.2.8299582. [DOI] [PubMed] [Google Scholar]
- MacNeil D. J., Occi J. L., Hey P. J., Strader C. D., Graziano M. P. Cloning and expression of a human glucagon receptor. Biochem Biophys Res Commun. 1994 Jan 14;198(1):328–334. doi: 10.1006/bbrc.1994.1046. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mayo K. E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992 Oct;6(10):1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- Siciliano S. J., Rollins T. E., DeMartino J., Konteatis Z., Malkowitz L., Van Riper G., Bondy S., Rosen H., Springer M. S. Two-site binding of C5a by its receptor: an alternative binding paradigm for G protein-coupled receptors. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1214–1218. doi: 10.1073/pnas.91.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unson C. G., Cypess A. M., Kim H. N., Goldsmith P. K., Carruthers C. J., Merrifield R. B., Sakmar T. P. Characterization of deletion and truncation mutants of the rat glucagon receptor. Seven transmembrane segments are necessary for receptor transport to the plasma membrane and glucagon binding. J Biol Chem. 1995 Nov 17;270(46):27720–27727. doi: 10.1074/jbc.270.46.27720. [DOI] [PubMed] [Google Scholar]
- Unson C. G., Gurzenda E. M., Merrifield R. B. Biological activities of des-His1[Glu9]glucagon amide, a glucagon antagonist. Peptides. 1989 Nov-Dec;10(6):1171–1177. doi: 10.1016/0196-9781(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Unson C. G., Macdonald D., Merrifield R. B. The role of histidine-1 in glucagon action. Arch Biochem Biophys. 1993 Feb 1;300(2):747–750. doi: 10.1006/abbi.1993.1103. [DOI] [PubMed] [Google Scholar]
- Unson C. G., Macdonald D., Ray K., Durrah T. L., Merrifield R. B. Position 9 replacement analogs of glucagon uncouple biological activity and receptor binding. J Biol Chem. 1991 Feb 15;266(5):2763–2766. [PubMed] [Google Scholar]
- Unson C. G., Wu C. R., Fitzpatrick K. J., Merrifield R. B. Multiple-site replacement analogs of glucagon. A molecular basis for antagonist design. J Biol Chem. 1994 Apr 29;269(17):12548–12551. [PubMed] [Google Scholar]